Abstract
The glass transition temperature (T g) and density of poly-(phthalazinone ether sulfone ketone) (PPESK A) were estimated by molecular dynamic (MD) simulation. A novel poly-(phthalazinone ether sulfone ketone) (PPESK B) was constructed by introducing nitrol and amini energetic groups into PPESK A, and T g and density were also simulated for PPESK B. The estimated T g values of PPESK A were very close to experimental results, while for PPESK B three estimated values differed by < 5 K. The interactions between explosives and polymer binders of polymer bonded explosives (PBXs) were simulated by MD. Comparison of the cohesive energy densities (CED) and solubility parameter (δ) values of PBXs, polymer binders, and mono-explosives indicate that, upon introducing polymer binders, the CED and δ values of PBXs decreased compared with those of corresponding mono-explosives. The binding energies (E bind) imply that 2,4,6-trinitrotoluene-based PBXs are more stable than 1,3,5-triamino-2,4,6-trinitrobenzene (TATB)-based PBXs. The mechanical properties, Young’s modulus E, shear modulus G, bulk modulus K, Poisson’s ratio γ and Cauchy pressure (C 12 –C 44) of the PBXs were assessed. The rigidity of the PBXs was found to be lower than that of mono-explosives. All K/G values were positive, indicating that PBXs are flexible. Based on these mechanical properties results, PBXs using PPESK B as a binder are superior to those using PPESK A as a binder. Due to the low C 12 –C 44 values of the PBXs, the ductility of the materials of the fracture surface is poorer, especially for TATB-based PBXs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research into energetic materials has great significance. Scientists are constantly striving to find explosives with high energy and low sensitivity, as such explosives can be applied widely to a variety of fields, and low sensitivity is crucial for transportation and storage [1]. Polymer bonded explosives (PBXs), as one kind of high energy–low sensitivity explosive, consist of 90–95 wt% energetic particles and 5–10 wt% polymer binders. Compared with mono-explosives, PBXs have many advantages, such as high energy, low mechanical sensitivity, good mechanical properties and workability [2, 3]. The polymer binders used in PBXs, such as polyisobutylene (PIB), ethylene fluorinated olefin copolymer (for example Kel-F and Vinton-A) and polystyrene (PS), are inert but exhibit lower adhesion with the explosive crystals. To address this problems, plasticizers are imported into these explosives, which not only enhance their adhesion properties but also reduce the thermal decomposition temperature of the PBXs [4, 5]. In addition, the reactions path for PBXs can be altered (due to inert binders adhering to explosive crystals), for example, by lowering the competitive reaction effect (evaporation and gas phase pyrolysis) and depressing the activation energy of the thermal decomposition reaction of the explosives [6, 7]. Traditional polymer binders have fewer energetic groups and influence the energy release of an explosive. Thus, many high energy and density polymer binders are increasingly being studied. The main method used to overcome the influence of energy release of the polymer binders [8] is to introduce energetic functional groups, such as nitrate ester (−ONO2), nitro (−NO2), nitramino (−NNO2) and azido (−N3), into the polymer chains. Furthermore, energetic thermal plastic elastomers, such as glycidyl azide polymer (GAP), poly-3-nitro methyl-3-methyloxetane (Poly NIMMO) [9, 10] and poly-3-bis(nitratomethyl)-3-methyloxetane (BNMO), not only elevate the explosive property, but also improve the oxygen balance of the system. However, synthesis of these polymers is still complex and their chemical preparation fraught with danger. Meanwhile, most of these binders are fatty chain polymers, thus their thermal decomposition temperatures are lower than those of explosive particles, which implies that they have low mechanical strength and weak chemical stability. So, it remains difficult to achieve binders with good physical properties and high energy at the same time.
