Abstract
Polyaniline (Pani) films prepared on Au wires were employed as substrates to deposit Pt, Pt-Ru, Pt-Os, Pt-Mo and Pt-Ru-Os or Pt-Ru-Mo by using appropriate working solutions and a potential-programmed perturbation. The atomic percentages of the different metals on Pani were determined by EDAX and their particle size and distribution by SEM. The catalytic activity was tested for adsorbed CO and CH3OH electrooxidation. Accordingly, the best binary and ternary metal combination resulted in Pt-Ru and Pt-Ru-Os.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The search for suitable anode materials for a methanol fuel cell confronts two important obstacles related to the qualities of the catalytic material and its distribution on a suitable matrix. For methanol oxidation, platinum has the highest catalytic activity. Unfortunately, after adsorption, methanol generates carbon monoxide, which adsorbs and blocks the Pt active sites and requires another oxygen atom to free the surface, giving CO2.Since water adsorbs at almost 0.6 V, Pt anodes require excessive polarization to satisfy the oxygen demand [1, 2]. For a direct methanol fuel cell (DMFC), the result is a loss of cell voltage and efficiency. In regard to this problem, many transition metals have been proposed as co-catalysts to improve the rate of CO and methanol oxidation because they exhibit one or several redox systems with redox potentials close to that of hydrogen or methanol [3, 4]. Thus, it has been found that Ru is one of the most effective Pt co-catalysts for methanol oxidation. The role of Ru is to provide oxygenated species to convert adsorbed methanol fragments on neighbouring Pt atoms into carbon dioxide [5, 6].
Lately, it has been claimed that a better performance than that of Pt-Ru should be obtained with multimetallic particles [7, 8]. Many authors have reported either Mo or Os as the most relevant third component to add to a given Pt-Ru composition [9]. Mo addition as the third component was studied by Lamy and co-workers [10], who reported a notably higher current density for methanol oxidation than after the addition of other elements such as Co, Ni or Fe.
Osmium is partially oxidized at −0.05 V and plays the role of oxygen transfer agent in a bi-functional mechanism [11]. Since Os is a noticeably reactive metal, with at least nine different oxidation states reported [12], it is important to take into account that the transformation of active oxides to inactive forms with higher oxidation states may cause a loss of efficiency [13].
For most of the electrode materials the electrochemical reaction is limited to the catalytic surface. One way of obtaining a better distribution of the catalytic particles is to disperse the material within a convenient electron conducting matrix, achieving efficient transport of charge from the underlying support electrode to the reaction site. Among conductive polymers [14, 15], polyaniline (Pani) is a particularly attractive material to be used as catalyst support because it is a conductor in its partially oxidized state (which occurs in the potential range where most organic fuels oxidize), adheres strongly to the electrode surface and also has high conductivity and durability under conditions applicable to the operation of fuel cells employing aqueous acidic electrolytes. The incorporation of metallic particles into polymer matrices has gained wide interest for electrocatalytic purposes [16, 17, 18, 19, 20].
A repetitive square wave potential signal (RSWPS) is conceived as a suitable method to obtain a better scattering of metallic particles [21], improving the electrocatalytic properties of the modified electrodes for methanol oxidation [18, 20]. In this work, different metals, namely Pt, Ru, Os and Mo, were examined as appropriate binary Pani-Pt-Mx and ternary Pani-Pt-Mx-My electrodes, applying the RSWPS technique. Our goal is to ascertain the composition that yields the best Pt-Mx and Pt-Mx-My relationship on the polymeric matrix to improve the oxidation of adsorbed CO and methanol, scrutinize its morphology and compare its electrocatalytic activity.
Experimental
Au wires of 0.08 cm2 geometric area were used as substrates for polymer film deposition. An Au foil was employed as counter-electrode and a saturated calomel electrode as reference. However, all the potentials in the text are referred to the reversible hydrogen electrode (RHE). The working electrodes were prepared by depositing a Pani film on an Au wire by applying cyclic voltammetry at 0.1 V s−1 between −0.04 V and 0.92 V in a 0.1 M aniline+0.5 M H2SO4 solution [22]. The film thickness was determined from the anodic charge involved between −0.04 and 1.24 V in a cyclic voltammogram run at 0.1 V s−1 in a 0.5 M H2SO4 solution. Pani films with average thickness of about 0.5 μm were employed.
