Abstract
Glasses in the system xGd2O3·(100-x)[GeO2·V2O5] with 0 ≤ × ≤ 20 mol% have been prepared from the melt quenching method. In this paper, we investigated changes in germanium coordination number in gadolinium-vanadate-germanate glasses through molar volume analysis, measurements of densities, investigations of FTIR and UV-VIS spectroscopy, calculations of density functional theory (DFT). Analyzing the structural changes resulted from the IR spectra we found that the gadolinium ions have a pronounced affinity toward [VO4] structural units which contain non-bridging oxygens necessary for the charge compensation. The introduction of the excess of oxygen yields the formation of [VO5] structural units. This attains maximum value at 5 mol% Gd2O3, in agreement with the density measurements. Further, the addition of the surplus of oxygen implies the transformation of [VO5] to [VO4] structural units and the formation of VO −34 orthovanadate structural units. The UV-VIS spectra show a broad UV absorption band located in the 300–500 nm region. These bands are assumed to originate from the combination of vanadium ions possibly present in the three states of valence. The presence of Ge-Ge wrong bonds attains its maximum values in the samples with x = 5 and 15 mol% Gd2O3 (bands centered in the 250–300 nm range). DFT calculations show the massive vibrations of the [VOn] structural units coupled with each other via [GeO6] and [GeO4] structural units. This leads to the splitting of the bridge modes and a multiplication of the number of these bands.

The optimized structure of the GeO2∙V2O5 glasses was used to perform the B3PW91/CEP-4G/ECP computations. Analyzing the structural changes resulted from the IR spectra we found that the gadolinium ions have a pronounced affinity towards [VO4] structural units which contain non-bridging oxygens necessary for the charge compensation. The UV-VIS spectra show a broad UV absorption band located in the 300–500nm region which can be associated with vanadium ions possibly present in the three stares of valence. FTIR and UV-VIS data will be used in the present research in order to compute a possible structural model of the GeO2∙V2O5 glasses network. DFT calculations show that there is a large displacement of the germanium atom from the centre of the octahedron, whose strongly asymmetry depends on the manner of connection with the surrounding polyhedron. This deformation will be expressed more clearly when one or two of the oxygen atoms in the vertex are not bridged, which is typical in the formation of the vitreous matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germanate glasses doped with rare-earth ions have been investigated extensively in the past because their physicochemical, optical and spectroscopic properties are advantageous for optoelectronic applications [1–4].
The structural chemistry of crystalline oxides such as V2O5 revealed that the structure of V2O5 is built of octahedrons, where the pentavalent vanadium has five-coordination with oxygen atoms. Although V+5 thus behaves like P+5 in forming the ions VO −34 and V2O −47 it shows a much greater tendency than P to form condensed oxy-ions.
On the other hand, the V2O5-rich glasses in which V2O5 acts as the network former have the network structure mainly consisting of corner-sharing branched [VO4] tetrahedral of the same structural units as found in phosphate glasses. The network structure was reported to be made up of unaffected [VO5] units as in vitreous V2O5 and affected [VO5] units with alkaline earth ions in contrast to the vanadate glasses formed by conventional network formers in which only unaffected [VO5] units are present [4–9]. These glasses are known to contain V+4 and V+5 ions where the electrical conduction was attributed to the hopping of 3d1 unpaired electron from V+4 to V+5 site which induces a polarization of the vanadium ion around it and form a polaron [10–14].
Experimental and computational procedure
The gadolinium-vanadate-germanate glasses with composition xGd2O3∙(100-x)[GeO2∙V2O5] where x = 0, 5, 10, 15, 20%moli were prepared by taking appropriate amounts of reagents grade GeO2, V2O5 and Gd2O3. After melting at 10000C for about 10 min in a corundum crucibles and introduce in an electric furnace, the melt was quenched rapidly to room temperature.
The samples were analyzed by means of X-ray diffraction using a XRD-6000 Shimadzu diffractometer, with a monochromator of graphite for the Cu-Kα radiation (λ = 1.54 Å) at room temperature.
The structure of the glasses was investigated by infrared spectroscopy using the KBr pellet technique. The IR spectra were recorded in the range 400–1200 cm−1 using a JASCO FTIR 6200 spectrophotometer.
The densities of the glass samples were measured accurately to the third decimal (±0,03 g cm−3) by the displacement method using CCl4 as an immersion liquid.
The starting structures have been built using the graphical interface of Spartan’04 [15] and preoptimized by molecular mechanics. Optimizations were continued at DFT level (B3PW91/CEP-4G/ECP) using the Gaussian’03 package of programs [16].