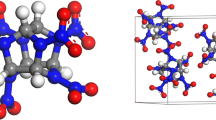
Nitrogen-containing heterocyclic polymers have been used in heat-resistant materials and fibers due to their comprehensive properties such as excellent thermal and chemical stability, high tensile strength, high elastic module and good flame retardant properties. However, such polymers, e.g., polyimide and polybenzimidazoles, are reported only rarely as energetic polymer binders despite their many merits. This is mainly because of their poor solubility, hard processibility and considerable cost. The core structure of the polymer phthalazinone (DHPZ) (molecular structure and 3D model are shown in Fig. 1a and b) has a twisted non-coplanar geometry, which may also exists in polymers. Several phthalazinone-containing polymers have been synthesized by Jian et al. [11, 12]. These polymers are hard to crystallize; on the other hand, they can endure high temperature and have good solubility, which are properties superior to traditional polymer binders. Based on these advantages, DHPZ-containing polymers, e.g., poly-(phthalazinone ether sulfone ketone) (PPESK), have been used as polymer binders. We wondered if these desirable properties of PPESK would combine well with explosives, and if the energy release of PBXs could somehow be improved. Herein, molecular dynamics (MD) simulation methods have been employed to study the interactions between explosives and polymer binder PPESK A (Fig. 1c), as well as the novel energetic DHPZ-containing polymer PPESK B (Fig. 1d). PPESK B was designed by introducing −NO2 and −NH2 groups into PPESK A. Initial simulations found that the optimal ratio of sulfone to ketone segments is 8:2, resulting in high T g values (ratios tested ranged from 0:10 to 10:0). Specific T g values and figures can be found in Table S1 and Fig. S1, respectively. Herein, a sulfone: ketone ratio of 8:2 for both the PPESK A and PPESK B were considered as the optimal polymer binder ratio for PBX for subsequent simulations.
TNT (2,4,6-trinitrotoluene) (Fig. 2a) is an insensitive explosive once widely used in weapons, e.g., during World War I and World War II, due to its good stability and high energy [13, 14]. The explosive 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) (Fig. 2c) is also an insensitive explosive, but has more energy and higher density than TNT [15]. PPESK A and PPESK B were chosen as polymer binders with TNT or TATB, respectively, to construct the PBX models. Interactions between binders and explosives were simulated based on these PBX models. Similar properties were subsequently simulated to the mono-explosives and the polymer binders.
Modeling and methods
Constructing models of amorphous cells polymers and PBXs
The COMPASS force field can be applied to the study of interactions of different components and condensed phase complexes. It works especially well for nitro-containing energetic complexes when using MD simulations [16, 17].
PPESK A and PPESK B amorphous cells (shown in Fig. 3) were constructed via five corresponding single chains; the ends of the polymer chains were saturated by chlorine atoms. The PPESK A and PPESK B amorphous cell models consist of 2450 and 2750 atoms, respectively.
To investigate the interactions of polymer binder and mono-explosives, a 3 × 6 × 2 TNT super cell (288 molecules and 6048 atoms in all) and a 5 × 5 × 5 TATB super cell (250 molecules and within 6000 atoms in all) were constructed using experimental data obtained from the Cambridge Crystallographic Data Center (CCDC). The TNT primitive cell belongs to the Pca21 space group and has the cell parameters a = 14.911 Å, b = 6.077 Å, c = 20.017 Å, α = β = γ = 90° [18]. However, the TATB primitive cell belongs to the 2P-1 space group and has the cell parameters a = 9.010 Å, b = 9.028 Å, c = 6.812 Å, α = 108.580°, β = 91.820°, γ = 119.970° [19]. Subsequently, the two super cells were cleaved along the three different crystal faces (1 0 0), (0 1 0) and (0 0 1), then 10 Å vacuum slabs were added for each new TNT super cell, and 12 Å vacuum slabs for the three TATB super cells. The optimized single chain PPESK A and PPESK B were placed into the six different boxes separately. The TNT-based PBX models are shown in Fig. 4 and the TATB-based PBX models are shown in Fig. S3. Taking, as an example, the PPESK A/TNT (1 0 0) PBX model, which contains 6538 atoms in all, the polymer binder accounts for 6.6 wt% of the PBX, and the mass ratios of the polymer binders were calculated in the same way for the other PBX models. For PPESK B/TNT [PPESK B (7.4 wt%)], PPESK A/TATB [PPESK A (6.5 wt%)] and PPESK B/TATB [PPESK B (7.5 wt%)], the ratios of all polymers are within the range of 5–10 wt%, meeting the requirement of binders for the PBX. Additionally, PPESK A/TNT(6.6 wt%) and PPESK A/TATB(6.5 wt%) have roughly the ratios ascribed to PBX and are similar to PPESK B. The construction of all models and simulations were fully simulated using Material Studio software [20].
