Abstract
The structures and stabilities of square–hexagon alternant boron nitrides (B x N x , x=12–36) vs their tube isomers containing octagons, decagons and dodecagons have been computed at the B3LYP density functional level of theory with the correlation-consistent cc-pVDZ basis set of Dunning. It is found that octagonal B20N20 and B24N24 tube structures are more stable than their square–hexagon alternants by 18.6 and 2.4 kcal mol−1, respectively, while the square–hexagon alternants of other cages are more stable. Trends in stability as a function of cluster size are discussed.

The octagonal B20N20 and B24N24 tube structures are more stable than their square-hexagon alternant cages
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boron nitrides B x N x have been studied extensively since they are the isoelectronic analogs of the carbon fullerenes. Boron nitride molecules [1, 2] and nanotubes [3–6] have been synthesized and/or characterized. For small molecules (x=3–10), ring isomers are the most stable form of boron nitride [7, 8]. For x>10, cages consisting of three-coordinate networks of boron and nitrogen are the most stable species [9, 10]. A wide variety of cage isomers exists, and the various classes of cages have been studied to determine which cages are the most stable. For example, fullerene-like cages with pentagons and hexagons in the cage network have been compared to cages with squares and hexagons containing alternate B–N bonds (alternant cage). Theoretical calculations comparing these two classes of molecules predict that the square–hexagon alternants are more stable than fullerene-like molecules for x=12 [11], x=13, 14 and 16 [12] and x=24 [13]. The energetic penalty associated with the pentagons and their inevitable non-alternate B–B and N–N bonds is the determining factor in the relative stability of these two classes. The most stable cages should have only polygons with an even number of atoms, so that full alternation of the boron and nitrogen atoms can take place, resulting in all the bonds being B–N bonds.
However, even-sided polygons include polygons larger than squares and hexagons, and molecules with octagons and larger even-sided polygons have been studied as well. For x=24, Pokropivny et al. [14] predicted that the most stable spheroidal structure has 12 squares, 8 hexagons and 6 octagons. More recently, a B24N24 cage with 8 squares, 16 hexagons and 2 octagons was shown [13] to be the most stable, but only slightly more stable than a square–hexagon alternant (6 squares and 20 hexagons).
An interesting result from the most recent study on B24N24 [13] is that the most stable octagon-containing molecule is an octagonal tube with exactly two octagons, more specifically with the octagons at each end of a tube consisting of squares and hexagons. Each such tube has a C4 axis of symmetry down the center of the molecule. In the current study, a stability comparison is made between these tube structures and alternant cages composed entirely of squares and hexagons. However, such tubes exist only if x=4n, where n is a positive integer. For molecule sizes where x=4n+2, the tube will be capped on one end by an additional ring of four alternating B and N atoms. Over a range of molecule sizes from x=12 to 36, calculations are carried out to determine how the stability relationship between these two classes of molecules varies with the size of the molecules. For each molecule size x, if F 4, F 6 and F 8 represent the number of squares, hexagons and octagons, respectively, the molecules are subject to the following mathematical bounds:
The alternant cages have F 4=6 and F 8=0, whereas the fully open tube structures have F 4=8 and F 8=2. The capped tube structures have F 4=7 and F 8=1, the one octagon on the uncapped end. Further, tube structures are chosen such that each octagon is adjacent to four squares that surround it in a fourfold symmetry (capped tubes only have a twofold symmetry overall because of the structure of the cap). Such square–octagon adjacency has been shown [13] to have a stabilizing influence on the molecules. Trends in stability will be discussed over the range of all even x from 12 to 36. The molecule size x=14 is excluded because no square–hexagon isomer exists for B14N14 without edge-sharing squares.
Also, the square–hexagon alternants will be compared to decagonal and dodecagonal tube-shaped molecules, at molecule sizes where such tube molecules exist. The decagonal tube has a ten-membered ring and five adjacent squares at each end, and the structure and energy of decagonal tubes will be calculated for x=20 and 30 (sizes at which the number of atoms is a multiple of ten). Likewise, the structure and energy of dodecagonal tubes, which have a 12-membered ring and six adjacent squares at each end, are calculated for x=18, 24, 30 and 36 (sizes at which the number of atoms is a multiple of 12). Trends of stability for decagonal and dodecagonal tubes will be discussed as a function of cluster size.
Computational details
Geometries of all the molecules in this study were optimized with the B3LYP density functional method [15, 16]. The basis set [17] was the correlation-consistent double-zeta (cc-pVDZ) set of Dunning. All optimized geometries were characterized as energy- minimum structures by frequency analysis. All calculations were carried out with the Gaussian 98 quantum chemistry software package [18].
Results and discussion
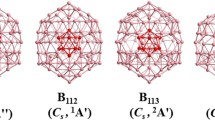
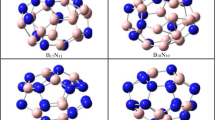
Twelve different molecule sizes were considered in this study, and the structures of the molecules are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12, respectively, for B x N x molecules with x=12, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34 and 36. Total energies for the square–hexagon alternants and tubes are given in Table 1, and their relative energies in Table 2. From the data, the following general trends are evident.
Octagonal tubes
For x=12, the octagonal tube is unstable by 133.8 kcal mol−1 above the square–hexagon alternant because the eight squares form four edge-sharing pairs (Fig. 1), which is a destabilizing feature that is inevitable for very short tubes. The separation of squares first occurs for x=16, but the octagonal tube is still 61.0 kcal mol−1 less stable than the square–hexagon alternant (Fig. 2). As the tube is lengthened, however, the octagonal tube becomes much more energetically competitive (x=18, Fig. 3), becoming more stable than the alternant at the intermediate sizes (x=20, 24, Figs. 4, 6). Toward the upper end of molecule sizes in this study, the octagonal tubes show signs of becoming less stable, but they are still close in energy to their square–hexagon alternants. It may be the case, however, that the gap widens again for very large sizes. The octagons do not incur a substantial energy penalty in the molecules in this study, and octagon-containing molecules are close in energy to alternants over a range of sizes.
