Abstract
Epithelial–myoepithelial carcinoma (EMCa) is a rare low-grade salivary malignancy. It is rare for EMCa to occur as the carcinomatous component of carcinoma ex-pleomorphic adenoma (PA). We examined one additional case of EMCa ex-PA, immunohistochemically and genetically. The patient was an 83-year-old female, who suffered from swelling of the right parotid region. Histologically, the tumor contained a hyalinized nodule, which displayed elastosis. The main tumor exhibited a bi-layered structure, involving inner ductal cells and clear outer myoepithelial cells. Immunostaining indicated that the inner cells were positive for epithelial membrane antigen, whereas the outer cells were positive for p40. On the genetic level, the carcinoma harbored no HRAS gene mutations, whereas fluorescence in situ hybridization (FISH) of the Pleomorphic Adenoma Gene1 showed splitting signals in the carcinomatous component. We diagnosed this case as EMCa ex-PA. It is necessary to differentiate EMCa ex-PA from myoepithelial carcinoma and clear cell carcinoma, and FISH is useful for such purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoma ex-pleomorphic adenoma (CXPA) is a relatively common malignant salivary gland tumor, which varies from low-grade to high-grade malignancy, and the carcinomatous component is most commonly composed of salivary duct carcinoma [1]. Approximately, 35% of the carcinomatous components of CXPA are composed of myoepithelial carcinoma (MC), and < 5% belong to other histological types, including mucoepidermoid carcinoma, squamous cell carcinoma, poorly differentiated carcinoma, and EMCa. Seethala et al. only encountered 1 case (4.9%) of EMCa ex-PA among 61 EMCa cases [2], and Sedassari et al. reported 3 new cases of EMCa ex-PA and found 9 further cases in their review of the literature [3]. Therefore, it is exceedingly rare for EMCa to arise from pre-/co-existing PA.
Epithelial–myoepithelial carcinoma (EMCa) is a salivary gland tumor, involving two different cell populations: inner ductal cells and outer myoepithelial cells, which classically have clear cytoplasm [4, 5]. Although rare cases of high-grade EMCa have been reported [6,7,8,9], most EMCa are low-grade tumors and have to be distinguished from pleomorphic adenoma (PA), especially from cellular PA, adenoid cystic carcinoma (AdCC), clear cell carcinoma (CCC), mucoepidermoid carcinoma, clear cell variant, and oncocytoma, clear cell variant. PA is the most common benign tumor of the salivary glands, whereas EMCa is a rare malignant salivary gland tumor, which accounts for < 1% of all salivary gland epithelial neoplasms and nearly 2% of malignant salivary gland tumors [4, 5]. Over 75% of EMCa develop in the parotid gland. EMCa exhibits a recurrence rate of 30%–50%, a lymph node metastasis rate of 15%–20%, and 5- and 10-year survival rates of 80%–94% and 72%–90%, respectively [7, 10, 11]. Recently, up to 33% of EMCa were reported to harbor GTPase HRas (HRAS) codon 61 mutations [9, 10]. El Hallani et al. reported that 80% of EMCa arose from PA, and progression to higher-grade EMCa with intact pleomorphic adenoma gene (PLAG) 1 and high mobility group A2 (HMGA2) genes was associated with the presence of tumor protein p53 (TP53) or F-box/WD repeat-containing protein 7 (FBXW7) mutations or SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1) deletion [11]. Urano et al. reported that no cases of EMCa ex-PA had HRAS mutations [12]. Thus, the relationship between EMCa and PA remains unclear.
Herein, we report a case of EMCa ex-PA, together with the results of immunohistochemical and genetical analyses.
Materials and methods
We selected EMCa cases from a pathology file of our institution, and furthermore we histologically selected one case of EMCa ex-PA.
Immunohistochemical analysis
We performed immunostaining on formalin-fixed, paraffin-embedded (FFPE) sections of the primary tumor using the antibodies listed in Table 1 and an Autostainer Link48 (DakoCytomation, Carpinteria, CA, USA), Leica BOND-MAX automated immunostainer (Leica, Bannockburn, IL, USA), or VENTANA BenchMark ULTRA automatic immunostainer (Roche Tissue Diagnosis, Oro Valley, AZ, USA). We used Image J (National Institutes of Health, Bethesda, MD, USA) to estimate the percentage of Ki-67-positive tumor cells. The results of the immunohistochemical examinations are summarized in Table 1.
