Abstract
The association of Cu–X (X = –H, –Cl, and –F) with H2CCHCHmYn and HCCCHmYn (Y = –Cl, –F, –OH, –CH3) has been studied at a high level of theory. The density functional theory (DFT) at B3LYP/6-311G(d,p)//B3LYP/6-311 + G(3df,2p) level has been chosen to calculate the structure and the relative stability of 24 different complexes. The interaction of Cu–F with the derivatives of ethylene and acetylene was found very strong, with interaction energies close to those of conventional covalent bonds. In all complexes, the most stable structure was found when Cu–X is positioned on the unsaturated CC bond, forming a three-membered ring that leads to longer CC bond distances. Both ethylene and acetylene complexes show similar trends of interaction energies with respect to the same moiety. All electronic indexes analyzed by means of the QTAIM, ELF, and NBO formalisms indicate that the strength of the interaction should increase with the number of withdrawing substituents in both series of compounds.

The p-Interaction of ethylene and acetylene derivative with fluoride copper. The ELF graphs and its 2D projection show the disynaptic basins of the electrostatic binding
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-covalent intermolecular forces play a major role in biological systems and mediating processes such as receptor-ligand interactions, enzyme bindings, and antigen-antibody recognition. When transition metals are involved, it is known that they form complexes with conjugated systems, this is especially true with cations; they bind to rich-electron π systems, phenomena that have been observed in the gas phase and solvated systems [1,2,3,4]. The most important feature of this type of non-covalent interactions is that they are mostly electrostatic [5,6,7,8,9].
Copper is an essential trace element found in small amounts in cells and tissues. It is required for structural and catalytic properties of a variety of important enzymes, including cytochrome oxidase, tyrosinase, p-hydroxyphenyl pyruvate hydrolase, dopamine beta hydroxylase, lysyl oxidase, and Cu–zinc superoxidase dismutase (Cu, Zn–SOD) [10]. Furthermore, amino acids, particularly histidine, methionine, and cysteine, when they are linked with Cu, allow absorption through an amino acid transport system [11]. For instance, although Cu+ complexes do not exhibit visible absorbance due to a full d-valence band, they absorb strongly in the UV region of the spectrum due to charge transfer between the metal and the associated ligands [11].
In biological systems, it is well known that Cu binds to proteins or cofactors via cation–π interactions. It is very important to state that biological systems rely on this type of interaction to create photoreceptors, link with peptides, and are capable to withdraw a system inside a hydrophobic cavity inside a protein [12, 13].
The oxidation state of the metal is also an important point to understand the behavior of the systems. Cu ions can exist in both an oxidized cupric (Cu2+) and reduced cuprous (Cu+) state. One of the relevant oxidation states of Cu to understand the intrinsic behavior of these complicated systems by investigating the interactions is Cu(I), which is also presented in biochemical media.
Due to the relation of copper with biological reactions, it is vital to study the interaction of this metal linked to a counter ion with model π C=C and C≡C involving electron-donating and electron-withdrawing substituents in order to understand the nature of the interactions of copper and these organic systems. Cu(I), due to its closed-shell electronic configuration [Ar]3d10, presents spherical symmetry, preferring linear bicoordination, trigonal tricoordination, and tetrahedral or tetracoordination structures.
Taking that as antecedent and based on the recent study on fluorinate ethylene and acetylene compounds towards CuF [14, 15], the present study will focus on the study of 2-substituted prop-1-ene and prop-1-yne derivatives towards CuX (X = H, F, Cl). The main aim is to explore the impact of substituting of position 2 of the ethylene and acetylene on the interaction between the double/triple bonds and halide/hydride copper. Replacing various substituents might contribute in different ways in the interacting zones by strengthening or weakening bonds. The main objective is to understand the different factors that are responsible for the CuX-π interaction using electron-donating and withdrawing substituents in the 2 position, especially those which could be observed between both interacting species when replacing the counter ion in CuX by F, Cl, and H.