MD simulations of PPESK A, PPESK B and PBXs
The amorphous polymers PPESK A and PPESK B consist of 2450 and 2750 atoms, respectively, and both polymer binders were optimized to get the lowest energy and stable configurations for further optimization. The specific MD simulation for both amorphous polymers was calculated in two steps: an equilibration state and a productive state. In the equilibration state, an annealing process to give relaxed amorphous polymers was simulated using the following conditions: isothermal (NVT) ensemble, 2000 ps total time step and 700 K. To obtain a stable structure for subsequent simulation, conditions of isobaric-isotherm (NPT) ensemble, 280 K and 2000 ps were set for the simulation. In the production state, the amorphous polymers were cooled gradually from 700 K to 280 K at 20 K intervals. At each specific temperature, the stable configuration of the MD simulation was used as the initial structure for the subsequent MD simulation. For the MD simulation process, PPESK A and PPESK B were simulated for 500 ps of the total time at each temperature under the isobaric-isotherm (NPT) ensemble; the first 300 ps was used for equilibration and the remaining 200 ps for data processing. The temperature and the pressure were controlled by an Anderson [21] thermostat and Barostat [22], respectively, with the time step set as 1 fs. Similarly, the same parameters were used for PBXs MD simulation, the difference being that total times of 2000 ps were used for the MD simulation.
Results and discussion
Glass transition temperatures and densities
The T g values of PPESK A and PPESK B were obtained by plotting temperature against density, free volume and volume. According to Fig. 4 (and as shown in Table 1), the highest value 568.4 K is obtained by the plot of free volume vs temperature, while the lowest value 543.2 K is from the plot of volume vs temperature. Among the values, 552.0 K, obtained by plotting density vs temperature, is closest to the experimental value of 557 K [14].
The simulated values are reliable because the difference between the simulated and experimental values is <15 K. The simulated T g values of PPESK B are 600 K, 596.6 K and 599.6 K (see Fig. 5) calculated three ways. The difference between the simulated values and experimental values of PPESK B is < 5 K. Comparing the values of two simulated polymer binders, those of PPESK B are about 40 K higher than those of PPESK A. Density of the polymer binders was also obtained via MD simulation, with the simulated value of PPESK A being 1.32 g cm−3 at 298 K, which is close to the experimental value of 1.35 g cm−3 [14]. For PPESK B, the estimated density value is 1.37 g cm−3,which is higher than that of PPESK A.
For each equilibrated PBX model, density values were obtained after MD simulation. The density values of TNT-based PBXs are shown in Table 2, and those of TATB-based PBXs are shown in Table S1. The density values of TNT-based PBXs follow the sequence: PPESK (Aor B)/TNT(0 0 1) > PPESK (Aor B)/TNT(1 0 0) > PPESK (Aor B)/TNT(0 1 0), and the same sequence is followed by TATB based PBXs. This implies that the simulated values of T g and the density of PPESK B are higher than the respective values of PPESK A. This is due mainly to the energetic groups nitro (−NO2) and amino (−NH2) of PPESK B. Specifically, the H atoms from the NH2 − groups in PPESK B can form hydration bonds with N atoms from the NO2 − groups in TNT.