It should be noticed that only the broken bonds at the model boundary were terminated by hydrogen atoms. The positions of boundary atoms were frozen during a calculation and the coordinates of internal atoms were optimized, to model the active fragment flexibility and its incorporation into the bulk.
Results and discussion
Density, molar volume and oxygen packing density
Density is a powerful tool capable of exploring the changes in the structure of glasses. Density is affected by the structural softening/compactness, change in geometrical configuration, coordination number, cross-link density and dimension of interstitial spaces of the glasses.
Figure 1 shows the compositional evolution of the density of gadolinium-vanadate-germanate glasses. This figure shows the presence of density maxima at x = 5 and 20 mol% Gd2O3. The changes in molar volume were particularly pronounced for x = 0% and appeared to be minimal at x = 5 and 20 mol%. We conclude that with increasing of the Gd2O3 content up to 5 mol%, the local vanadium environment in the glass changes from tetrahedral to pentagonal geometry due to the fact that the coordination number of vanadium atoms changes from 4 to 5. Further the addition of the modifier gadolinium oxide to the vanadium germanate glass network introduces a surplus oxygen into the vitreous network and the need to accommodate the glasses network. The additional oxygen may be incorporated into the network as non-bridging oxygen by breaking oxygen bridges and in these conditions the four-coordinated germanium atoms attain maximum value.
The oxygen packing density is the ratio of measured density per molecular weight and number of oxygen ions in a formula unit [17]. For x ≤ 5 mol% and x = 20%moli the oxygen packing density increases which clearly indicates that the glassy network becomes tightly packed when more Gd2O3 is introduced to the vitreous matrix.
In the case of pure V2O5 glass it has been reported [14] that V+5 ions exhibit both four and fivefold coordination states, depending on the sample preparation conditions. The IR spectrum of pure crystalline and amorphous V2O5 is characterized by the intense band in the 980–1020 cm−1 region, related to vibration of isolated V = O vanadyl groups [18, 19] while the band located at about 950–970 cm−1 was attributed to [VO4] units [20–23]. If V = O mode position at around 1020 cm−1 is preserved it can be concluded that V = O bond is not directly influenced and the coordination number and symmetry of the [VO4] and [VO5] structural units do not change.
FTIR spectroscopy
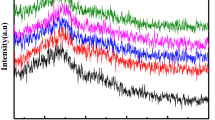
FTIR spectra obtained for the samples are shown in Fig. 2. The examinations of these spectra show that the increase of Gd2O3 content modify the characteristic IR bands as follows:
-
i)
It can be seen that the IR band in the wavenumber region of ∼800 cm−1 is found for all samples and its intensity attains maximum value at 20 moli% Gd2O3. This band can be due to the Ge-O stretching vibrations of the [GeO4] tetrahedral structural units involving non-bridging oxygen [10, 11]. This suggests that the glass network modification has taken place mainly in the vanadate part whereas the germanate part remained unmodified and its network consists mainly from the [GeO4] structural units with interconnected through Ge-O-Ge bridges in [GeO4] structural units.
-
ii)
The bands located at about 480 cm−1 and 530 cm−1 attain their maximum values at x = 10 mol% Gd2O3. These bands are attributed to the angular deformation vibration of the V-O bond [24]. This higher capacity of migration of the gadolinium ions inside the glass can be explained by a process of conversion of the [VO5] to [VO4] structural units.
-
iii)
With the increase of Gd2O3 content, a sharp decreasing trend was observed in wavenumber of the bands centered at about 1025 cm−1, which belong to vibration of isolated V = O bond. This may be attributed to the electrostatic field of the strongly polarizing Gd+3 ions. The increase of Gd+3 content leads to strengthening the electron cloud around oxygen in [VOn] units, and consequently causes a shift of stretching vibration of V-O-Ge and/or V-O-Gd to lower wavenumber. The intensity of this band attains its maximum value at 5% Gd2O3.
The shifting of the high-frequency band of the V = O bond toward lower wavenumber can be attributed to changes in the structure of vanadate network produced by the addition of Gd2O3. When Gd2O3 is introduced, Gd+3 ions go either to interstitial or substitutional sites in vanadate chains. This would influence the isolated V = O bonds and thus affect the vibrations of V = O bonds depending upon the position of the gadolinium ions. If gadolinium ions occupy substitutional sites, the V-O-V bonds will break up and form V-O-Gd bridges which restrict the influence of gadolinium ions on V = O bonds, yielding to preserve the position of the vibrational frequency at 1025 cm−1 in the IR spectrum.