Genetical analysis
According to the previously described method [14], HRAS gene mutation was examined; briefly, DNA was extracted from an FFPE sample of the primary tumor using the QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany). The polymerase chain reaction (PCR) was performed with the KOD FX enzyme (Toyobo, Osaka, Japan). The PCR products were electrophoresed, and each purified product was directly sequenced using an HRAS-F primer with the BigDye Terminator v3.1 cycle sequencing kit (Thermo Fisher Scientific, Waltham, MA, USA) and a 3730 × 1 DNA analyzer (Thermo Fisher Scientific). Fluorescence in situ hybridization (FISH) of FFPE sections of the main tumor was performed to examine the splitting of the PLAG1 and EWSR1 genes using appropriate probes (SureFISH 8q12.1 PLAG1 3′BA RD probe [G10097R-8] and SureFISH 8q12.1 PLAG1 5′BA 625 kb GR probe [G100998G-8], Agilent, Santa Clara, CA, USA, and Vysis LSI EWSR1 (22q12) Dual Color Break Apart Rearrangement probe [07J71-001], Abbott Laboratories, IL, USA), according to the manufacturers’ guidelines.
Results
Clinical history
An 83-year-old Japanese female suffered from swelling of the right parotid region. She had undergone partial resection and adjuvant chemotherapy for right mammary carcinoma 13 years ago, and no recurrence or metastasis had been detected since. She had noticed a movable elastic-hard mass in the right parotid region. A fine-needle aspiration biopsy (FNAB) examination was performed, and the cytological diagnosis was “PA, suspected”. As after 1 year, the mass had rapidly enlarged, she was admitted to our hospital, and another FNAB examination was conducted. The cytological diagnosis was “malignant tumor, suspected”, and computed tomography (CT) revealed a large relatively well-demarcated mass in the right parotid gland (Fig. 1a). Right total parotidectomy was performed, and at that time, pathological diagnosis was “EMCa”. Nine months later, fluorodeoxyglucose-positron emission tomography (FDG-PET) demonstrated local recurrence. Therefore, tumor resection and additional radiotherapy (60 Gy) were conducted. No recurrence or metastasis was detected for 6 years. However, although we could not be examined histologically, CT subsequently revealed primary lung cancer. Radiotherapy (48 Gy) was administered, but radiation-induced pneumonitis occurred. Therefore, the radiotherapy was stopped. Multiple liver metastases were detected on PET 2 years later, and the patient selected best supportive care. She died at home 2 months later. No autopsy was permitted.
Pathological findings
The primary tumor at the total parotidectomy
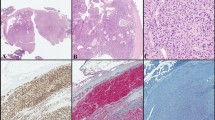
Macroscopically, a relatively well-demarcated, lobulated, grayish-white tumor was seen within the parotid gland, and a well-defined yellowish nodule was observed in the main tumor (Fig. 1b).
Histologically, the main tumor consisted of regions of trabecular and nest-like growth separated by a hyalinized stroma. Approximately, half of the tumor was made up of round to oval cells with moderate amounts of clear cytoplasm and round to oval, eccentrically placed, mildly pleomorphic, and vesicular nuclei with small prominent nucleoli (Fig. 2a). Periodic acid–Schiff (PAS)–Alcian blue (AB) staining indicated that they contained large amounts of PAS-positive glycogens, and contained AB-positive myxoid stroma. In a residual area, bi-phasic structures were observed, i.e., inner cuboidal to flattened ductal cells were seen in the central areas of the nests, and they were surrounded by clear round to ovoid cells. Cellular atypia or mitosis was seen in some of the clear outer cells, and focal necrosis was also noted (Fig. 2b). Keratinization and myoepithelial anaplasia were sometimes observed (Fig. 2c). The bi-phasic tumor was considered to be an EMCa. The yellowish nodule was composed of a markedly hyalinized, hypocellular nodule, and Elastica van Gieson (EvG) staining showed moderate elastosis in the nodule (Fig. 2d). The nodule was considered to be a pre-existing PA. No perineural invasion was seen, but some lymphovascular invasion was observed. The surgical margins were focally positive for the carcinomatous component. The pathological stage of the primary tumor was pStage II (pT2N0M0).