Capped octagonal tubes
Capping one end of the octagonal tube with a square (to produce molecules with x=4n+2 as opposed to 4n) also does not incur an energetic penalty for the molecules. Capped tubes, with a single octagon, are very close in energy to the square–hexagon alternants over a wide range. For x=18, 22, 26 and 30, the capped octagonal tubes are within 9–22 kcal mol−1 of the square–hexagon alternants. The capped octagonal tube is not as stable for x=34, which may be a special case since the smaller sizes do not show a strong trend upward in energy for the capped octagonal tubes. For B28N28, the square–hexagon alternant structure (Fig. 8) is the most stable isomer, and structures with one or more octagons are higher in energy among ten isomers containing squares, hexagons and octagons [19]. For B32N32, the square–hexagon alternant structure (Fig. 10) is the most stable isomer, and other isomers are higher in energy [20].
Decagonal and dodecagonal tubes
The decagonal tubes for x=20 and 30 show much more favorable stability for the longer x=30 tube relative to the respective square–hexagon alternants as these molecule sizes. However, the x=30 decagonal tube is still over 50 kcal mol−1 higher in energy than the corresponding square–hexagon alternant, so that studies of x=40 and 50 would probably be required to establish whether these tubes become energetically competitive with molecules composed entirely of squares and hexagons. The dodecagonal tubes also show a trend of increasing stability with increasing length, and are very much higher in energy at all molecule sizes relative to the square–hexagon alternants. Even for x=30 and 36, these tubes are about 200 kcal mol−1 above the square–hexagon molecules, with no sign of significant stabilization. The dodecagonal tubes would likely remain high in energy even at sizes larger than the range studied here.
Conclusion
Boron nitride cages are the most stable if all the polygons have an even number of atoms, but even within that framework, several types of molecules exist with comparable stability, as found previously. Squares and hexagons are not the only structural features that lead to stable molecules. A systematic investigation of square–hexagon alternants and tubes of various diameters reveals that octagonal tubes are comparable in stability to square–hexagon alternant over a wide range of molecule sizes. Larger tubes are not as stable, however. Tubes with a decagonal structure are less stable than octagonal tubes, and the decagonal tubes will be comparable in stability to alternants only at large sizes or perhaps not at all. The dodecagonal tubes are very unstable relative to square–hexagon alternants and octagonal tubes across the range of sizes in this study and, in all likelihood, at larger sizes as well. The general trend is toward decreasing tube stability with increasing tube diameter. Therefore, tubes based on openings larger than 12-membered rings are likely even less stable.
References
Jensen F (1993) Chem Phys Lett 209:417–422
Silaghi-Dumitrescu I, Lara-Ochoa F, Bishof P, Haiduc I (1996) J Mol Struct (Theochem) 367:47–54
Chopra NG, Luyken RJ, Cherrey K, Crespi VH, Cohen ML, Louie SG, Zettl A (1995) Science 269:966–967
Terrones M, Hsu WK, Terrones H, Zhang JP, Ramos S, Hare JP, Castillo R, Prassides K, Cheetham AK, Kroto HW, Walton DRM (1996) Chem Phys Lett 259:568–573
Loiseau A, Williame F, Demoncy N, Hug G, Pascard H (1996) Phys Rev Lett 76:4733–4740
Saito Y, Maida M (1999) J Phys Chem A 103:1291–1293
Martin JML, El-Yazal J, Francois JP, Gijbels R (1995) Chem Phys Lett 232:289–294
Martin JML, El-Yazal J, Francois JP (1996) Chem Phys Lett 248:95–101
Strout DL (2000) J Phys Chem A 104:3364–3366
Strout DL (2001) J Phys Chem A 105:261–263
Wu HS, Xu XH, Zhang FQ, Jiao H (2003) J Phys Chem A 107:6609–6612
Strout DL (2004) Chem Phys Lett 383:95–98
Wu HS, Jiao H (2004) Chem Phys Lett 386:369–372 (This relationship has been confirmed further: Zope RR, Baruah T, Pederson MR, Dunlap BI (2004) Chem Phys Lett 394:300–304)
Pokropivny VV, Skorokhod VV, Oleinik GS, Kurdyumov AV, Bartnitskaya TS, Pokropivny AV, Sisonyuk AG, Sceichenko DM (2000) J Solid State Chem 154:214–222
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R.L, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98. Gaussian Inc, Pittsburgh, PA
Wu HS, Cui XY, Qin XF, Jiao H (2005) J Mol Struct (Theochem) 714:153–155
Wu HS, Cui XY, Xu XH (2005) J Mol Struct (Theochem) 717:107–109
Acknowledgments
D.L.S. acknowledges the Alabama Supercomputer Authority for a grant of computer time on the Cray SV1 and SGI Altix operated in Huntsville, AL and the National Institutes of Health (NIH/NCMHD 1P20MD000547-01) for support. We also thank the Natural Science Foundation of China (20471034) for support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr. Paul von Ragué Schleyer on the occasion of his 75th birthday
Rights and permissions
About this article
Cite this article
Wu, HS., Cui, XY., Qin, XF. et al. Boron nitride cages from B12N12 to B36N36: square–hexagon alternants vs boron nitride tubes. J Mol Model 12, 537–542 (2006). https://doi.org/10.1007/s00894-005-0042-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0042-6