Pani electrodes were decorated with either Pt, binary Pt-Ru, Pt-Os and Pt-Mo or ternary Pt-Ru-Os and Pt-Ru-Mo particles using a RSWPS between a lower potential E l=−0.2 V and an upper potential E u=0.6 V with a frequency of 2.5 kHz for a time t=10 min. The working solutions to co-deposit the metals were aqueous acid solutions, namely 0.1 M HClO4 containing 9×10−3 M H2PtCl6, 1.5×10−2 M RuCl3 and either OsO4 (1×10−4 M<OsO4<1×10−2 M) or (NH4)6Mo7O24 (1×10−4 M<(NH4)6Mo7O24<10−2 M).
The electrocatalytic behaviour of the electrodes was followed through the stripping peak potential of adsorbed CO, E peakCO, or by measuring the quasi-steady oxidation currents of 0.5 M CH3OH in 0.1 M HClO4 at different anodic potentials. The CO stripping voltammogram was run at 0.01 V s−1, after CO adsorption at −0.04 V from a CO-saturated 0.5 M H2SO4 solution for 10 min, and subsequent bubbling of N2 for another 10 min. All experiments were performed at room temperature. Real areas were determined considering the anodic charge involved under the CO stripping peak.
Electrodispersed metals on Pani electrodes were characterized by SEM and their compositions determined by EDAX.
Results and discussion
CO stripping
The electrocatalytic behaviour of Pani-Pt electrodes
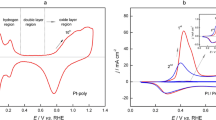
Pt deposits on Pani are actually achieved because the reduction of Pt(IV) to Pt(0) occurs at potentials above that corresponding to the first oxidation potential peak (E peak ca. 0.44 V) of the polymer that acts as a mediator in the metal deposition process. Pani is conductive in the oxidized state and non-conductive in the reduced state [14]. An anodic peak of CO electrooxidation, E peakCO, at ca. 0.73 V is detected in a cyclic voltammogram recorded at 0.01 V s−1 after CO adsorption (Fig. 1a). According to SEM micrographs (Fig. 1b), the particles are dispersed in the bulk of Pani agglomerates, exhibiting an average size of ca. 90 nm. Although we measured the cathodic charge involved in the deposition process, we could not calculate the size of the catalytic particles as for potentiostatic Pt deposition experiments [23], because the RSWPS signal changes the applied potential value periodically and its cathodic potential value allows not only Pt deposition but also hydrogen evolution.
The electrocatalytic behaviour of Pani-Pt-Mx electrodes
Catalytic Pani-Pt-Mx electrodes (where Mx stands for Ru, Os or Mo) were tailored by applying the RSWPS to Pani substrates immersed in a suitable aqueous acidic solution.
The electrocatalytic behaviour of Pani-Pt-Ru electrodes
To prepare Pani-Pt-Ru electrodes, the E u value found for RSWPS Pt deposits on Pani [18] was modified, since at potentials above 0.7 V, corrosion and loss of Ru has been reported [5].
The stripping voltammogram of adsorbed CO on a Pani-Pt-Ru electrode obtained after 10 min RSWPS is shown in Fig. 1c. The effect of Ru addition on Pt is measured throughout the shift of the onset of CO oxidation towards lower potentials on Pani-Pt-Ru compared to Pani-Pt electrodes [18]. Although a sole Ru deposit on Pani is impossible [20], Pt-Ru deposits on Pani are obtained by taking into account that Pt is easily reduced on Pani films and that the potential of zero charge is ca. 0.2 V for Pt and ca. −0.25 V for Ru [24]. Thus, at E l=−0.2 V, Pt would be negatively charged and the deposition of Ru on Pt should be more rapid than on Ru alone [25]. Moreover, the formation of alloys is accompanied by a change in the free energy of the components. Hence, the equilibrium potential of each metal should shift towards more positive values. It is pointed out that for any Pani-Pt-Ru combination the stripping CO peak shifts towards more negative values when compared to the one obtained with only Pt on the electrode (E peakCO=0.73 V) [18]. Moreover, the more cathodic E peakCO at ca. 0.45 V corresponds to a Pani-Pt-Ru electrode, with 49 at% Ru measured by EDAX [20]. This Ru amount on the surface is close to the optimum value reported to electrooxidize CO, that is 50 at% Ru [5]. For higher Ru percentages, Ru atoms covered the deposited Pt and a coalescence of the deposited particles resulted in larger particles; consequently, real area values diminished [20].