Histological findings. The tumor had a bi-phasic structure, involving inner small eosinophilic cells (arrows) and outer ovoid clear cells (a) (hematoxylin and eosin [H&E] staining). The tumor contained a necrotic area (H&E) and it sometimes showed keratinization (b, inset). The clear cells of the tumor frequently showed marked structural and cellular atypia, such as swollen and irregular nuclei, prominent nucleoli, and irregularly shaped cytoplasm, with a myxoid stroma (H&E: c, inset: higher magnification). The yellowish nodule was composed of a hypocellular hyalinized stroma, which displayed some calcification (H&E). It also exhibited moderate elastosis (EvG staining. d, inset). The clear ovoid cells, which harbored more cellular atypia than the main region of the primary tumor, predominated in the recurrent tumor (e) (H&E)
The recurrent tumor at the additional resection
The recurrent tumor had a similar histology, but contained smaller amounts of ductal cells, and it was predominantly composed of proliferating clear myoepithelial cells (Fig. 2e).
Immunohistochemical analysis
The inner cells were positive for epithelial membrane antigen (EMA) and cytokeratin (CK) 7, whereas the outer cells were positive for p63, p40, alpha-smooth muscle actin (ASMA), CK5/6, and vimentin (Figs. 3a, b). The tumor cells were weakly positive for S-100 protein. Most of the tumor was weakly positive for p53, but some areas, which exhibited myoepithelial anaplasia, were strongly positive for p53 (Fig. 3c). The Ki-67 labeling indices of these elements were 12.2% and 64.2%, respectively (Fig. 3d).
Immunohistochemical findings. The inner cells were positive for EMA (a). The outer cells were positive for p40 (b). Most of the cancer cells were weakly positive for p53, whereas some atypical cells in the region of myoepithelial anaplasia were strongly positive for p53 (c, inset). Similarly, most of the carcinoma cells had low Ki-67 labeling rates, but some cancer cells, which displayed more marked atypia, demonstrated a relatively high Ki-67-labeling rate (d, inset)
Genetic analysis
No point mutations were detected in codon 12, 13, or 61 of the HRAS gene (Fig. 4a). FISH analysis indicated that most of the tumor cells showed split signals for the PLAG1 gene, but no split signals for EWSR1 gene (Figs. 4b, c).
The results of the genetic analyses. The sequencing of genomic DNA from the tumor sample showed no mutations at the hot spot (codon 61: red arrowhead) of the HRAS gene (a). A FISH examination detected the splitting of the PLAG1 gene in the carcinoma cells (b). A FISH examination did not detect any splitting of the EWSR1 gene in the carcinoma cells (c)
We diagnosed this tumor as EMCa ex-PA, widely invasive, and the EMCa harbored a high-grade component.
Discussion
EMCa was first proposed by Donath et al. in 1972 [15] and was first listed as an independent tumor entity in the second edition of the WHO Classification in 1991 [16]. Despite its rarity, EMCa is a well-recognized low-grade malignant tumor of the salivary gland. Typical EMCa have bi-phasic glandular structures, involving eosinophilic inner ductal cells and clear myoepithelial abluminal cells, and exhibit invasive growth with a lobular pattern. This characteristic histology and the corresponding immunohistochemical findings can facilitate a correct diagnosis [17]. An immunohistochemical examination of EMCa indicated that the luminal cells were positive for ductal cell markers, such as EMA and CK7, whereas the clear abluminal cells were positive for myoepithelial markers, such as ASMA, CK5/6, CK14, and vimentin [18]. Therefore, EMCa are composed of two cell populations. Various histological variants of EMCa are known to exist, including EMCa with high-grade transformation (dedifferentiated EMCa) [19,20,21], oncocytic EMCa [22], EMCa ex-PA [2, 13, 14], double clear EMCa [4, 23], EMCa with myoepithelial anaplasia [2], and sebaceous EMCa [2, 24, 25]. In addition to these histological variants, the cribriform pattern, basaloid pattern, papillary-cystic pattern, squamous metaplasia with keratinization, psammomatous pattern, and Verocay-like pattern have been reported [2]. The present case consisted of both inner ductal cells and outer myoepithelial cells, and the former component was immunopositive for EMA and CK7, while the latter component was immunopositive for myoepithelial markers, such as CK5/6 and ASMA. A hyalinized nodule, which exhibited elastosis, was seen in the tumor, and it was considered to be a pre-existing PA [1]. Therefore, the tumor was finally diagnosed as an EMCa ex-PA. As a necrotic area and myoepithelial cells, which exhibited increased histological atypia, were observed, the EMCa in the present case was considered to display myoepithelial anaplasia, and it also had a high-grade component. A high Ki-67 labeling index and the nuclear accumulation of p53 were observed in these areas. The recurrent tumor displayed a similar histology, but the myoepithelial component predominated. Therefore, our findings suggest that high-grade components of EMCa might recur.