Computational details
Density functional theory (DFT) was chosen for geometry optimization and vibrational frequency calculations of the molecules, since previous theoretical works show a good performance for this kind of systems [14]. The geometry of the monomers and complexes under survey has been fully optimized using B3LYP [16, 17] functional combined with 6-311G(d,p) basis set. The same level of theory was used to calculate the harmonic vibrational frequencies to confirm that the stationary points found correspond to local minima of the potential energy surface. Frequency calculations were also used to evaluate the zero-point energy (ZPE), thermal correction of enthalpy, and thermal correction of free energy. To ensure reliable energies, single-point calculations on the optimized geometries were achieved at B3LYP/6-311 + G(3df,2p) level of theory. The basis set superposition error has not been considered in our calculations since the basis set considered is so large that this error could be negligible [18, 19].
Previous studies stated that the B3LYP optimized geometries are very close to those given by CCSD(T); both methodologies differ by less than 4 kJ/mol when optimizing this type of structures [15]. Therefore, for our calculations, we have chosen B3LYP to optimize geometries and obtain the energies of the systems. All the calculations have been carried out with the Gaussian09 computational package [20].
The study of the electronic density and the chemical bonding between the interacting entities was done by means of three population analysis techniques: QTAIM (quantum theory of atoms in molecules) [21, 22], ELF (electron localization function) [23], and NBO (natural bond orbital) [24]. Each one of these techniques has been performed in order to answer specific issues. For the QTAIM, we focused on the electron density and its laplacian values at the most important bond critical points (BCPs) of the systems. Within the ELF techniques proposed by Becke and Edgecombe and which allow the division of the density in regions where electron pairs are localized, we performed a detailed inspection of the interacting areas. The NBO was used in order to understand the orbital exchange between the metal and the double and triple bonds by means of the second-order orbital interaction energies. The QTAIM calculations were carried out with the AIMall [25] computational package. The calculations of NBO were done by means of the NBO6 series of programs [26], whereas ELF results were obtained by TOPMOD suit of programs [27].
To get the electronic factors that contribute to the energy binding between the unsaturated organic moiety with CuY, the ETS-NOCV energy decomposition scheme has been used. This scheme, originated from a combination of the extended transition state (ETS) [28] energy decomposition approach with the natural orbitals for chemical valence (NOCV) analysis [29, 30], presents the binding energy as the sum of four energy components divided into two categories: attractive and repulsive. For the attractive components, we find electrostatic interaction energy, ΔEelstat, and the orbital interaction energy, ΔEorb, which correspond to the classical electrostatic interaction and the orbital interactions between the involved fragments. For the repulsive components, we can cite Pauli repulsion energy, ΔEPauli, and the steric energy, ΔEster, which account for the repulsive interactions between the occupied orbitals and the hindrance of the fragments. To obtain this decomposition, Amsterdam Density Functional (ADF) suite of programs [31] has been used by applying the ETS-NOCV procedure at B3LYP/TZ2P level of theory [16, 17] on the optimized structures.
In order to account for the stability of these complexes, we estimate their dissociation (De) and their interaction (Eint) energy. De is defined as the difference between the energy of the complex and the energy of the two subunits separated at infinite distance in their electronic ground states, and Eint is the difference between the energy of the complex and the energy of the monomers at the geometry they have within the complex. To obtain the interaction energies, we calculate the deformation energy (Edef) of the subunits which is given as the energy difference between the monomers at the equilibrium geometry and at the geometry in the complex. Edef is added to the De to obtain the final interaction energies, using the following equation coming from the thermodynamic cycle shown in Scheme 1.
Results and discussion
As mentioned above, our main objective is to study the interaction of CuY (Y = H, Cl, F) with 2-subtituted prop-1-ene and prop-1-yne. We have calculated eight derivatives of ethylene and acetylene; to get in this objective, three types of substituents have been chosen: (i) an electronic donor substituent reflected by methyl and tert-butyl substituents in position 2, (ii) an electronic acceptor substituent done by replacing one or the three hydrogen atoms in the earlier methyl by fluorine or chlorine atoms, and (iii) a resonant electronic donor such as hydroxyl group(s) attached to position 2 instead of one or the three hydrogen atoms. It is worth noting that two of the considered substituents of (i) are bulky in order to analyze their structural influence towards CuY. Since many of the species under study could present different conformational structures, a previous exploration of the possible rotamers was done. In Tables 1 and 2, the values of the dissociation (De), deformation (Edef), and interaction (Eint) energies of the acetylene and ethylene-CuY complexes released on the most stable conformer are reported.