Richarz et al. [26] demonstrated that the composition of Pt-Ru alloy prepared by electrochemical co-deposition depends linearly on the concentration of Pt(IV) and Ru(III) ions in solution. We assumed that the same relationship is accomplished by the other elements; therefore we denote the composition of the electrode by specifying the concentration in mM of Pt(IV), Ru(III) and Mx [Mx=Mo(VI) or Os(VIII) in the electrodeposition solution]. Thus, Pani-Pt-Ru (9:15) means that the electrode has been prepared from an electroplating solution containing 9 mM H2PtCl6 and 15 mM RuCl3 in 0.1 M HClO4 and Pani-Pt-Ru-Os (9:15:1) indicates that the electrode has been prepared from a solution containing 9 mM H2PtCl6, 15 mM RuCl3 and 1 mM OsO4 in 0.1 M HClO4.
Electrocatalytic Pani-Pt-Os or Pani-Pt-Mo electrodes
In Fig. 2, the CO stripping voltammograms on Pani-Pt-Ru (9:15) (Fig. 2a), Pani-Pt-Os (9:10) (Fig. 2b) and Pani-Pt-Mo (9:10) (Fig. 2c) electrodes are depicted to compare their catalytic activities. The E peakCO value resulted in 0.49, 0.55 and 0.65 V, respectively. Ball et al. [27] reported that although Pt-Mo may oxidize adsorbed CO at low potentials, it appears that only a small number of sites are active because the value of E peakCO is similar to that observed with Pt. According to Mukerjee and Urian [28], both Mo and Ru play a distinct role in CO oxidation. On Ru there is a competition between oxide formation and CO adsorption, whereas Mo oxides show no affinity for CO. Therefore, the binary Pt-Ru combination was the most appropriate for adsorbed CO electrooxidation.
The catalytic Pt-Ru-Mx electrodes (Mx=Os or Mo)
Stripping voltammograms of adsorbed CO on different modified electrodes, namely Pani-Pt-Ru and Pani-Pt-Ru-Os, recorded at 0.01 V s−1, show just a slight shift of the onset of the CO peak towards lower potentials for Pani-Pt-Ru-Os in comparison to Pani-Pt-Ru electrodes. Profiting from the same argument used to confirm the role of Ru in Pt-Ru binary catalysts, it has been proposed that the presence of Os in the Pt-Ru assembly produces active oxides which are capable of oxidizing carbon-containing species such as CO formed on Pt sites [29]. According to our results, both metals, Ru and Os, contribute to enhance the cathodic shift of CO oxidation.
No positive effect of Mo addition to Pani-Pt-Ru deposits on adsorbed CO oxidation was found.
Methanol electrooxidation on polymer-modified electrodes
Current densities measured in any Pani-Pt-Ru and Pani-Pt-Ru-My electrodes are referred to real areas determined through the CO oxidation charge, taking as reference that 420 μC=1 cm2. According to Vielstich and co-workers [30], the oxidative stripping of a CO layer at saturated coverages seems to be a very suitable normalization procedure for the study of Pt-based porous catalysts.
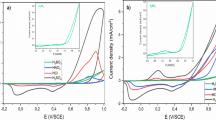
In Fig. 3, the quasi-stationary current densities at 0.5 V for methanol oxidation are plotted as a function of Ru percentage on the surface; the highest current density corresponds to 32 at% Ru. It is emphasized that Gesteiger et al. [31] reported for 0.5 M methanol oxidation at 25 °C an optimum Ru surface concentration fairly low (10 at%) on sputter-cleaned Pt-Ru alloy electrodes. Moreover, in co-deposited Pt-Ru layers, Vielstich and co-workers [30] found that an electrode with 25 at% Ru has the highest catalytic activity for 0.1 M methanol oxidation.