Chiosea et al. and Urano et al. reported that HRAS mutations were frequently observed in EMCa (26.7% and 82.7%, respectively) [12, 13]. Furthermore, they concluded that the detection of HRAS mutations was of high diagnostic value for EMCa. Although HRAS gene mutations were observed in de novo cases of EMCa, EMCa that arose from preexisting PA had no HRAS mutations or a lower percentage of HRAS mutations [12], which implies that the tumorigenic mechanism of EMCa differs according to the histological origin of the tumor. In the present case, the tumor did not harbor any HRAS mutations, which was compatible with the findings obtained in the above-mentioned studies, and the EMCa was determined to be the carcinomatous component of a CXPA. On the other hand, the reported prevalence of preexisting PA in EMCa ranges widely from 1.6% to 80% [2, 11,12,13], but only low frequency EMCa is accompanied by preexisting PA (personal communication). Although El Hallani et al. reported that 30 (77%) of 34 EMCa cases involved preexisting PA [12], their criteria for “preexisting PA” were considered to be too wide, and they had not excluded pseudo-EMCa. For examples, they considered that because of only existence of a tiny hyalinized nodule at the periphery of the main tumor, such a case was included into EMCa ex-PA, or it is possible that when the normal ductal cells were involved in the tumor of MC, they even included such a case into the EMCa ex-PA. There might be some divergence in how the histomorphological features of PA and the carcinomatous components of EMCa ex-PA are interpreted. For example, the bi-phasic tubular pattern of PA occasionally resembles that of EMCa, and the differences between MC ex-PA and high-grade EMCa might be indiscernible in some cases. In the current case, we considered whether the carcinomatous component of the CXPA was an EMCa or MC, but as it immunohistochemically exhibited both ductal and myoepithelial differentiation, we concluded that it was an EMCa, which was relatively rare. We believe that in case of diagnosis of EMCa ex-PA, 1) the existence of a relatively large hyalinized nodule with elastosis and 2) the bi-phasic structures by inner ductal cells and outer clear myoepithelial cells were necessary to diagnosis.
On the basis of morphological and molecular evidence of preexisting PA, El Hallani et al. indicated that four subsets of EMCa exist [11]: (a) EMCa with morphological evidence of preexisting PA, but intact PLAG1 and HMGA2 genes (31%); (b) carcinomas with PLAG1 gene rearrangements (23%); (c) carcinomas with HMGA2 gene rearrangements (26%); and (d) de novo carcinoma without histomorphological or molecular evidence of PA (21%). HRAS mutations were more common in EMCa with intact PLAG1 and HGMA2 genes than in other subtypes. Furthermore, high-grade EMCa harbored TP53 and FBXW7 mutations and SMARCB1 deletions [11]. The present case exhibited break-apart PLAG1 gene signals and belonged to subset (b). The invasive carcinoma component contained a high-grade element, which showed strong immunopositivity for p53. However, we could not perform PLAG1 immunostaining. In previous studies, PLAG1 gene rearrangements were seen in 38.1% to 44.4% of PA [26, 27], whereas HMGA2 gene rearrangements were observed in approximately 20% of PA [28]. Although PLAG1 gene rearrangements were relatively common in PA, such gene rearrangements are rare in CXPA (33.3%) [29]. Immunohistochemically, 92.5% of PA showed immunopositivity for PLAG1, whereas PLAG1 expression was only detected in 35% of CXPA [30]. Marked alterations in the PLAG1 gene, such as gene deletion or chromosomal changes, might occur as part of the malignant changes seen in PA. Further alterations might induce the loss of PLAG1 expression in CXPA.