-
iv)
The bands located at about 980 cm−1 is attributable not only to the [VO5] structural units but also to the branched [VO4] structural units having one V = O double bond. In accordance with the mechanism suggested above the gadolinium ions rest between vanadates chains and their influence on the V = O bond is important. This band shows a maximum intensity at x = 5 mol%.
-
v)
v) An increasing trend was observed in the strength of the bands centered at ∼870 cm−1. This band is usually assigned to the stretching vibrations of VO −34 orthovanadate structural units.
The added Gd2O3 also gives rise to the formation of non-bridging oxygens, thereby creating VO −34 units. It is obvious that the oxygen, which becomes non-bridging and acquires a negative charge, will move closer to the connected vanadium, consequently reducing the positive charge on the vanadium and thereby resulting in a decrease in the binding of other oxygens attached to this particular ion; the length of the V = O bond therefore increases. Also, due to the irregular and random distribution of atoms in the glass structure, the gadolinium ions take positions interstitially that are more symmetrical among other units.
Analyzing the structural changes resulted from the IR spectra, we found that the gadolinium ions have a pronounced affinity toward [VO4] structural units which contain non-bridging oxygens necessary for the charge compensation. The introduction of the excess of oxygen yields the formation of [VO5] structural units. This attains maximum value at 5 mol% Gd2O3, in agreement with the density measurements. Further, the addition of the surplus of oxygen implies the transformation of [VO5] to [VO4] structural units and the formation of VO −34 orthovanadate structural units. The formation of free orthovanadate units creates smaller network cavities, the increase of local densification and density (for sample with x = 20 moli%).
UV-VIS spectroscopy
The UV-VIS absorption spectra of xGd2O3∙(100-x)[GeO2∙V2O5] glasses are shown in Fig. 3. The examinations of these spectra show that the characteristic UV-VIS bands are modified namely:
-
i)
For samples with x ≥ 5 mol% Gd2O3, it follows from the spectrum that the glass matrix absorption begins at about 250 cm−1. These bands are very sharp in the lead germanate with x = 5 and 15 mol%, and shifted with increasing of gadolinium oxide content to longer wavenumbers (275 nm). All these bands can be assigned to the Ge-Ge wrong bonds [25].
This shifting can be interpreted considering the chemical bonding property of the ligand (oxygen) that exerts influences on the positions the absorption peaks due to the so-called nephelauxetic effect (meaning cloud expanding effect) as defined by Jorgensen [26]. As a consequence of this effect when a paramagnetic ion (gadolinium ion) is introduced into glass, parameters such as the electrostatic interaction of the electron, spin-orbit interaction and potential at the ion site due to its environment the Hamiltonian expression are reduced from their free-ion values [27]. The chemical bonding property of the ligand or the nephelauxetic effect can be elucided in terms of electronegativity because it reflects the degree of covalent bonding characteristics of different hosts of Gd+3 ions [28].
The changes in the features of the absorption bands can be explained as a consequence of the appearance of additional absorption shoulder due to photoinduced color centers in the glass such as the formation of germanate-gadolinium paramagnetic defect centers in the glasses.
The data above seem to confirm that the presence of gadolinium ions, well known as glass network modifiers and necessary for the ion-exchange, modify the structure of the glasses with x = 5 and 15 mol% Gd2O3. A striking evidence is that the bands situated in the 250–300 nm region, is not detectable if gadolinium ions are present in the amounts corresponding to x = 20 mol%. This could be due to the formation of a gadolinium germanate-like structure, which is able to eliminate or hide the presence of Ge-Ge wrong bonds absorbing at 250 cm−1. If any Ge-Ge wrong bonds are present in these glasses, they do not absorb at 250 cm−1 anymore.
-
ii)
It follows from the spectrum that all the glasses samples show a strong absorption extending from about 300 to 530 nm. This broad UV absorption is assumed to originate from the combination of vanadium ions possibly present in the three valences. Trivalent vanadium ions exhibit a characteristic UV band at 350–400 nm, vanadyl tetravalent ions possess an absorption at about 420 nm and pentavalent ions are known to belong to d0 configuration and exhibit only UV bands.
The bands located at about 360 nm correspond to the V+5 ions. These results show that the incorporation of Gd+3 ions in the host matrix have directly influenced V = O bonds. The V = O bonds are not conserved. The UV-VIS spectrum is dominated by the charge transfer of the type O−2 → V+5 [29, 30].
-
iii)
For the sample with x = 1%moli Gd2O3, our results reveal a low resolution of the bands located in the range from 600–800 nm. These are due to the V+4 ions.
