Abstract
Insulinoma-associated protein 1 (INSM1) is an important biomarker of Achaete-scute homolog-like 1-driven pathways. For diagnosis of pancreatic neuroendocrine tumors (PanNET), chromogranin A (CGA), synaptophysin (SYP), and neural cell adhesion molecule (NCAM) were also considered as potential biomarkers. However, it is often difficult to diagnose it immunohistochemically. Hence, we examined the expression pattern of INSM1 in pancreatic solid tumors. We detected INSM1, CGA, SYP, and NCAM immunohistochemically, in 27 cases of NET [pure type: 25 cases, mixed adenoneuroendocrine carcinoma (MANEC): 2 cases]. We included 5 cases of solid-pseudopapillary neoplasm (SPN), 7 cases of acinar cell carcinoma (ACC), and 15 cases of pancreatic ductal adenocarcinoma (PDAC) as the control group. Nuclear expression of INSM1 was found in all PanNET pure type cases. However, expression of INSM1 was negative in PDAC, ACC, and SPN in all cases, whereas faint expression was seen in the cytoplasm from SPN. MANEC comprises of two components: neuroendocrine carcinoma and adenocarcinoma components. The NET component was positive for INSM1 expression, whereas the PDAC component does not express INSM1, which aids in distinguishing these components. Our results suggest that INSM1 is a useful immunohistochemical marker for diagnosing pancreatic neuroendocrine tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumors (PanNET) are pancreatic solid tumors that have neuroendocrine differentiation and express chromogranin A (CGA), synaptophysin (SYP), and neural cell adhesion molecule (NCAM), which are known neuroendocrine markers [1,2,3]. From histological findings, it is possible to easily diagnose PanNET in many cases because of characteristic chromatin patterns (salt and pepper appearance) and other histological structures (for example, microtrabecular–gyriform) [1, 2]. In contrast, it is difficult to morphologically distinguish between duct formation and pseudopapillary like structures in histological specimens [4, 5], and immunohistochemical (IHC) studies demonstrated that solid-pseudopapillary neoplasm (SPN) was positive for SYN and NCAM, and that acinar cell carcinoma (ACC) is occasionally partially positive for neuroendocrine markers [6, 7]. Thus, distinguishing such tumors is difficult using existing markers, and markers with higher specificity are required.
Insulinoma-associated protein 1 (INSM1) is used as a biomarker for human small cell lung carcinoma and it is an important factor in ASCL1-driven pathways [8]. INSM1 is inhibited by Notch-Hes 1 signaling pathway, which is associated with tumor growth and development, and it has been confirmed to promote expression of SYP, CGA, and NCAM [8]. Therefore, INSM1 has recently started to gain attention as an important indicator in small cell lung carcinoma. INSM1 may play an important role as a modulator for PanNET, which is thought to be linked to Notch-Hes 1 signaling pathway [9, 10]. However, there are no reports related to INSM1 expression in pancreatic solid tumors.
In this study, we have used pancreatic solid tumors to compare and evaluate the expression pattern of CGA, SYP, NCAM, and INSM1, which are widely recognized as important biomarkers of PanNET, and we have evaluated the efficacy of using INSM1 as a biomarker of PanNET.
Materials and methods
Patients
For the study, excision biopsies of pancreatic tail and head were acquired from 27 NET cases at the Kurume University Hospital during the period from 1994 to 2016 (Fig. 1). The control subjects consisted of 15 pancreatic ductal adenocarcinoma (PDAC) cases, 7 ACC cases, and 5 SPN cases in which the patients underwent surgical removal of the pancreas at the Kurume University Hospital. The excised tissues were fixed using 10% buffered formalin and sectioned at 4-µm thickness, followed by hematoxylin–eosin staining. Next, a histopathological evaluation was performed by three pathologists (M.T., M.N., and Y.N.). The expression behavior of CGA, SYP, and NCAM was evaluated in NET, SPN, ACC, and PDAC cases using IHC staining (Fig. 1). The NET grade classification was carried out in accordance with the 2010 World Health Organization (WHO) classification followed by Ki-67 staining and mitotic count [2]. Each NET was classified into either grade 1 (mitotic count <2/10 high power field (HPF), MIB-1: ≤2%), grade 2 (mitotic count: 2–20/10HPF, K-67 LI: 3–20%), or neuroendocrine carcinoma (mitotic count >20/10HPF, MIB-1: >20%). We referred to two tumor-node-metastasis (TNM) systems in the American Joint Committee on Cancer (AJCC) Staging Manual and the European Neuroendocrine Tumor Society (ENETS) for information concerning the T factor and stages of progression [1]. All procedures were approved by the Ethics Review Committee for Animal Experimentation of Kurume University School of Medicine (no. 16072).