In the present case, proliferating clear myoepithelial cells predominated, especially in the recurrent tumor. The clear cell variant of MC and CCC were included as differential diagnoses. The frequency of EWSR1 gene rearrangement in these tumors was reported to be 24% and 87%, respectively [31, 32]. As no break-apart EWSR1 gene signals were detected in the current case, it was considered to not be a clear cell variant of MC or CCC. However, it could not be completely ruled out that it was an EWSR1-intact clear cell variant of MC. The primary tumor exhibited a bi-phasic structure, and the immunohistochemical results also indicated that the tumor was composed of two cell populations. Therefore, we considered that the carcinomatous component was composed of an EMCa, rather than an EWSR1-intact clear cell variant of MC. AdCC frequently showed EMCa-like bi-layered structures, but the typical histology (i.e.; cribriform pattern) could be found in even such a case, and AdCC does not harbor PLAG1 gene rearrangement. Our case was entirely different from AdCC due to the histological and genetical examinations.
Conclusions
In summary, it is considered rare for EMCa to arise from a pre-/co-existing PA, despite the report by El Hallani et al. [13]. During the diagnosis of EMCa ex-PA, clear cell variants of MC and CCC should be excluded, and genetic analyses, such as PCR of the HRAS gene and FISH, are diagnostically useful.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EMCa:
-
Epithelial–myoepithelial carcinoma
- PA:
-
Pleomorphic adenoma
- FISH:
-
Fluorescence in situ hybridization
- CXPA:
-
Carcinoma ex-pleomorphic adenoma
- MC:
-
Myoepithelial carcinoma
- AdCC:
-
Adenoid cystic carcinoma
- CCC:
-
Clear cell carcinoma
- HRAS:
-
GTPase HRas
- PLAG1:
-
Pleomorphic adenoma gene 1
- HMGA2:
-
High mobility group 2
- TP53:
-
Tumor protein p53
- FBXW7:
-
F-box/WD repeat-containing protein 7
- SMARCB1:
-
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1
- FNAB:
-
Fine needle aspiration biopsy
- CT:
-
Computed tomography
- FGD-PET:
-
Fluorodeoxyglucose-positron emission tomography
- PAS:
-
Periodic acid–Schiff
- AB:
-
Alcian blue
- EvG:
-
Elastica van Gieson
- FFPE:
-
Formalin-fixed, paraffin-embedded
- EMA:
-
Epithelial membrane antigen
- CK:
-
Cytokeratin
- ASMA:
-
Alpha-smooth muscle actin
- PCR:
-
Polymerase chain reaction
References
Williams MD, Ihrler S, Seethala R (2017) Carcinoma ex pleomorphic adenoma. In: El Naggar A, Chan JK, Grandis J, Takata T, Slootweg P (eds) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon, pp 176–177
Seethala RR, Barnes EL, Hunt JL (2007) Epithelial–myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol 31:44–57
Sedassari BT, dos Santos HT, Mariano FV, de Silva Lascane NA, Altemani A, Sousa S (2015) Carcinoma ex pleomorphic adenoma of minor salivary glands with major epithelial–myoepithelial component: clinicopathologic and immunohistochemical study of 3 cases. Ann Diag Pathol 19:164–168
Ellis GL, Auclair P (2008) Epithelial–myoepithelial carcinoma. Tumors of the salivary glands (AFIP Atlas of Tumor Pathology: Series 4). American Registry of Pathology, Washington, DC, pp 309–322
Seethala R, Bell D, Fonseca I, Katabi N (2017) Epithelial–myoepithelial carcinoma. In: El Naggar A, Chan JK, Grandis J, Takata T, Slootweg P (eds) WHO Classification of Head and Neck Tumours. International Agency for Research on Cancer, Lyon, pp 175–176
Sentani K, Ogawa I, Uraoka N, Ikeda M, Hayashi N, Hatttori T, Hattori Y, Oue N, Takata T, Yasui W (2015) High-grade epithelial–myoepithelial carcinoma of the parotid gland with mucous cell differentiation. Pathol Int 65:490–494