CED and solubility parameters
The CED and solubility parameters are used to estimate the miscibility of different components. According to the “like dissolves like” theory, the closer the values of the solubility parameters, the better the miscibility of the two composites [23, 24]. The solubility parameter (δ) values of TNT, TATB, PPESK A, PPESK B and PBXs are displayed in Table 3. The PPESK A and PPESK B solubility parameters (δ) values are very similar, indicating that the two compounds have similar miscibility. The δ value of the two mono-explosives are very close, the δ value of the TNT is 31.761 (J/cm3)½ while the δ value of the TATB is 33.669 (J/cm3)½. The difference value δ (Δδ) of the PPESK (A or B) and the mono-explosive (TNT or TATB) is about Δδ = 15 (J/cm3)½. The differences for PBXs [PPESK (A or B)/(TNT or TATB)] and mono-explosive (TNT or TATB) are less than 10 (J/cm3)½ (Δδ) except for PPESK B/TATB(0 1 0) [Δδ = 11.23 (J/cm3)½]. Furthermore, the solubility parameters of TATB-based PBXs decreased more than those of TNT-based PBXs compared with their corresponding mono-explosives, indicating that the miscibility of the PBXs has been improved by introducing polymer binders (PPESK A or PPESK B) into the mono-explosive (TNT or TATB).
Binding energy
The binding energy (E bind) is used as a measure of the interaction between different components. The E bind value is the inverse of the E inter, which is obtained from the equilibrium state of the total energy PBXs minus the equilibrium state energy of the polymer binder E PPESK, and then deduct the equilibrium state energy of the mono-explosive E explosive. The relationship of these energies is shown in Eq. (1) [25], where E explosive, E PPESK and E PBX represent the equilibrium state energy of mono-explosive (TNT or TATB), PPESK (A or B) and PBXs, respectively. The stability of composite surfaces is positively correlated with E bind values. The PPESK A/TNT and PPESK B/TNT E bind values are shown in Table 4, while for PPESK A/TATB and PPESK B/TATB, the E bind values can be seen in Table S3. For TNT-based PBX, the E bind values of PPESK A/TNT follow the sequence: PPESK (A)/TNT(0 1 0) > PPESK (A)/TNT(0 0 1) > PPESK (A)/TNT(1 0 0). However, this is different for PPESK B/TNT PBXs, where the E bind values follow the sequence PPESK (B)/TNT(1 0 0) > PPESK (B)/TNT(0 1 0) > PPESK (B)/TNT(0 0 1). Among the E bind values, the PPESK (B)/TNT(1 0 0) is the greatest, which indicates that PPESK B has the most stable state on the TNT (1 0 0) crystal surface. Furthermore, the E bind values of TATB-based PBXs follow the sequence: PPESK (A or B)/TATB(0 0 1) > PPESK (A or B)/TATB(1 0 0) > PPESK (A or B)/TATB(0 1 0). These results imply that polymer binders are stable on the TATB(0 0 1) crystal surface but are unstable on the TATB(0 1 0) crystal surface. Compared with the E bind values of TNT- and TATB-based PBXs, the E bind values of TATB-based PBXs are lower than those of TNT-based PBXs, which indicates that the stability of TNT-based PBXs is superior to that of TATB-based PBXs, due to the low surface energy polarization of the TATB crystal [26]. Thus, interface modifications of the TATB crystals need to be improved and several methods to enhance the particle surface roughness, such as γ-radiation grafting methods or using microwave and ultraviolet radiate to deal with TATB powder, have been tested [27].
Mechanical properties
The mechanical properties of PBXs are closely connected with their preparation and use. The elastic properties consist of the elastic coefficient, Young’s modulus (E), Poisson’s ratio (γ), etc. The parameters of material stress and strain tensor depend mainly on σ I and ε I. For the statistical mechanics of the elasticity, Hooke’s law is shortened as in Eq. (2), where σ I is stress, ε j is strain, and C ij is the 6 × 6 elastic coefficient matrix. The elastic coefficient of materials are the 36 elastic constants in matrix (3). Due to the existence of strain energy and the symmetry of the matrix, C ij = C ji, only 21 elastic constants are needed to analyze the stress and strain properties of these materials [28, 29].