The first conspicuous fact is that the interaction between all the studied compounds with the different copper-halides is very strong. This is reflected by the higher absolute values of the dissociation energies observed which values are close to the energy of a conventional covalent bond. In our three sets of complexes, Cu–F set gives the strongest complexes with dissociation energies in a range 130–150 kJ mol−1, while Cu–H complexes are the weakest with a De in the range 60–83 kJ mol−1. Dissociation energies of Cu–Cl complexes have been found to be oscillating between 105 and 131 kJ mol−1, which makes them stronger compared with Cu–H complexes, yet, still weaker than Cu–F complexes [15]. This is due mainly to the increased electronegativity of the atom bonded to copper when going from proton to fluorine through chlorine atom.
Exploring the structural changes due to this interaction, the contribution of fluorine compared with chlorine or hydrogen lead to great perturbations of the organic moiety when the interaction takes place. Hereafter, the interaction of Cu–Y brings almost no perturbation to double or the triple bond in acetylene or ethylene. The lost of planarity in the former and the angular changes in the later are good reflections of these interactions. For Cu–F interaction, the angle α, following Scheme 2, in the prop-1-ene is around 162° while for Cu–H the same angle is estimated at about 165°. For acetylene set of compounds and taking prop-1-yne as a reference, the angle α is about 160° for copper fluorine while for copper hydride it is around 164°. This demonstrates that although the interaction is strong, the hybridization of the unsaturated carbon apparently presents slight changes.
To understand why geometry does not substantially change under substitution, we tried to explore the deformation energy. In this step, we will expand Scheme 1 to include two new energy components. The interaction between copper halide and acetylene/propene can be viewed also by considering the deformation of the complex besides the deformation of the monomer. So De can be partitioned into two energy components: the interaction energy and the deformation energy.
Taking into account the possibility of two processes, these values can be associated with the monomer deformation, Edef or to the complex deformation, Edef(c). The interaction energy is, thus, related to the interaction of the undistorted monomers Eint or the interaction to get the deformed one Eint(c). Since the copper halide is linear, its deformation energy appears negligible (around 0.5 kJ/mol). For this objective, in Figs. 1 and 2, we have selected some significant derivatives of ethylene and acetylene. The main reason is to highlight the intrinsic transformation with and without Cu–Y association. So the most important changes can be obtained on the unsaturated species.
The analysis of the values represented in Figs. 1 and 2 shows that the energy cost to deform the complex is always smaller than the one needed to deform the isolated monomer. Independently of the substituent nature and the type of the unsaturated organic compounds, Edef appears always higher than Edef(c). The exploration of the variation between these two values appears to be the reasonable way to analyze the different effects affecting the complexation. Hence, to analyze the substituent effect on the interaction of acetylene and ethylene with CuY, it seems sufficient to examine the changes involving the hydride derivative. In fact, avoiding the halogen effect on the copper, the major contributions may be provided by the substituent attached to position 2.
In the acetylene–CuH and ethylene–CuH complexes, the differences between Edef and Edef(c) increase when going from the least to the most electronegative substituent. The withdrawing electronic effect of the substituent appears the most important factor that accentuates the gap between these two values along the studied set of compounds. Indeed, if we take the tert-butyl substitution in prop-1-ene, the difference between Edef and Edef(c) is about 3 kJ/mol, when CuH is involved, whereas in the CF3 substitution this difference attains about 10 kJ/mol (see Fig. 1).
To recover the halogen effect on copper in the interaction, we can trace the variation of the gap between Edef and Edef (c) on the terc-butyl derivative. In fact, switching the hydrogen by chlorine, the difference increases from 3 to approximately 6 kJ/mol. If we take CuF, the difference between these values is incremented to attain about 9 kJ/mol. These variations can be generalized for all the compounds under scrutiny with the exception of ethylene substituted by C(OH)3 where Edef (c) is observed higher than Edef and so the gap is negative. This exception can be understood by the OH hydrogen bonds that take place when CuCl interacts with the organic moiety, also by the lack of rotational freedom of this substituent. The deformation in this case does not only involve the structure of the complex but the weakening or the breaking of the hydrogen bond between chlorine and one of hydroxyl hydrogen.