Alos L, Carrillo R, Ramos J, Baez JM, Mallofre C, Fernandez PL, Cardesa A (1999) High-grade carcinoma component in epithelial–myoepithelial carcinoma of the salivary glands clinicopathological, immunohistochemical and flow-cytometric study of three cases. Virchows Arch 434:291–299
Daa T, Kashima K, Gamachi A, Nakayama I, Yokoyama S (2001) Epithelial–myoepithelial carcinoma harboring p53 mutation. APMIS 109:316–320
Vazquez A, Patel TD, D’Aguillo CM, Abdou RY, Farver W, Baredes S, Eloy JA, Park RC (2015) Epithelial–myoepithelial carcinoma of the salivary glands: an analysis of 246 cases. Otolaryngol Head Neck Surg 153:569–574
Gore MR (2018) Epithelial–myoepithelial carcinoma: a population-based survival analysis. BMC Ear Nose Throat Disord 18:15
El Hallani S, Udager AM, Bell D, Fonseca I, Thompson LDR, Assaad A, Anaimy A, Luvison AM, Miller C, Seethala RR, Chiosea S (2018) Epithelial–myoepithelial carcinoma: frequent morphologic and molecular evidence of preexisting pleomorphic adenoma, common HRAS mutation in PLAG1-intact and HMGA2-intact cases, and occasional TP53, FBXW7, SMARCB1 alteration in high-grade cases. Am J Surg Pathol 42:18–27
Urano M, Nakaguro M, Yamamoto Y, Hirai H, Tanigawa M, Saigusa N, Shimizu A, Tsukahara K, Tada Y, Sakurai K, Isomura M, Okamura Y, Yamagichi H, Matsubayashi J, Nagao T (2019) Diagnostic significance of HRAS mutations in epithelial–myoepithelial carcinomas exhibiting a broad histopathologic spectrum. Am J Surg Pathol 243:984–994
Chiosea SI, Miller M, Seethala RR (2014) HRAS mutations in epithelial–myoepithelial carcinoma. Head Neck Pathol 8:146–150
Cros J, Sbidian E, Hans S, Roussel H, Scotte F, Tartour E, Brasnu D, Laurent-Pluig P, Bruneval P, Blons H, Badoual C (2013) Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumors. Ann Oncol 24:2624–2629
Donath K, Seifert G, Schmiz R. Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts (1972) Epithelial–myoepithelial carcinoma of the intercalated ducts. Virchows Arch A Pathol Pathol Anat 356:16–31
Seifert G (ed) (1996) Epithelial–myoepithelial carcinoma. Histological Typing of Salivary Gland Tumours (World Health Organization International Histological Classification of Tumours), 2nd edn. Springer, Washington DC
Nagao T, Sato E, Inoue R, Oshiro H, Takahashi R, Nagai T, Yoshida M, Suzuki F, Obikane H, Yamashina M, Matsubayashi J (2012) Immunohistochemical analysis pf salivary gland tumors: application for surgical pathology practice. Acta Histochem Cytochem 45:269–282
Tralongo V, Daniele E (1998) Epithelial–myoepithelial carcinoma of the salivary glands: a review of literature. Anticancer Res 19:603–608
Fonseca I, Felix A, Soares J (2000) Dedifferentiation in salivary gland carcinomas. Am J Surg Pathol 24:469–471
Kusafuka K, Takizawa Y, Ueno T, Ishiki H, Asano R, Kamijo T, Iida Y, Ebihara M, Ota Y, Onitsuka T, Kameya T (2008) Dedifferentiated epithelial–myoepithelial carcinoma of the parotid gland: a rare case report of immunohistochemical analysis and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:85–91
Roy P, Bullock MJ, Perez-Ordonez B, Dardick I, Weinreb I (2010) Epithelial–myoepithelial carcinoma with high grade transformation. Am J Surg Pathol 34:1258–1265
Seethala RR, Richmond JA, Hoschar AP, Barnes EL (2009) New variants of epithelial–myoepithelial carcinoma: oncocytic-sebaceous and apocrine. Arch Pathol Lab Med 133:950–959
Pai RR, Sahu K, Kini AU (2008) Clear cell predominant epithelial–myoepithelial carcinoma of the palate—role of immunohistochemistry. Indian J Otolaryngol Head Neck Surg 60:163–165
Shinozaki A, Nagao T, Endo H, Kato N, Hirokawa M, Mizobuchi K, Komatsu M, Igarashi T, Yokoyama M, Masuda S, Sano K, Izumi M, Fukayama M, Mukai K (2008) Sebaceous epithelial–myoepithelial carcinoma of the salivary gland: clinicopathologic and immunohistochemical analysis of 6 cases of a new histologic variant. Am J Surg Pathol 32:913–923