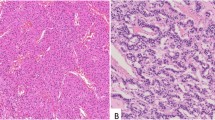
Pathological findings of PanNET. a Solid tumors with clear boundaries and a membrane with a yellow–white tone accompanied by internal hemorrhaging were confirmed visually. b H.E. staining of solid tumors with membranes. c Tumor proliferated in an alveolar and funicular manner and small blood vessels were seen to be interpositioned between alveolars. The tumor cells were between small and medium sizes. The nucleus varied in size, and was oval-shaped and irregular. The tumor cells were positive for d CGA, e SYP, and f NCAM
Immunohistochemical analysis
Staining slides were prepared using the primary pancreatic lesion from 27 NET cases [pure type: 25 cases, mixed adenoendocrine carcinoma (MANEC): 2 cases], 5 SPN cases, 7 ACC cases, and 15 PDAC cases. We used 4-µm-thick sections of formalin-fixed, paraffin-embedded tissues. The sections were mounted on coated glass slides and incubated with anti-rat monoclonal antibody against Ki-67 (1:100, clone MIB-5) (DAKO Cytomation, code no. M7249) for IHC analysis with the use of BenchMark ULTRA (Ventana Automated Systems, Inc., Tucson, AZ, USA). In brief, each slide was heat-treated for 64 min using Ventana’s CC1 retrieval solution, and incubated for 32 min with Ki-67 antibody. This automated system used the ultraVIEW DAB Detection Kit (Ventana Automated Systems) with 3,3ʹ diaminobenzidine (DAB) as the chromogen. IHC staining for INSM1 (1:400, clone A-8) (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA), chromogranin A (1:400, clone DAK-A3) (DAKO Corporation, Carpinteria, CA, USA), synaptophysin (1:1, clone 27G12) (Nichirei, Tokyo Japan), and CD56 (1:200, clone 1B6) (Leica Microsystems, Newcastle-upon-Tyne, UK) was performed on the same fully automated Bond-III System (Leica Microsystems) using onboard heat-induced antigen retrieval with epitope retrieval solution 2 for 20 min at 99 °C, and incubated with the antibody for 30 min at room temperature for INSM1 or 15 min for other antibodies. This automated system uses a Bond Polymer Refine Detection Kit with DAB and horseradish peroxidase-polymer as a secondary antibody; incubation with the secondary antibody was performed for 30 min at room temperature. All IHC analyses were evaluated by two experienced observers who were unaware of the patients’ conditions.
From the INSM1 expression, we determined that only the results for nuclear expression were positive (Fig. 2a, b). We used an Allred score system to evaluate staining by obtaining a total score (TS) that is calculated from a population score (PS) and an intensity score (IS) (Fig. 2c–e) [11]. The scores for INSM1, SYP, CGA, and NCAM were determined to be: 0 = no staining, 1+ = 1–10% positive cells, 2+ = 11–30% positive cells, and 3+ = over 50% positive cells. Here, 0 and 1+ were determined to be negative, and 2+ and 3+ were determined to be positive. β-Catenin staining was performed for the SPN cases which were confirmed for nuclear expression (data not shown).
INSM1 staining for PanNET. H.E. staining of tumor cells around the existing pancreatic tissue. a Expression of INSM1 nuclei was apparent in the tumor cells. b Nuclear expression of INSM1 was seen in tumor cells. There were no signs of normal acinar cells. Allred scores for INSM1. c Intensity score (IS) 3, d IS 2, and e IS 1
Statistical analysis
For NCAM, CGA, SYP, and INSM1, the sensitivity, defined as the probability of a positive score in NET patients, was calculated with the Pearson–Cropper 95% two tailed confidence intervals, and was compared using the McNemar test. Similar analysis was conducted for the specificity, which was defined as the probability of a negative score in non-NET patients for SPN, ACC, and PDAC. The two-sided significance level of 5% was employed and no multiplicity adjustment was used owing to the exploratory nature of the present study.