Summarizing the deformation energy is important in the complexation process; however, it is not perceptible in the structural changes that the organic moiety suffers. It is apparently clear that all the alterations are mostly electronic. Hence, to get deeply on this, different analyses have been done through various population analysis methods. The electronic changes when going from the interaction of CuH to CuF can be displayed by the amount of the electronic charge transferred from the acetylene or ethylene to copper halide. For example, the estimated natural charge transferred to CuH is about 0.15 |e| when considering prop-1-ene whereas it rounds about 0.19 |e| when the same compound is associated to CuF. Apparently these two values are close; however, if we analyze the natural charge on copper in each case, the differences appear more perceptible. This shows the great effect of the halogen electronegativity on the strength of the interaction. In fact, the natural charge of copper appears close to unity when copper fluorine is considered while in CuH the metal holds a natural charge slightly more than the half of unity. The alteration is more noticeable if we focus on the CuY linkage after complexation (see table 1S in supporting info).
By analyzing the linkage in CuY, we can also understand the reactivity of these complexes. If we focus primarily on CuH, for the isolated molecule, the bond appears close to covalency with a Wiberg bond index (WBO) close to 1. For complexes throughout the different substituents, this bond appears electrostatic with an appreciable covalent character. The WBO in the set of acetylene and ethylene is in the range between 0.690 and 0.760. On the other hand, in the copper halide and precisely in CuF, the strength of the interaction evolves to a pronounced electrostatic bonding between the metal and the halogen. In fact, if in the isolated species the Wiberg index is close to 0.6 in the complex, this quantity oscillates from 0.3 to 0.35. So, our findings agreed with theoretical and experimental evidence that states that π systems bind metal cations by electrostatic interactions. This can be ratified by the energy decomposition applying ETS-NOCV procedure. Within this analysis, the contribution of the electrostatic interaction appears very important in both type of systems (see Figs. 3, 4).
If we focus deeply on the results displayed in Figs. 3 and 4, it is clear that the steric and orbital interactions participate in less extent than Pauli and the electrostatic components. Analyzing the effect of each substituent on the interaction of CuY might show the differences between complexes. So, the highest energy contribution to the electrostatic component for ethylene complexes is observed for R–C(OH)3 and C(CH3)3 within CuF. It seems that contrary to chemical intuition, which would predict that steric contributions are the most important, they counterbalance the effect with Pauli repulsion, where CuY species are the ones that are driving all the changes. With respect to the steric contribution, it is higher for C(F)3 + CuF and C(Cl)3 + CuF association. This might be expected due to the electronic repulsion coming from the valence shell electrons on both ethylene and copper monomers.
Regarding the energy decomposition of acetylene complex, listed by substituent and copper salt, the behavior along the set of studied compounds is slightly similar as the ethylene complexes with some marked differences in certain substituents (see Fig. 4). The major electrostatic energy is located at CF3 + CuCl and C(CH3)3 + CuCl. Again here, it seems that copper salt plays a major role in these systems; the largest contribution is with the bulkiest and free rotating substituent C(CH3)3, which also presents a marked Pauli energy, due to the repulsions between electrons of the π cloud and halide copper.
It is worth noting that the orbital energy component in the interaction energy for all the studied compounds has an important participation in the attractive component of the total energy of the association between hydride/halide copper and the organic moiety. In fact, a percentage of the orbital energy oscillates from 32 to 40% of the attractive energy, which shows a great stabilizing electronic exchange between Cu–Y orbitals and the π/π* orbitals of the double/triple bond of ethylene and acetylene. In fact, the examination of the perturbation at the second order in NBO population analysis shows higher energy between empty and occupied orbitals of the involved fragments. Thus, for the CuH interacting with propylene, the electronic donation from copper πC=C orbital to the anti-bonding σ*Cu–H orbital is estimated about 152 kJ/mol while the retro-donation from the Cu 4d orbital to the anti-bonding π*C=C accounts for about 150 kJ/mol. These values are substantially increased when the hydrogen attached to the metal is replaced by fluorine showing a great electronic exchange in the linkage of copper halide with ethylene derivative. The electronic donation from πC=C to σ*Cu–F orbital reaches about 202 kJ/mol where CF3 is involved while the back-donation form Cu 4d to π*C=C orbital attains about 217 kJ/mol. In the case of propyne, the energy of the electronic donation and back-donation involving the same orbitals is smaller than its ethylene counterparts yet still considerably higher.