In order to take into account the metal concentration of the different composite electrodes, their atom % values found through EDAX are presented in Table 1. It is interesting to point out that Pani-Pt-Ru-Os (9:15:1) and (9:15:0.1) and also Pani-Pt-Ru-Mo (9:15:0.1) electrodes show only Pt and Ru according to EDAX reports, and a higher Pt concentration and a lower Ru concentration compared with the Pani-Pt-Ru (9:15) electrode. The composite electrodes were employed for methanol oxidation in the following sections.
Pani-Pt-Ru-Mo electrodes
The effect of different Mo concentrations in the solution containing predetermined values of H2PtCl6 and RuCl3 concentrations on the catalytic activity of Pani-Pt-Ru surfaces for 0.5 M methanol oxidation is shown in Fig. 4. Thus, the best performance was achieved with a Pani-Pt-Ru-Mo of (9:15:1). According to Table 1, the electrode composition was 57.5 at% Pt, 36.1 at% Ru and 6.4 at% Mo. Other combinations such as Pt-Ru-Mo (9:15:10) and Pt-Ru-Mo (9:15:0.1) show a minor electrocatalytic effect for methanol oxidation. It has been claimed that Pt-Mo showed no enhancement for methanol oxidation because Mo oxy-hydroxides are inhibited from efficient removal of CO and CHO species, in contrast to Ru oxides [28].
Pani-Pt-Ru-Os electrodes
Different concentrations of OsO4 in the electroplating Pt(IV)+Ru(III) solution influence the catalytic properties of the tailored Pani-Pt-Ru-Os electrodes Thus, the oxidation current densities measured for methanol in the potential range 0.45–0.65 V with electrodes with different Pt-Ru-Os combinations on the surface are shown in Fig. 5. In order to compare the catalytic performance, a Pani-Pt-Os (9:10) electrode was added to the plot. The EDAX composition was 51 at% Pt and 49 at% Os and its electrocatalytic activity was poor. In this respect, Pani-Pt-Ru-Os (9:15:10) showed a better catalytic activity; the electrode composition determined by EDAX (Table 1) was 39 at% Pt, 30 at% Ru and 31 at% Os. The best electrocatalyst was the electrode Pani-Pt-Ru-Os (9:15:0.1); the EDAX analysis of this electrode indicates 56 at% Pt, 44 at% Ru but no at% value for Os was found. It seems as if the Os concentration on the surface was below the detection limit of the technique (≤1%).
Comparison between different ternary catalysts
The current density values associated with 0.5 M CH3OH oxidation on Pani-Pt-Ru and on each of the best combination Pani-Pt-Ru-Mx prepared surfaces are plotted as a function of the applied potential in Fig. 6.
Regarding the picture, we reach the conclusion that the best Pani-Pt-Mx-My catalyst for methanol oxidation is Pani-Pt-Ru-Os (9:15:0.1), meaning that Os as the third component gives a better performance in comparison to Mo.
Conclusions
-
1.
The RSWPS routine proved to be an appropriate method to electrodisperse metals on Au/Pani films.
-
2.
A co-deposition of Ru and Pt ions from suitable combinations of H2Cl6Pt and RuCl3 solutions on Pani films produces Pani-Pt-Ru electrodes exhibiting catalytic particles around 90 nm in size and noticeable catalytic properties for CO and methanol oxidation.
-
3.
The best Ru concentration on Pani-Pt-Ru electrodes, measured by EDAX, resulted in 49 at% for CO oxidation and 32 at% for methanol oxidation
-
4.
Co-deposition of Os with Pt and Ru on Pani gives the best catalyst for CO and CH3OH oxidation.
-
5.
Co-deposition of Os and Pt on Pani gives a poor catalyst for CH3OH oxidation.
-
6.
Co-deposition of either Pt and Mo, or Pt and Ru and Mo, on Pani produces worse catalysts when compared to Pani-Pt-Ru for both CO and CH3OH oxidation.