As the symmetry of the materials increases, the independent elastic coefficients decrease. Isotropic materials have only two independent elastic coefficients, C 11 and C 12. The elastic constant C ij values are shown in Table 5, which can be divided into three parts C 11, C 22, C 33; C 44, C 55, C 66; and C 12 , C 13 , C 23 , as the other elastic constant values are almost zero. For these three parts of TNT-based PBXs, elastic constant values have decreased to some extent compared to those of pure TNT, meaning improved isotropy for PBXs. Similarly, the elastic constants C ij of TATB based PBXs are shown in Table S4. The C ij values can be divided into three parts C 11, C 22, C 33; C 44, C 55, C 66 and C 12, C 13, C 23, while the other values are almost zero or below zero, except for C 15 and C 35. Since the polymer binders have been introduced into TNT or TATB, the ratio of the binder of the PBXs can be divided into two groups [PPESK A/TNT (6.6 wt%), PPESK A/TATB (6.5 wt%) and PPESK B/TNT (7.4 wt%) PPESK B/TATB (7.5 wt%)]. Using the same polymer binder, the elastic constant values of TATB-based PBXs are lower than those of TNT-based PBXs, which implies that the isotropy of TATB-based PBXs is superior to that of TNT-based PBXs with the same polymer binder at a similar ratio. In addition, upon comparing elastic values, PBX isotropy was improved compared to that of mono-explosives. The modulus values of the materials indicate to what degree the materials resist deformability. This value is closely related to the plasticity and fracture properties of the materials, which act as indicators to assess the rigidity of the materials. For ideal isotropic materials, the elastic constants can be simplified as two independent coefficients (λ and μ) [30, 31]. Additionally, the mechanical parameters can be calculated based on the elastic constants. For E, shear modulus (G), bulk modulus (K) and Cauchy pressure (C 12–C 44), all of these values are obtained from the two independent coefficients (λ and μ), and Eq. (4) implies a relationship between the modulus and the two independent coefficients. The G values are closely connected with rigidity and yield strength of the materials. The K values indicate the degree of fracture of the materials.
The γ values of TNT- or TATB-based PBX are almost positive except for PPESK A/TNT(0 0 1) and PPESK A/TATB(0 0 1). Most Poisson’s ratio (γ) values of the PBXs are within the range 0.2–0.4, which indicates that they have more plastic properties than their corresponding mono-explosives. Moreover, the K/G values and the Cauchy pressure C 12–C 44 are two measurements of the ductility of the materials. The difference is that the former is based on degree of plastic deformation, while the latter is based on morphology of the fracture surface of the materials. The E, G and K values of TNT-based PBXs are lower than those of pure TNT, which means that the rigidity of the PBXs are reduced compared to the mono-explosives. The K/G values of PPESK A/TNT(1 0 0), PPESK B/TNT(0 1 0) and PPESK B/TNT(0 0 1) are higher than the corresponding values of pure TNT, but the other values of PBXs are the opposite. For PBXs such as PPESK A/TNT (1 0 0), PPESK B/TNT (0 1 0) and PPESK B/TNT(0 0 1), ductility has been improved. The K and G values decrease a lot compared with the corresponding mono-explosives; the specific values are displayed in Table 6.
For the Cauchy pressure values of the PBXs, only PPESK A/TNT (1 0 0) (0.91) and PPESK B/TNT(0 0 1) (0.09) values are positive (C 12–C 44), while the other values of the PBXs are negative, as shown in Table 6. The E, K, G and Cauchy pressure values of TATB-based PBXs are presented in Table S5. The rigidity of TATB-based PBXs decreased much than that of TNT-based PBXs upon adding the same polymers to the mono-explosive. The K/G ratio and Cauchy pressure (C 12–C 44) values of TATB-based PBXs are similar to those of TNT-based PBXs. The K/G values of TATB-based PBXs are all positive, indicating that TATB-based PBXs exhibit plastic deformation. However, Cauchy pressure (C 12–C 44) values for TATB-based PBXs are low, implying that ductility of the materials in terms of morphology of the fracture surface are worse. This is due to the lower surface energy polarization of the TATB crystal; this problems can be addressed by dealing with TATB crystals.