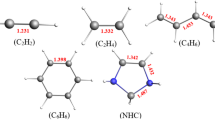
It is thus evident that the electronic donation and back-donation from empty to occupied orbitals play an important role in the orbital energy component of the interaction energy. Furthermore, it can be related to the deformation of the fragments under interaction. As it has been cited above, even though there are no appreciated structural changes that justify sufficiently the values of the deformation energies, the electronic and orbital reorganizations upon association are essential in the interaction of hydride/halide copper with ethylene and acetylene. To understand the effect of the substituent on the deformation energy, we can trace the variation of donation and back-donation energy of the set of compounds under study. That is, the energy deformation correlates well with the energy of the electronic back-donation from Cu(4d) to π*C=C in ethylene (see Fig. 5a). In acetylene, the correlation is not as good as in the previous case yet still significant. This can be explained basically, by the presence of the triple bond so the anti-bonding π*C=C orbital might be oriented differently for some compounds. The main conclusion that we can keep from this relationship is that the perturbation is raised by the 4d orbital of the metal which jointly with the electronegative effect of the halogen are enhancing or reducing the interaction between the halide/hydride copper and the organic moiety. The ELF analysis together with the QTAIM analysis could corroborate this assumption. Figure 6 presented the ELF analysis on some representative isomers that are included in this work, such as ethylene and acetylene complexes with CuH and CuF; the substituents of the unsaturated chain were chosen to address the difference on the electronegativity present of the bulkier substituent CX3 (X = H, F, OH) and analyzing the change of electronegativity on the bond formed with the copper salt.
In ethylene, it can be seen that all the CuH complexes present almost identical zones, where the basin of the π–Cu bond is well defined as a zone with an electronic charge between the π bond and the copper salt. These basins are with an ELF value of about 0.5 by simple observation of Fig. 6. The electron density for this zone can be estimated quantitatively if the QTAIM is checked. In fact, the electron density at the bond critical points of the bonds formed between copper and the carbon atoms of the ethylene are about 0.09 a.u. showing a strong interaction in this zone. This value is practically constant for the different substituents. When CuF–ethylene complex is analyzed, a yellow region, in the ELF analysis, appears on the interacting carbon atoms showing the increase of the interaction gained by the fluoride copper. The ELF is about 0.7 in this case proving a presence of higher interaction between these moieties. The electron density at the BCPs of the bonds involved appears also increased. In this case, the values are about 0.1 u.a.
In acetylene, the CuH and CuF complexes present the same characteristics as in the case of ethylene. The disynaptic basins of the interaction between copper and triple bond appear clearly within an electronic density on the BCP about 0.112 u.a. The effect of the substituent in this case is more notorious than in the case of ethylene. So between acetylene and ethylene, substituted by t-butyl group in their interaction with CuF, the density at the BCP between carbon and the metal appears increased by about 0.012 u.a. The π-cloud of the triple bond is probably responsible for the augmentation of the interaction strength and so its interaction energy in agreement with the above discussion.
Conclusions
We have studied the CuX–π interaction with unsaturated systems such as ethylene and acetylene using different electron-donating and withdrawing substituents at B3LYP/6-311 + G(3df,2p)//B3LYP /6-311G(d,p) level.
The substituent withdrawing effect was revealed to be the most important factor directing the differences of the interaction energy along the separated structures (Cu–X and the unsaturated system). The deformation energy of the complex appears especially more perturbed when a strong withdrawing substituent as CF3 is involved. Even though the deformation energy is important when forming these complexes, the structural perturbation is not redundant since the bonding of Cu–H, Cu–Cl, and Cu–F still quite constant upon complexation.
When analyzing the electronic changes, the halogen impact on the Cu charge is important, the changes on the Wiberg indexes indicate that the complexation bonds between ethylene, acetylene, and copper systems are mostly electrostatic, confirming previous experimental evidence.