The presence of Ge-Ge wrong bonds attains its maximum values in the samples with x = 5 and 15 mol% Gd2O3. The UV-VIS spectra of all samples are dominated by the charge transfer of the type O−2 → V+5 (the V+5 ions have d0 configuration) at about ∼380 nm (with an edge energy of about 3.18 eV) [29, 30]. By increasing the gadolinium oxide content up 20 mol%, Gd+3 ions rest between vanadates chains may affect the isolated V = O bonds.
DFT calculations
These data will be used in the present research in order to compute a possible structural model of the GeO2∙V2O5 glasses network. Similar methodology has previously been reported to study other glasses [31–34]. The study on structural modifications of the vitreous network and the equilibrium geometry were found by optimization (Fig. 4).
There are some sites in our model (Fig. 4), namely:
-
i)
Germanium atom is in hexagonal coordination. The Ge-O bonds are subdivided into four groups: a bond has shorter interatomic distance (1.78 Å), two bonds have short interatomic distances (1.82 Å), one has intermediate interatomic distances (1.88 Å) and two have longer interatomic distances (2.65 Å). Considering all six bonds, the [GeO6] polyhedron is irregular with four Ge-O bond lengths ranging from 1.78 to 1.88 Å and with two oxygen atoms remaining relatively far away from germanium cations (2.65 Å). These bonds are too large to be accepted as chemical bonds in the usual sense.
Therefore, if only oxygen atoms with Ge-O distances less than 1.88 Å are taken into account, the coordination polyhedron of the germanium is equal to four. However, from crystallographic point of view, coordination polyhedron of the germanium atoms is equal to six.
This shows that there is instability between the nonequivalent Ge-O bonds in the octahedron. In essence, there is a large displacement of the germanium atom from the center of the polyhedron, whose strongly asymmetry depends on the manner of connection with the surrounding polyhedron. This deformation will be expressed more clearly when one or two of the oxygen atoms in the vertex are not bridged, which is typical in the formation of the vitreous matrix.
-
ii)
The germanium site is coordinated to four O atoms. The Ge-O bonds are subdivided into two groups: two bonds have short interatomic distances (1.77–1.79 Å) and two have longer interatomic distances (1.80–1.83 Å).
-
iii)
The vanadium ions are distributed into two crystallographic sites: the [VO4] tetrahedrons and [VO5] square pyramids. The [VO4] tetrahedral units are higher distorted around the vanadium center (the vanadium-oxygen distances are ranging from 1.62 to 2.02 Å). The [VO5] square pyramids are considerably distorted around vanadium center and the V-O bond distances are ranging from 1.64 to 3.78 Å. Such behavior was reported for two-dimensional layered vanadate compounds [35, 36].
The vibrational properties of these glasses inferred from quantum chemical simulation are interpreted through a comparison with the experimental spectrum (Fig. 5). The evolution of vibrational spectrum of the proposed model is important for understanding the broadening-effect of the glasses from the experimental FTIR spectrum. Our results show that the Ge-O and V-O stretching vibration region of the proposed model is similar to the same region of the glass.
The massive vibrations of the [VOn] structural units can be coupled with each other via [GeO6] and [GeO4] structural units. This leads to the splitting of the bridge modes and a multiplication of the number of these bands.
The distribution of the electronic states of the HOMO, HOMO-1, LUMO and LUMO+1 can be seen in Fig. 6. An interesting finding in these systems is that:
-
i)
The HOMO and HOMO-1 give character of electron donor for the [VO4], [VO5] structural units of the vanadate glasses network and the [GeO6] structural units. The HOMO energy, EHOMO is −9.90 eV.
-
ii)
The LUMO and LUMO+1 give the character of electron acceptor for the [GeO4] structural units of the germanate network and the [VOn] structural units of the vanadate network. The LUMO energy, ELUMO is −2.59 eV.
There is a change transfer between the vanadium atoms tetra- and penta-coordinated. Also, a charge transfer was yielded between germanium atoms and vanadate network. This can be explained considering that the vanadate network is flexible to form the appropriate coordination environments with structural units of opposite charge such as gadolinium ions.
Conclusions
Homogeneous glasses of xGd2O3·(100-x)[GeO2·V2O5] system were obtained within 0 ≤ x ≤ 20 mol%. In this paper, we investigated changes in structural properties of the gadolinium-vanadate-germanate glasses through molar volume analysis, measurements of densities, investigations of FTIR and UV-VIS spectroscopy and calculations of density functional theory (DFT).
Our results show that the gadolinium ions have a pronounced affinity toward [VO4] structural units which contain non-bridging oxygens necessary for the charge compensation. The formation of [VO5] structural units attains maximum value at 5 mol% Gd2O3, in agreement with the density measurements. Further, the addition of the surplus of oxygen implies the transformation of [VO5] to [VO4] structural units and the formation of VO −34 orthovanadate structural units.