References
Kunimatsu K (1986) J Electroanal Chem 213:149
Wilhelm S, Iwasita T, Vielstich W (1987) J Electroanal Chem 238:383
Anderson AD, Grantscharova E, Seong S (1996) J Electrochem Soc 143:2075
Wang Y, Fachini R, Cruz G, Zhu Y, Ishikawa Y, Colucci JA, Cabrera CR (1999) J Electrochem Soc 146:1613
Gasteiger HA, Markovic MN, Ross PN Jr, Cairns E (1993) J Phys Chem 97:12020
Iwasita T, Nart FC, Vielstich W (1990) Ber Bunsenges Phys Chem 94:1030
Reddington E, Sapienza A, Gurrar B, Viswanathan R, Sarangapani S, Smotkin ES, Mallouk TE (1998) Science 280:1735
Gotz M, Wendt H (1998) Electrochim Acta 43:3637
Liu R, Iddir H, Fan Q, Hou G, Bo A, Ley K, Smotkin ES, Sung YE, Kim H, Thomas S, Wieckowski A (2000) J Phys Chem B 104:3518
Lima A, Coutenaceau C, Leger J-M, Lamy C (2001) J Appl Electrochem 31:379
Crown A, Moraes IR, Wieckowski A (2001) J Electroanal Chem 500:333
Llopis JF, Colom F (1976) Osmium. In: Bard AJ (ed) Encyclopedia of electrochemistry of the elements, vol 6. Dekker, New York, p 235
Zhu R, Cabrera CR (2001) Electrochem Solid State Lett 4:A45
Stilwell DE, Park Su-Moon (1988) J Electrochem Soc 135:2254
Inzelt G (1990) Mechanism of charge transport in polymer-modified electrodes. In: Bard AJ, Rubinstein I (eds) Electroanalytical chemistry, vol 18. Dekker, New York, p 89
Ocon Esteban P, Leger J-M, Lamy C, Genies E (1984) J Appl Electrochem 19:462
Hable CT, Wrigton MS (1991) Langmuir 7:1305
Castro Luna AM (2000) J Appl Electrochem 30:1137
Bouzek K, Mangold KM, Juttner K (2000) Electrochim Acta 46:661
Kessler T, Castro Luna AM (2002) J Appl Electrochem 32:825
Zubimendi JL, Andreasen G, Triaca WE (1995) Electrochim Acta 40:1305
Biaggio S, Oliveira C, Aguirre MJ, Zagal J (1994) J Braz Chem Soc 5:203.
Croissant MJ, Napport T, Leger J-M, Lamy C (1998) Electrochim Acta 43:2447
Raub E, Muller K (1967) Fundamentals of metal deposition. Elsevier, Amsterdam
Gorbunova KM, Polukarov YuM (1969) Electrodepostion of alloys. In: Khomutov NE (ed) Electrochemistry electrodeposition of metals and alloys. Israel Program for Scientific Translations, Jerusalem, pp 28–70
Richarz F, Wohlmann B, Vogel U, Hoffschulz H, Wamdelt K (1995) Surf Sci 335:361
Ball S, Hodgkinson A, Hoogers G, Maniguet S, Thompsett D, Wong B (2002) Electrochem Solid State Lett 5:A31
Mukerjee S, Urian R (2002) Electrochim Acta 47:3219
Petrii OA, Kalinin VD (1999) Russ J Electrochem 35:627
Iúdice De Souza JP, Iwasita T, Nart FC, Vielstich W (2000) J Appl Electrochem 30:43
Gesteiger H, Markovic N, Ross PN Jr, Cairns EJ (1994) J Electrochem Soc 141:1795
Acknowledgements
The authors are grateful to Comisión de Investigaciones de la Provincia de Bs As (CIC) and Agencia Nacional de Promoción Científica y Tecnológica de Argentina for financial support. T.K. and A.M.C.L. are members of the research career at CIC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Wolf Vielstich on the occasion of his 80th birthday in recognition of his numerous contributions to interfacial electrochemistry
Rights and permissions
About this article
Cite this article
Kessler, T., Castro Luna, A.M. Catalytic polyaniline-supported electrodes for application in electrocatalysis. J Solid State Electrochem 7, 593–598 (2003). https://doi.org/10.1007/s10008-003-0360-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-003-0360-5