The ETS-NOCV index indicates that the electrostatic interactions are very important in both unsaturated systems, especially on CuF complexes and ethylene, being higher with C(OH)3 and C(CH3)3. Pauli repulsion also plays an important role, counterbalancing the interactions. In acetylene, the same behavior is obtained, the most important interactions are electrostatic and Pauli repulsion, being higher on CuF with C(OH)3 and CCl3.
NBO second perturbation energy values indicate that the most important electrostatic effect which stabilizes the complex is between Cu–X orbitals and π/π* orbitals of ethylene and acetylene. Being the most important is the back-donation of the Cu 4d orbital to π* at the unsaturated systems.
The Cu 4d–π* interaction highly correlates with Edef, indicating the nature of the deformation energy on the complex formation, being higher on Cu–F complexes with ethylene and acetylene. The perturbation is raised by the 4d orbital of the metal along with the electronegativity of the halogen and hydride, enhancing or reducing the interaction. ELF and QTAIM analysis showed that CuF presents the higher interaction with ethylene, and higher electron densities at the BCPs of the involved bonds. With acetylene complex, the same behavior is observed.
References
Du X, Fan R, Wang X, Qiang L, Wang P, Gao S, Zhang H, Yang Y, Wang Y (2015) Combined effect of hydrogen bonding and π···π stacking interactions in the assembly of indium(III) metal-organic materials: structure-directing and aggregation-induced emission behavior. Crystal Growth and Design 15(5):2402–2412
Moncho S, Brothers EN, Hall MB (2015) Addition of ethylene to a π-conjugated two-dimensional nickel-based organometallic framework with implications for olefin separation. J. Mol. Mod. 21(5)
Sakamoto R, Wu KH, Matsuoka R, Maeda H, Nishihara H (2015) π-Conjugated bis(terpyridine)metal complex molecular wires. Chem. Soc. Rev. 44(21):7698–7714
Yamabe K, Nomura N, Goto H (2019) Biological quorum sensing molecule-metal complex produces π-conjugated polymer. Inter. J. Poly. Mat. Poly. Biomat. 68(13):805–809
Barrientos L, Miranda-Rojas S, Mendizabal F (2019) Noncovalent interactions in inorganic supramolecular chemistry based in heavy metals. Quantum chemistry point of view. Int. J. Quant. Chem. 119(2)
Oliveira V, Cremer D (2017) Transition from metal-ligand bonding to halogen bonding involving a metal as halogen acceptor a study of Cu, Ag, Au, Pt, and Hg complexes. Chem. Phys. Lett. 681:56–63
Lamsabhi AM, Alcamí M, Mó O, Yáñez M (2003) Gas-phase reactivity of uracil, 2-thiouracil, 4-thiouracil, and 2,4-dithiouracil towards the Cu+ cation: a DFT study. ChemPhysChem 4(9):1011–1016
Lamsabhi AM, Alcamí M, Mó O, Yáñez M, Tortajada J (2004) Association of Cu2+ with uracil and its thio derivatives: a theoretical study. ChemPhysChem 5(12):1871–1878
Lamsabhi AM, Alcamí M, Mó O, Yáñez M, Tortajada J (2006) Gas-phase deprotonation of uracil-Cu2+ and thiouracil-Cu2+ complexes. J. Phys. Chem. A 110(5):1943–1950
Kaur K, Sharma A, Capalash N, Sharma P (2019) Multicopper oxidases: biocatalysts in microbial pathogenesis and stress management. Microbiol. Res. 222:1–13
Abed F, Rayah H, Bouamrane R, Al-Taiar AH (2015) Spectroscopic studies of charge transfer complexes of some amino acids with copper (II). Oriental J. Chem. 31(1):493–497
De Gregorio G, Biasotto F, Hecel A, Luczkowski M, Kozlowski H, Valensin D (2019) Structural analysis of copper(I) interaction with amyloid β peptide. J. Inorg. Biochem. 195:31–38
Li XW, Zhao XH, Li YT, Wu ZY (2019) Synthesis and crystal structure of bicopper(II) complexes: the influence of bridging ligands on DNA/BSA binding behaviors and in vitro antitumor activity. Inorg. Chim. Acta 488:219–228
Arslancan S, Lamsabhi AM, Mó O, Yáñez M (2018) Complexes between cyclopentene and cyclopentyne derivatives with HCu and FCu: the importance of cyclization effects. Int. J. Quantum Chem. 118(9):e25489
Sánchez-Sanz G, Alkorta I, Elguero J, Yáñez M, Mó O (2012) Strong interactions between copper halides and unsaturated systems: new metallocycles? Or the importance of deformation. Phys. Chem. Chem. Phys. 14(32):11468
Becke AD (1993) Density-functional thermochesmistry. 3. The role of exact exchange. J. Chem. Phys. 98(7):5648–5652
Lee CT, Yang WT, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B 37(2):785–789
Inada Y, Orita H (2007) Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: evidence of small basis set superposition error compared to Gaussian basis sets. J. Comput. Chem., vol 29. John Wiley & Sons, Ltd.
