Abstract
Hepatic ATP-binding cassette A1 (ABCA1) transporter is the modulator of intrahepatic cholesterol levels via the efflux of cholesterol into plasma. This study aimed to determine the expression of hepatic ABCA1 levels in a cholestatic rat model and patients with primary biliary cholangitis (PBC). A cholesterol efflux study was conducted with Abca1 knock down using siRNA in WIF9 cells. Cholesterol levels in the ABCA1 siRNA cells in the medium were significantly decreased compared with those in controls (P < 0.05). Hepatic ABCA1 mRNA levels were significantly higher in BDL rats than in control rats (P < 0.05). Furthermore, the protein expression level of hepatic ABCA1 was also significantly increased by 200% in BDL rats (P < 0.05). In PBC patients, expression of hepatic ABCA1 mRNA was 2.2-fold higher than that in controls (P < 0.05). The level of hepatic liver X receptor (LXR)β mRNA was correlated with ABCA1 mRNA levels in PBC patients. The expression of hepatic ABCA1 transporter was upregulated in both the cholestatic rat model and PBC patients. Upregulated hepatic ABCA1 may lead to efflux of cholesterol into plasma, thus explaining the mechanism of cholestasis leading to hypercholesterolemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with cholestatic disorders have lipid abnormalities, including high total serum cholesterol [1], but the mechanism underlying this phenomenon is unknown. The liver is the most important modulator of cholesterol homeostasis. Hepatic cholesterol and bile acid metabolism are important to physiological processes such as digestion, lipid and vitamin uptake and distribution, steroid hormone and cell membrane fraction, and toxin elimination.
ATP-binding cassette transporter A1 (ABCA1), also known as cholesterol efflux regulatory protein (CERP), is a protein that is encoded by the ABCA1 gene in humans (Abca1 in rodents). Mutations in this gene have been associated with Tangier disease and familial high-density lipoprotein (HDL) deficiency [2]. This transporter is a major regulator of cellular cholesterol and phospholipid homeostasis [3]. With cholesterol as its substrate, this protein functions as a cholesterol efflux pump in the cellular lipid removal pathway. ABCA1 mediates the efflux of cholesterol and phospholipids to lipid-poor apolipoproteins (ApoA1 and ApoE), which then form nascent HDL. ABCA1 transporter is highly expressed in the liver, and is located at the basolateral membrane of hepatocytes [4]. Several molecular biological studies have shown that ABCA1 transporter is involved in the regulation of cholesterol efflux from hepatocytes to plasma in vivo and in vitro [5,6,7,8,9]. Transgenic mice overexpressing Abca1 have an increase in serum cholesterol levels [10], while the Abca1 knockout mice have a marked decrease in plasma total cholesterol [11]. Confocal fluorescence microscopy has shown that the ABCA1-green fluorescent protein (GFP) fusion protein in WIF-B cells, a suitable model for in vitro studies of polarized hepatocytes structure and function, is located on the basolateral membrane of the hepatocytes [4]. Expression of ABCA1-GFP stimulated efflux of WIF-B cell cholesterol into the culture medium [4]. ABCA1 may participate in the regulation of the levels of intracellular hepatic cholesterol. The Wisconsin hypoalpha mutant (WHAM) chicken is an animal model with a naturally occurring mutation in the Abca1 gene [12]. WHAM chickens have a 70% reduction in serum cholesterol levels [13, 14]. Serum cholesterol concentration in Abca1 knockout mice using ABCA1 short interfering RNA (siRNA) was also reduced [15].
Bile acids are derived from cholesterol, and bile acid synthesis pathways involve signaling molecules that regulate cholesterol homeostasis in mammals. Nuclear hormone receptors function as ligand-activated transcription factors with pivotal roles in the regulation of cholesterol and bile acid metabolism [16]. In general, both serum and hepatic bile acid concentrations are increased in cholestatic disorders in human and animal models [17]. Bile acids activate hepatic fetoprotein transcriptional factor (FTF), which induces multidrug resistance-associated protein 3 (MRP3) to excrete and transport bile acids [18, 19]. Moreover, bile acids activate farnesoid X receptor (FXR) to inhibit transcription of the gene encoding cholesterol 7-hydroxylase (CYP7A1) [20], which is the rate-limiting enzyme in the bile acid synthesis pathway that catalyzes conversion of cholesterol into bile acids [21]. Cholesterol is converted to bile acids by liver X receptor α (LXRα)-mediated stimulation of CYP7A1 transcription, and CYP7A1 is regulated by LXRα [22]. Using quantitative analyses for the characterization of interacting systems, we investigated nuclear protein with DNA binding with the hepatic CYP7A1/LXR element in the rat model of cholestasis using the electrophoretic mobility shift assay (EMSA).