Conclusions
To overcome the drawbacks of non-energetic groups, and the low solubility and processing temperature of traditional polymer binders, poly-(phthalazinone ether sulfone ketone) (PPESK A) and the energetic PPESK B can be used as polymer binders. The T g values of the amorphous cells of polymers PPESK A and PPESK B were estimated by three means: density, free volume and volume. The T g values of PPESK A obtained by these three methods are close to the experimental values, and the corresponding estimated T g values of PPESK B are all within a difference of 5 K. The density values of the two polymers are similar, but the value for PPESK B is higher than that for PPESK A. For equilibrated PBXs, the density values follow the sequence PPESK (A or B)/Explosive (TNT or TATB)(0 0 1) > PPESK (A or B)/Explosive (TNT or TATB)(1 0 0) > PPESK (Aor B)/Explosive (TNT or TATB) (0 1 0). The CED and δ values of polymers binders (PPESK A or B), mono-explosives and PBXs were simulated, and those of PBXs found to be lower than those of the corresponding mono-explosives. The E bind of TNT-based PBXs fall within one of two different situations depending on the different polymer binders. With PPESK A as the binder for PBXs, E bind values follow the sequence PPESK (A)/TNT(0 1 0) > PPESK (A)/TNT(0 0 1) > PPESK (A)/TNT(1 0 0), whereas, with PPESK B as the binder for PBXs, the E bind values have the following sequence: PPESK (B)/TNT(0 1 0) > PPESK (B)/TNT(0 0 1) > PPESK (B)/TNT(1 0 0). In addition, E bind values of TATB-based PBXs follow the sequence: PPESK (A or B) /TATB(0 0 1) > PPESK (A or B)/ TATB(1 0 0) > PPESK (A or B)/TATB(0 1 0). Generally, E bind values for TNT-based PBXs are higher than those of TATB-based PBXs because of the properties of the TATB crystal. Finally, the mechanic properties of TNT- and TATB-based PBXs were simulated. The E values of the PBXs were lower than corresponding values of the mono-explosives, which indicates that the rigidity of the PBXs is decreased relative to mono-explosives. The rigidity of TNT-based PBXs is higher than that of TATB-based PBXs. The K/G ratio values are positive for TNT- and TATB-based PBXs, indicating that the PBXs exhibit plastic deformation. The Cauchy pressure values are low, especially for TATB-based PBXs, which mean that the ductility of the materials on the fracture surface are worse. These properties are related to the structure of the TATB crystal.