Results
Clinicopathological findings of the pancreatic neuroendocrine tumors
The patient backgrounds are shown in Table 1. The average age was 56.7 ± 14.8 years. There were 13 males (including 2 MANEC cases) and 14 females. Lesions occurred on the pancreatic head (8 cases) and tail (19 cases), and the average tumor size was 26.1 ± 18.8 mm. The grade classification was determined to be G1 in 20 cases (74%), G2 in 4 cases (15%), NEC in 1 case (4%), and MANEC in 2 cases (7%). There were 10 cases (37%) that were found to have a tumor infiltrating the lymph ducts and veins. A T factor evaluation for the AJCC and ENETS showed that they comprised 21 cases (78%) and 6 cases (22%) of pT1/T2, respectively, and 20 cases (74%) and 7 cases (26%) of T3/T4, respectively. Five cases (19%) exhibited metastasis to the lymph nodes, whereas one case (4%) exhibited metastasis outside the regional lymph nodes. With respect to distant metastasis, there were 4 cases (15%) that exhibited metastasis to the liver and the regional lymph nodes and 3 cases (11%) that exhibited metastasis to the liver only. The stage of progression for the AJCC and ENETS was Stage I/II for 21 cases (78%) and 20 cases (74%), respectively, and Stage III/IV for 6 cases (22%) and 7 cases (26%), respectively. In our follow-up, we found that 25 patients (93%) survived and 2 (2%) did not. There were 3 patients (11%) that experienced a relapse.
INSM1 expression for solid pancreatic tumors
The expression results for INSM1 in pure type NET cases (n = 25) are shown in Table 2. The pure type NET cases comprised PS4/5 in 23 cases (92%) and IS3 in 21 cases (81%). Twenty cases (77%) were found to have PS5+IS3. In contrast, there were no PS1/2 cases observed and 2 cases (8%) were found to have PS3. There were 4 cases (16%) with IS1/2. Table 3 shows the results from a comparison of the IHC staining of the solid pancreatic tumors by histological type. The INSM1 in pure type NET was found to be positive in all pure type cases (25 cases). There were 4 cases (80%) with SPN, in which we found expression of INSM1. However, there were cases where faint expression was seen in the cytoplasm (Fig. 3). In contrast, the results were negative for INSM1 in all the ACC and PDAC cases. Among the pure type NET cases, CGA and SYP expressions were found to be positive in 22 cases (88%) and 25 cases (96%) and negative in 3 cases (12%) and 1 case (4%), respectively. Among the SPN cases, CGA expression was negative in all 5 cases and the SYP was positive in 2 of the 5 cases and negative in the remaining 3 cases. The results for CGA and SYP were negative in all the ACC and PDAC cases. There were 18 pure type cases (72%) that were positive for NCAM, and all the SPN cases were positive for NCAM. However, the NCAM was negative in all the ACC and PDAC cases. Among the MANEC, the two cases showed the expression of INSM1. There was no expression of INSM1 in the PDAC cases (Fig. 4). The NET in the 2 MANEC cases showed that 1 case was positive for CGA and both cases were positive for SYP. In contrast, the PDAC component showed that the two cases were negative for both CGA and SYP. NCAM was positive in the MANEC NET component.
INSM1 staining for SPN. H.E. staining showed a pseudorosette structures and pseudopapillary shapes and exhibited tissue images that were similar to NET. b Although INSM1 was observed in the cytoplasm of the SPN, only faint nuclear expression was observed. The results from staining with CGA, SYP, and NCAM in SPN showed that c CGA was negative, and d SYP and e NCAM were positive
Sensitivity and specificity of INSM1 for pancreatic solid tumors
Table 4 shows the evaluation of sensitivity and specificity associated with PanNET. The trends showed that INSM1 sensitivity in NET was significantly higher compared with that of NCAM and higher than that of CGA. In contrast, there were no statistically significant differences found in SYP. INSM1 specificity in pancreatic solid tumors was high in SPN, there was a significant difference with NCAM, and there was no expression of INSM1 in any of the ACC or PDAC cases. In SYP, there were no significant differences seen in INSM1 and specificity.