The broader UV absorption bands located in the 300–500 nm region corresponds to the vanadium ions present in the three states of valence. In the sample with x = 5 and 15 mol% Gd2O3, the Ge-Ge wrong bonds attains its maximum values. DFT calculations show the massive vibrations of the [VOn] structural units coupled with each other via [GeO6] and [GeO4] structural units.
References
Guinhos FC, Nobrega PC, Santa-Cruz PA (2001) J Alloys Compd 323&324:358–361
Bueno LA, Melnikov P, Messaddeg Y, Ribeiro SJL (1999) J Non-Cryst Solids 247:87–91
Klimesz B, Dominiak-Dzik G, Lisiecki R, Ryba-Romanowski W (2008) J Non-Cryst Solids 354:515–520
Wright AC, Yarker CA, Johnson PAV (1985) J Non-Cryst Solids 76:333–350
Sen S, Ghosh A (2001) J Phys Condens Matter 13:1979–1986
Sen S, Ghost A (1999) J Non-Cryst Solids 258:29–33
Dimitriev V, Dimitrov Y, Arnaudov M, Topalov D (1983) J Non-Cryst Solids 57:147–156
Sayer M, Mansingh A (1972) Phys Rev B 6:4629–4643
Mott NF (1968) J Non-Cryst Solids 1:1–18
Rada M, Culea E, Rada S, Maties V, Pascuta P (2010) J Mater Sci 45(6):1487–1494
Chung CH, Mackenzie JD (1980) J Non-Cryst Solids 42:357–370
Owen AE (1977) J Non-Cryst Solids 25:370–423
Sen S, Ghosh A (1999) Phys Rev B 60(22):15143–15149
Mendialdua J, Casanova R, Barbaux Y (1995) J Electron Spectrosc Relat Phenom 71:249–261
Spartan’04, Wavefunction Inc, 18401 Von Karman Avenue, Suite 370 Irvine, CA 92612
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision A1. Gaussian Inc, Pittsburgh
Miyata H, Fujii K, Ono T, Kubokawa Y, Ohno T, Hatayama F (1987) J Chem Soc Faraday Trans 83:675–685
Rada S, Bosca M, Culea E, Rada M, Dan V, Maties V (2009) Struct Chem 20:801–805
Dimitrov V (1987) J Solid State Chem 66:256
Khattak GD, Tabet N, Wenger LE (2005) Phys Rev B 72:104203–104215
Rada S, Culea M, Rada M, Culea E (2008) J Mater Sci 43(18):6122–6125
Rada S, Culea E, Rus V, Pica M, Culea M (2008) J Mater Sci 43:3713–3716
Manara D, Grandjean A, Pinet O, Dussossoy JL, Neuville DR (2007) J Non-Cryst Solids 353:12–23
Mandal S, Hazra S, Das D, Ghosh A (1995) J Non-Cryst Solids 183:315–319
Atkins RM, Mizrahi V (1992) Electron Lett 28:1743–1744
Jorgensen CK, Pappalardo R, Schmidtker HH (1963) J Chem Phys 39:1422–1430
Patek K (1970) Glass lasers. Butterworth, London
Katzin L, Barnet ML (1964) J Phys Chem 68:3779–3785
Keller DE, Visser T, Soulimani F, Konigsberger DC, Weckhuysen BM (2007) Vibrat Spectrosc 43:140–151
Gao X, Wachs IE (2000) J Phys Chem B 104:1261–1268
Rada S, Culea M, Culea E (2008) J Phys Chem A 112(44):11251–11255
Rada S, Culea M, Neumann M, Culea E (2008) Chem Phys Lett 460:196–199
Rada S, Culea E (2009) J Mol Struct 929:141–148
Rada S, Ristoiu T, Rada M, Coroiu I, Maties V, Culea E (2010) Mater Res Bull 45:69–73
Sun C, Wang E, Xiao D, An D, Xu L (2007) J Mol Struct 840:53–58
Zhou Y, Qiao H (2007) Inorg Chem Commun 10:1318–1320
Acknowledgments
The financial support of the Ministry of Education and Research of Romania-National University Research Council (CNCSIS, PN II-IDEI 183/2008, contract number 476/2009) is gratefully acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rada, S., Chelcea, R. & Culea, E. Experimental and theoretical investigations on the structure-properties interrelationship of the gadolinium-vanadate-germanate glasses. J Mol Model 17, 165–171 (2011). https://doi.org/10.1007/s00894-010-0706-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0706-8