Moreover, LXR agonist (T0901317) stimulated hepatic ABCA1 transporter via upregulated LXRβ [23]. Using qualitative analyses for the characterization of interacting systems, we investigated the protein-DNA binding site for LXR/retinoid X receptor (RXR) direct repeat 4 (DR4) element using southwestern histochemistry (SWH), as the LXR/RXR DR4 element is the transcription factor that regulates the expression of ABCA1 gene by binding to LXR/RXR DR4 element [24]. SWH is used to localize transcription regulatory factors that bind to specific sequences of DNA and regulate the transcriptional activity of the genes, using a haptenized double-stranded DNA [25].
The aim of this study was to clarify that an increase in hepatic ABCA1 transporters leads to hypercholesterolemia in primary biliary cholangitis (PBC) patients and in a cholestatic rat model. PBC is the most common cholestatic liver disease. Hence, we studied the liver tissue samples from PBC patients.
Methods
Animals and animal treatment
Male Wistar rats were obtained from KYUDO (Fukuoka, Japan) and maintained in the Fukuoka University Center for Experimental Animals. All rats were housed in a temperature and humidity controlled environment under a constant light–dark cycle, and provided regular rat chow with free access to water. The experimental protocols were approved by the Animal Care and Use Committee of Fukuoka University (Approval No. 0401603, 2003) according to criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, revised 1985). Rats underwent bile duct ligation (BDL) or sham operation as described previously [26]. Lipopolysaccharide (LPS) from Salmonella typhimurium (2 mg/kg body weight; Sigma Chemical Co., St Louis, MO, USA) was injected intraperitoneally and animals were killed 16 h after injection as described elsewhere [27,28,29]. Controls were injected with the vehicle (saline) alone. After pentobarbital anesthesia, all animals were sacrificed at 7 days, and livers were harvested for analysis.
PBC patients
This part of the study involved PBC patients at Fukuoka University Hospital, Japan. In accordance with the regulations of the Human Investigations Committee of Fukuoka University, informed consent was obtained from all patients. The diagnosis of PBC (n = 44) was based on the following criteria: (a) abnormal biochemical tests with preferential elevation of serum ALP and GGT; (b) presence of antimitochondrial antibodies with M2 (AMA) specificity as confirmed by ELISA or immune blotting; and (c) evidence of chronic non-suppurative destructive cholangitis (CNSDC) at histology [30]. None of the patients had undergone treatment with anti-hyperlipidemic drug. Liver samples were obtained by percutaneous needle biopsy. The PBC staging was used Scheuer’s histological staging system.
Real-time reverse transcriptase (RT)-PCR analysis
TaqMan® real-time quantitative polymerase chain reaction (PCR) assay was performed on an ABI Prism 7500EAST Sequence Detection System, according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA, USA). The following primers and probes were used for the TaqMan® RT-PCR assay (Applied Biosystems, Foster City, CA, USA): ABCA1, MRP3, FTF, CYP7A1, and LXRβ.
SiRNA of ABCA1 transporter study
WIF-B9 cells were cultured in a humidified 7% CO2 incubator at 37 °C as described previously [31]. siRNA against ABCA1 was purchased from Dharmacon (siGENOME SMART pools; Thermo Fisher Scientific, Pittsburgh, PA, USA). SiRNA transfection with Dharmacon FECT4 (Thermo Fisher Scientific) was performed according to the manufacturer’s protocol.
Cholesterol concentrations in the medium
Cholesterol levels were determined by a commercial company (SRL, Tokyo, Japan) in media of pre- and post-ABCA1 siRNA cells, and Control.
Western blot analysis
Blots were incubated for 1 h at room temperature with ABCA1 (1:1000) (Novus Biologicals Inc., Littleton, CO, USA), MRP3 (1:2000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), FTF (1:1000) (Santa Cruz Biotechnology, Inc.), and mouse, rabbit, or goat polyclonal IgG antibodies. Blots were washed and then incubated with anti-mouse, -rabbit, or -goat horseradish peroxidase-conjugated antibody (1:2000) (Santa Cruz Biotechnology, Inc.). The immune complexes were detected with the ECL™ Western Blotting Analysis System kit (Amersham Biosciences, Little Chalfont, UK).