Discussion
The INSM1 which we evaluated here is a zinc finger transcription factor that was extracted from the human insulinoma subtraction library [12]. INSM1 has been reported to be a useful marker in neuroendocrine tumors, parathyroid tumors, phenochromacytoma, medullary thyroid carcinoma, neuroblastoma, and retinoblastoma [8, 10, 12,13,14]. In small cell lung carcinoma research, the expression of INSM1 and INSM1 genes has been confirmed to be important factors for modulating neuroendocrine factors, and reports state that IHC staining has shown INSM1 nuclear expression in all cases of small cell lung carcinoma [8]. Fujino et al. reported an investigation to see if differentiation of the Notch-Hes 1 pathway is related to the neuroendocrine system, and assumed the possibility that INSM1 may also be an important factor in PanNET, which is said to develop from cells secreted from within the pancreas [8, 10].
Types of pancreatic tumors include PanNET, SPN, ACC, and PDAC. PanNET is a rare disease even among pancreatic tumors [2]. The variability in tissue form (gland formation, gyriform, etc.), and cell morphology make PanNET difficult to diagnose definitively [15]. SYP, CGA, and NCAM have been considered to be useful markers with high sensitivity and specificity for the pathological diagnosis for PanNET. However, NET might be difficult to accurately distinguish from ACC or SPN using IHC staining [16]. In fact, we found that 3 cases were negative for CGA and 1 case was negative for SYP in PanNET, as shown in Table 3. In contrast, INSM1 was positive for nuclear expression in all PanNET cases. Therefore, we suggest that INSM1 is a useful marker for diagnosis for PanNET, and its sensitivity might be greater than CGA and SYP.
PDAC has the worst prognosis of all pancreatic tumors, and accurate diagnosis of PDAC is important. However, MANEC has both components, NET and PDAC, with each component comprising at least 30% of the tumor, making diagnosis complicated [17, 18]. In this study, INSM1 expression was negative in all PDAC, which has been confirmed to be a useful IHC marker. Conversely, INSM1 expression was positive in MANEC, suggesting that the tumors contained a neuroendocrine component. We clearly verified the difference in expression between the adenocarcinoma and neuroendocrine components. Thus, INSM1 is a more efficient marker that can also be used to confirm the presence of neuroendocrine components. Moreover, we propose that INSM1 plays the role of a marker that is useful to exclude PDAC.
SPN has an unclear differentiation, low-grade malignancy potential, and the initial diagnosis for SPN is important using immunohistochemistry [2, 19, 20]. However, SPN sometimes demonstrates SYP and NCAM expression. In fact, NCAM appeared in all cases and SYP appeared in 2 cases among the SPN cases that we evaluated, which made it difficult to determine PanNET and other pancreatic solid tumor. In our study, INSM1 appeared negative in all SPN cases and it is useful to make critical diagnosis for pancreatic solid tumors. On the other hand, 4 cases of SPN were positive for cytoplasmic expression based on the stainability of INSM1, although INSM1 acts as a regulator and controlling factor in SYP, CGA, and NCAM. We suggest that INSM1 is useful in diagnoses in comparison to SYP, CGA, and NCAM. However, we anticipate that future studies will involve evaluation of cases with faint INSM1 expression in SPN.
Although NEC is an extremely rare tissue type, it is known to be highly malignant, and value can be obtained from diagnosing NEC [21, 22]. In our study, NEC was found in 1 case with INSM1 expression. However, no significant differences were found between NET and NEC, and it is unclear if immunostaining can be used to detect malignancy. This result may indicate that it is critical that malignancy evaluations be performed using Ki-67 staining and mitotic counts while simultaneously proceeding with INSM1-based diagnoses.
In this study, we used INSM1 in addition to chromogranin A and synaptophysin, which are useful in immunohistochemically staining of PanNET. Our data lead us to conclude that INSM1 can improve the efficiency in diagnosing pancreatic solid tumors and can be applied to small samples such as pancreatic biopsy tissue and cytopathology.