Stalhammer G, Elmberger G (2014) Sebaceous epithelial–myoepithelial carcinoma of the parotid gland: a case report of a new histologic variant. Ann Diag Pathol 18:248–252
Asahina M, Saito T, Hayashi T, Fukumura Y, MItan K, Yao T (2019) Clinicopathologic effect of PLAG1 fusion genes in pleomorphic adenoma and carcinoma ex pleomorphic adenoma with special emphasis on histological features. Histopathology 74:514–525
Matsuyama A, Hisaoka M, Nagao Y, Hashimoto H (2011) Aberrant PLAG1 expression in pleomorphic adenomas of the salivary gland: a molecular genetic and immunohistochemical study. Virchows Arch 458:583–592
Katabi N, Ghossein R, Ho A, Dogan S, Zhang L, Sung Y-S, Antonescu CR (2015) Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol 46:26–33
Chiosea SI, Thompson LDR, Weinreb I, Bauman JE, Mahaffey AM, Miller C, Ferris RL, Gooding WE (2016) Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma, PLAG1 or HGMA2 rearrangements, and common genetic alterations. Cancer 122:3136–3144
de Brito BS, Giovanelli N, Egal ES, Sanchez-Romero C, Nascimento JS, Martins AS, Tincani AJ, Del Negro A, Gondak RO, Almeida OP, Kowalski LP, Altemani A, Mariano FV (2016) Loss of expression of Plag1 in malignant transformation from pleomorphic adenoma to carcinoma ex pleomorphic adenoma. Hum Pathol 57:152–159
Skalova A, Weinreb I, Myrcza M, Simpson RHW, Laco J, Agamy A, Vazmitel M, Majewska H, Vancek T, Talarcik P, Manajlovic S, Losito SN, Steiner P, Klimkova A, Michal M (2015) Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol 39:338–348
Shah AA, LeGallo R, van Zante A, Frierson HF Jr, Mills SE, Berean KW, Mentrikoski MJ, Stelow EB (2013) EWSR1 genetic rearrangements in salivary gland tumors. A specific and very common feature of hyalinizing clear cell carcinoma. Am J Surg Pathol 37:571–578
Acknowledgements
The authors thank Mr. Naofumi Ishikawa, Ms. Yoko Yamazaki, Mr. Tomohiro Iwasaki, Ms. Aki Kubota, Mr. Kensuke Shimazaki, Ms. Chinatsu Tsuchiya, Mr. Kazuki Hirata, Mr. Koji Takahashi, Ms. Nobuyo Tsujino and Mr. Yohei Saguchi, who are medical technologists at the Department of Pathology, Shizuoka General Hospital, Shizuoka, Japan, for their excellent technical assistances. The authors are grateful to Dr. Masato Nakaguro at the Department of Diagnostic Pathology and Laboratory Medicine, Nagoya University Hospital, Aichi, Japan, for his help with the genetic analyses, including the PCR analysis, direct sequencing, and fluorescence in situ hybridization.
Funding
This work was supported in part by a Grant-in-Aid for Medical Research Support Project of Shizuoka Prefectural Hospital Organization in 2019 of Japan (to KK).
Author information
Authors and Affiliations
Contributions
KK designed and drafted the manuscript, and KK, AM, MS and KA made the histopathological diagnosis and the analysis of the immunohistochemical results. MY provided the clinical data. KK also estimated the genetic changes. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests relating to this study.
Ethical approval
This study was approved by the institutional review board of Shizuoka General Hospital (SGHIRB#2019007).
Consent to participate
All subjects signed informed consent forms before participating.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kusafuka, K., Yamashita, M., Muramatsu, A. et al. Epithelial–myoepithelial carcinoma ex-pleomorphic adenoma of the parotid gland: report of a rare case with immunohistochemical and genetic analyses. Med Mol Morphol 54, 173–180 (2021). https://doi.org/10.1007/s00795-020-00262-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-020-00262-6