Kobko N, Dannenberg JJ (2001) Effect of basis set superposition error (BSSE) upon ab initio calculations of organic transition states. J. Phys. Chem. A 105(10):1944–1950
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Frisch H, Trucks MJ, Schlegel GW, Scuseria HB, Robb GE, Cheeseman MA, Scalmani JR, Barone G, Mennucci V, Petersson B, Nakatsuji GA, Caricato H, Li M, Hratchian X, Izmaylov HP, Bloino AF, Zheng J, Sonnenberg G, Hada JL, Ehara M, Toyota M, Fukuda K, Hasegawa R, Ishida J, Nakajima M, Honda T, Kitao Y, Nakai O, Vreven H, Montgomery TJ, Peralta JA, Ogliaro JE, Bearpark F, Heyd M, Brothers JJ, Kudin E, Staroverov KN, Kobayashi VN, Normand R, Raghavachari J, Rendell K, Burant A, Iyengar JC, Tomasi SS, Cossi J, Rega M, Millam N, Klene JM, Knox M, Cross JE, Bakken JB, Adamo V, Jaramillo C, Gomperts J, Stratmann R, Yazyev RE, Austin O, Cammi AJ, Pomelli R, Ochterski C, Martin JW, Morokuma RL, Zakrzewski K, Voth VG, Salvador GA, Dannenberg P, Dapprich JJ, Daniels S, Farkas AD, Foresman Ö, Ortiz JB, Cioslowski JV, Fox J (2017) Gaussian 09. Revision E01 edn. Gaussian Inc,, Wallingford, CT
Bader RFW (1990) Atoms in molecules. Clarendon Press, Oxford
Matta CF, Boyd RJ (eds) (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. Wiley-VCH Verlag, Weinheim
Becke AD, Edgecombe KE (1990) A simple measure of electron localization n atomic and molecular-systems. J. Chem. Phys. 92:5397–5403
Weinhold F, R Landis C (2005) Valency and bonding
Keith TA (2018) AIMAll http://aim.tkgristmill.com 17.11.14 edn., Overland Park
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F (2013) NBO http://nbo6.chem.wisc.edu/. 6.0 edn., Theoretical chemistry institute, University of Wisconsin, Madison
Silvi B (2010) TopMod http://www.lct.jussieu.fr/pagesperso/silvi/topmod.html. GNU edn. The TopMod
Ziegler T, Rauk A (1977). Theor. Chim. Acta 46:1–10
Bickelhaupt FM, Baerends EJ (2007) Kohn-Sham density functional theory: predicting and understanding chemistry. In: Lipkowitz KB, Boyd DB (eds) Reviews in computational chemistry. John Wiley & Sons, Inc., pp 1–86
Kitaura K, Morokuma K . Int. J. Quant. Chem. 10:325 (1976)
A. Vu Theoretical chemistry in Amsterdam density functional (ADF) program, http://www.scm.com.(2016)
Acknowledgments
This work has been partially supported by the FONDECYT REGULAR 1170837 (BH) DGI Projects no. CTQ2015-63997-C2. A generous allocation of computing time at the Centro de Computación Científica of the UAM is also acknowledged (AML).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25.4 kb)
Rights and permissions
About this article
Cite this article
Arslancan, S., Herrera, B. & Lamsabhi, A.M. On the nature of the interaction of copper hydride and halide with substituted ethylene and acetylene. J Mol Model 26, 61 (2020). https://doi.org/10.1007/s00894-020-4320-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4320-0