Electrophoretic mobility shift assays
Liver nuclei were prepared from BDL and sham rats at the given timepoints according to the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL, USA) DIG Gel Shift Kit, 2nd Generation (Roche Diagnostics GmbH, Penzberg, Germany) according to the manufacturer’s protocol with CYP7A1-LXR element, sense (5′-GATCCCTTTGGTCACTCAAGTTCAAGTGGATC-3′) and antisense (5′-CTAGGGAAACCAGTGAGTTCAAGTTCACCTAG-3′) probes (Sigma Aldrich, Tokyo, Japan).
Southwestern histochemistry
SWH was used to elucidate DNA and its binding nuclear protein interactions, according to a previously described technique with modification [18, 25]. ABCA1 LXR/RXR DR4 element, transcriptional factor of ABCA1, and sense (5′-ACTGGGCTTTGACCGATAGTAACCTCTGCGCTCG-3′) and antisense (5′-CGAGCGCAGAGGTTACTATCGAAAGCCCAGT-3′) probes (Sigma Aldrich) were annealed by heating at 80 °C for 2 min. The probes were subjected to digoxigenin (DIG) oligonucleotide 39-end labeling (Roche Diagnostics GmbH). Liver sections were dewaxed, rehydrated, incubated with 5 mmol/L levamisole for 30 min, and fixed with 0.2% paraformaldehyde. They were then digested with 0.5% pepsin in 1 M HCl for 30 min, washed with HEPES, incubated with 0.1 mg/mL DNAse I for 30 min, and washed again with HEPES buffer.
Confocal immunofluorescence microscopy
Indirect immunofluorescence was conducted on liver specimens from sham-operated and BDL rats and PBC patients [18, 20, 32]. ABCA1 antiserum was used at a dilution of 1:100. The secondary antibody was Alexa546 anti-rabbit immunoglobulin G (Molecular Probes, Eugene, OR, USA). All fluorescent imaging was performed on a Zeiss LSM5 PASCAL confocal scanning microscope (Carl Zeiss Japan, Tokyo, Japan). Digital images were processed with Adobe Photoshop (Adobe, San Jose, CA, USA).
Serum and hepatic lipid profiles
Hepatic total bile acids (Direct Spectrophotometry: TEST WAKO®) and hepatic cholesterol (cholesterol oxidase DAOS: E-TEST WAKO®) concentrations were determined using commercially available kits (Wako Pure Chemical Industries, Ltd, Osaka, Japan) according to the manufacturer’s protocol. Serum total cholesterol, HDL cholesterol, and phospholipid levels were analyzed by a commercial company (SRL).
Statistical analysis
All data were expressed as the mean ± standard deviation. Data were subjected to analysis of variance, and statistical significance was accepted at P < 0.05. Differences between specific groups were determined using an unpaired Student’s t test.
Results
Cholesterol efflux study in cells treated with ABCA1 siRNA
We confirmed the inhibition of ABCA1 expression of membrane protein levels by 50% and mRNA levels by 59% in the ABCA1 siRNA transfected WIF-B9 cells (ABCA1 siRNA cells) using immunoblot and real-time RT-PCR, respectively (Fig. 1a). In the ABCA1 siRNA cells, culture medium cholesterol levels were significantly decreased by 22% compared with those in controls (Fig. 1b, P < 0.05) after applying the background subtraction method (Fig. 1b, P < 0.001).
Cholesterol efflux study in cells treated with ABCA1 siRNA. a Levels of ATP-binding cassette transporter A1 (ABCA1) mRNA (left) and membrane protein (right) in ABCA1 short interfering RNA (siRNA) cells (n = 3) and control (n = 6). ABCA1 expression of mRNA levels was inhibited by 59% and membrane protein levels were reduced by 50% in the ABCA1 siRNA transfected WIF-B9 cells (ABCA1 siRNA cells). b Culture medium cholesterol levels (μg/mL) in ATP-binding cassette transporter A1 (ABCA1) short interfering RNA (siRNA cells) (n = 3) and mock (n = 6). Background subtraction (right): the amount of medium cholesterol levels (post ABCA1 siRNA cells)–(pre ABCA1 siRNA cells). In the ABCA1 siRNA cells, culture medium cholesterol levels were significantly decreased by 22% compared with those in controls (left, P < 0.05) and culture medium cholesterol levels were significantly decreased after applying the background subtraction method (right, P < 0.001)
Increased bile acids upregulate Mrp3 via FTF in BDL rats
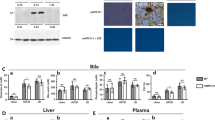
As shown in Table 1, hepatic and serum total bile acid concentrations in the BDL rats were significantly higher than in sham rats. Both mRNA and hepatic Mrp3 membrane protein levels were significantly increased in BDL rats compared with those of sham rats (Fig. 2a). Otherwise, hepatic Mrp3 mRNA and membrane protein levels were decreased in BDL rats (data not shown; previously described) [26]. The expressions of hepatic FTF mRNA and nuclear protein were significantly increased in BDL rats compared with sham rats (Fig. 2b, P < 0.01).