References
Shu YJ, Huo JC (2011) Theory of explosives. Chemical Industry, Beijing
Sun GX (1984) Polymer mixed explosives. National Defense Industry, Beijing
Sun YB, Hui JM, Cao XM (1995) Military mixed explosives. Weapon Industry, Beijing
Wang Z, Li G, Lao Y, Li H (2005) Study on thermal stability of PBX-HKF by accelerating rate calorimeter. Chin J Energ Mater 13(2):113–114
Elbeih A, Pachman J, Zeman S (2010) Thermal stability and detonation characteristic of pressed and elastic explosive on the basis of selected cyclic nitramines. Cent Eur J Chem 7:115–129
Singh C, Felix SP, Soni P (2005) Study on energetic compounds, Part 31. Thermolysis and kinetic of RDX andsome of its plastic bonded explosives. Theor Chim Acta 426:131–139
Singh C, Felix SP, Pandey DK, Agrawal JP, Sikder AK (2005) Study on energetic compounds, Part XXXIX. Thermal analysis of a plastic bonded explosive containing RDX and HTPB. J Therm Anal Calorim 79:631–635
Zhang JQ, Zhu H, Zhang W, Zhang JQ, Yang SJ (2006) Research progress of energetic binder system used in solid propllants. Chem Propell Polym Mater 4(3):6–9
He LM, Xiao ZL, Zhang XZ, Jing DQ (2003) The research and development on energetic binders for propellants abroad. Chin J Energ Mater 11(2):99–102
Wu YG, Wu XQ, Zhang L, Zhang CF (2008) Research progresses of energetic adhesive system for gunpowder. China Adhesives 17(8):47–50
Jian XG, Liao GX, Wang JY (2002) Research progress of poly(arylene ether ketone)s and poly(arylene ether sulfone)s containing phthalazinone moieties. China Plastic 16(4):11–15
Jan XG, Wang JY (2012) Progress on high performance engineering plastics containing phthalazinone moieties and their applications. Materials China 31(2):16–22
Agrawal JP, Hodgson RD (2007) Organic chemistry of explosives. Wiley, New York
Davis TL (1953) The chemistry of powder and explosives: complete in one volume. Wiley, New York
Cooper PW (1996) Explosives engineering. Wiley-VCH, New York
Ou YX, Meng Z, Liu JQ (2007) Review of the development of application technologies of CL-20. Chem Ind Eng Prog 26(12):1690–1694
Qiu L, Xiao HM, Zhu WH, Xiao JJ, Zhu W (2006) Ab initio and molecular dynamics studies of crystalline TNAD (trans-1,4,5,8-tetranitro-1,4,5,8-tetra azadecalin). J Phys Chem B 110(22):10651–10661
Carper WR, Davis LP, Extine MW (1982) Molecular structure of 2,4,6-trinitrotoluene. J Phys Chem 86:459
Cady HH, Larson AC (1965) The crystal structure of 1,3,5-triamino-2,4,6-trinitrobenzene. Acta Crystallogr 18:485–496
Accelrys Software. Materials Studio Accelrys Software Inc, San Diego, CA
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72(4):2384–2393
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Scatchard G (1931) Equilibria in non-electrolyte solutions in relation to the vapor pressures and densities of the components. Chem Rev 8(2):321–333
Watt JP, Davies GF, O’Connell RJ (1976) The elastic properties of composite materials. Rev Geophys Space Phys 14:541–563
Qiu L, Zhu WH, Xiao JJ, Zhu W, Xiao HM, Huang H, Li JS (2007) Molecular dynamics simulations of trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecalin-based polymer-bonded explosives. J Phys Chem B 111:1559–1566
Sharma J, Garrett WL, Owens FJ, Vogel VL (1982) X-ray photoelectronic structure, ultraviolet, and isothermal decomposition of 1,3,5-triamino-2,4,6-trinitrobenzene. J Phys Chem 86(9):1657–1661
Wang XC, Huang H, Nie FD (2001) The investigation of surface modification of TATB particle. Chin J Expl Propell 1(24):33–35
Wu JL (1993) Elasticity. Tongji University Press, Shanghai
Xiao JJ, Huang H, Li JS, Zhang H, Zhu W, Xiao HM (2007) MD simulation study on the mechanical properties of HMX crystals and HMX/F2311 PBXs. Acta Chim Sinica 65(17):1746–1750.
Watt JP, Davies GF, O’Connell RJ (1976) The elastic properties of composite materials. Rev Geophys Space Phys 14:541–563
Weiner JH (1983) Statistical mechanics of elasticity. Wiley, New York
Acknowledgments
The authors appreciate financial support from the National Natural Science Foundation of China (grant. no. 51373159).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 1029 kb)
Rights and permissions
About this article
Cite this article
Shu, Y., Yi, Y., Huo, J. et al. Interactions between poly-(phthalazinone ether sulfone ketone) (PPESK) and TNT or TATB in polymer bonded explosives: a molecular dynamic simulation study. J Mol Model 23, 334 (2017). https://doi.org/10.1007/s00894-017-3492-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3492-8