References
Yang M, Zeng L, Zhang Y, Wang WG, Wang L, Ke NW, Liu XB, Tian BL (2015) TNM staging of pancreatic neuroendocrine tumors: an observational analysis and comparison by both AJCC and ENETS systems from 1 single institution. Medicine (Baltimore) 94:e660
Klimstra DS, Arnold R, Capella C, Hruban RH, Kloppel G, Komminoth P, Solcia E, Rindi G (2010) Neuroendocrine neoplasm of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Thise ND (eds) WHO classification of tumors of the digestive system. IARC Press, Lyon, pp 322–326
Kasajima A, Yazdani S, Sasano H (2015) Pathology diagnosis of pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci 22:586–593
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS (2015) The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 39:683–690
Meriden Z, Shi C, Edil BH, Ellison T, Wolfgang CL, Cornish TC, Schulick RD, Hruban RH (2011) Hyaline globules in neuroendocrine and solid-pseudopapillary neoplasms of the pancreas: a clue to the diagnosis. Am J Surg Pathol 35:981–988
Ohike N, Kosmahl M, Kloppel G (2004) Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch 445:231–235
Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S (2000) Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol 24:1361–1371
Fujino K, Motooka Y, Hassan WA, Ali Abdalla MO, Sato Y, Kudoh S, Hasegawa K, Niimori-Kita K, Kobayashi H, Kubota I, Wakimoto J, Suzuki M, Ito T (2015) Insulinoma-associated protein 1 is a crucial regulator of neuroendocrine differentiation in lung cancer. Am J Pathol 185:3164–3177
Wang H, Chen Y, Fernandez-Del Castillo C, Yilmaz O, Deshpande V (2013) Heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors: a critical look at notch signaling pathway. Mod Pathol 26:139–147
Fujino K, Yasufuku K, Kudoh S, Motooka Y, Sato Y, Wakimoto J, Kubota I, Suzuki M, Ito T (2017) INSM1 is the best marker for the diagnosis of neuroendocrine tumors: comparison with CGA, SYP and CD56. Int J Clin Exp Pathol 10(5):5393–5405
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Goto Y, De Silva MG, Toscani A, Prabhakar BS, Notkins AL, Lan MS (1992) A novel human insulinoma-associated cDNA, IA-1, encodes a protein with “zinc-finger” DNA-binding motifs. J Biol Chem 267:15252–15257
Breslin MB, Zhu M, Lan MS (2003) NeuroD1/E47 regulates the E-box element of a novel zinc finger transcription factor, IA-1, in developing nervous system. J Biol Chem 278:38991–38997
Lan MS, Breslin MB (2009) Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J 23:2024–2033
Carstens PH, Cressman FK Jr (1989) Malignant oncocytic carcinoid of the pancreas. Ultrastruct Pathol 13:69–75
Ohara Y, Oda T, Hashimoto S, Akashi Y, Miyamoto R, Enomoto T, Satomi K, Morishita Y, Ohkohchi N (2016) Pancreatic neuroendocrine tumor and solid-pseudopapillary neoplasm: key immunohistochemical profiles for differential diagnosis. World J Gastroenterol 22:8596–8604
Tang LH, Basturk O, Sue JJ, Klimstra DS (2016) A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol 40:1192–1202
Murata M, Takahashi H, Yamada M, Song M, Hiratsuka M (2017) A case of mixed adenoneuroendocrine carcinoma of the pancreas: immunohistochemical analysis for histogenesis. Medicine (Baltimore) 96:e6225
Hirabayashi K, Kurokawa S, Maruno A, Yamada M, Kawaguchi Y, Nakagohri T, Mine T, Sugiyama T, Tajiri T, Nakamura N (2015) Sex differences in immunohistochemical expression and capillary density in pancreatic solid pseudopapillary neoplasm. Ann Diagn Pathol 19:45–49
Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N (2009) E-cadherin/beta-catenin and CD10: a limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol 132:831–839
Yachida S, Zhong Y, Patrascu R, Davis MB, Morsberger LA, Griffin CA, Hruban RH, Laheru D, Iacobuzio-Donahue CA (2011) Establishment and characterization of a new cell line, A99, from a primary small cell carcinoma of the pancreas. Pancreas 40:905–910
Girardi DM, Silva ACB, Rego JFM, Coudry RA, Riechelmann RP (2017) Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: a systematic review. Cancer Treat Rev 56:28–35
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tanigawa, M., Nakayama, M., Taira, T. et al. Insulinoma-associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor. Med Mol Morphol 51, 32–40 (2018). https://doi.org/10.1007/s00795-017-0167-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-017-0167-6