Study in cholestatic rat models. a Expression of hepatic multidrug resistance-associated protein 3 (Mrp3) mRNA and membrane protein levels in cholestatic rat models. Both mRNA (left) and hepatic Mrp3 membrane protein (right) levels were significantly increased in bile duct ligation (BDL) rats compared with those of sham rats. b Expression of hepatic fetoprotein transcriptional factor (FTF) mRNA (left) and nuclear protein levels (right) in cholestatic rat models. The expressions of FTF mRNA (left) and nuclear protein (right) were significantly increased in bile duct ligation (BDL) rats compared with those in sham rats. c Electrophoretic mobility shift assay (EMSA): the binding capacity of liver X receptor (LXR) element on hepatic cholesterol 7α-hydroxylase (Cyp7a1) (left) and the expression of hepatic Cyp7a1 mRNA levels (right) in a cholestatic rat model. This binding capacity was significantly reduced in bile duct ligation (BDL) rats to 38% of sham levels (left); this correlated with the observed reduction in the expression levels of hepatic Cyp7a1 mRNA (right). d Immunolocalization study of hepatic ATP-binding cassette transporter A1 (Abca1) in cholestatic rats. Abca1 expression appeared to be restricted to the basolateral membrane of hepatocytes in bile duct ligation (BDL) rats. e Expression of hepatic ATP-binding cassette transporter A1 (Abca1) mRNA (left) and membrane protein (right) levels in cholestatic rat models. The expression of hepatic Abca1 [both mRNA levels (left) and membrane protein levels (right)] was significantly increased in bile duct ligation (BDL) rats
LXRα abundance and DNA binding to the Cyp7a1 promoter in BDL rats
The EMSA study indicated the binding capacity of LXR element on the hepatic Cyp7a1 gene. This binding capacity was significantly reduced in BDL rats to 38% of sham levels; this also correlated with the observed reduction the expression levels of hepatic Cyp7a1 mRNA (Fig. 2c).
ABCA1 expression in BDL rats
The immunofluorescence study was performed to analyze the distribution pattern of Abca1 expression in the liver. As shown in Fig. 2d, Abca1 expression appeared to be restricted to the basolateral membrane of hepatocytes in BDL rats. The expression of hepatic Abca1 (both mRNA levels and membrane protein levels) was significantly increased in BDL rats (Fig. 2e). Abca1 was labeled with the same antibodies used for immunoblots. The upregulated Abca1 was accompanied by notably higher serum cholesterol levels in BDL rats than in sham rats (Table 1). Serum HDL cholesterol levels were lower in BDL rats than in sham rats (Table 1). Abca1 is also a transporter of phospholipids from hepatocytes to sinusoidal blood, and serum phospholipid concentrations in BDL rats were also significantly increased compared with sham rats (Table 1).
PBC patients study (Table 2)
Hepatic ABCA1 mRNA expression in liver biopsy samples was significantly increased in PBC patients compared with controls (Fig. 3a, P < 0.05). In PBC patients, there were no significant differences in hepatic ABCA1 mRNA expression among each PBC stage (data not shown). Confocal immunofluorescence study showed that ABCA1 transporter protein was expressed on the basolateral membrane of hepatocytes in PBC patients (Fig. 3b). Immunohistochemical localization of ABCA1 was no change among each PBC stage. As LXRβ regulates the expression of ABCA1 transporter, we showed that hepatic LXRβ mRNA levels were correlated with hepatic ABCA1 mRNA levels in PBC patients (Fig. 3c, r = 0.64). There was no correlation between hepatic ABCA1 mRNA levels and serum total cholesterol in all PBC patients (data not shown).
Study in PBC patients. a Expression of hepatic ATP-binding cassette transporter A1 (ABCA1) mRNA levels in primary biliary cholangitis (PBC) patients (n = 35). We obtained liver samples of PBC patients by percutaneous needle biopsy. Hepatic ABCA1 mRNA expression was significantly increased in PBC patients compared with controls. b Immunolocalization study of hepatic ATP-binding cassette transporter A1 (ABCA1) in primary biliary cholangitis (PBC) patients. ABCA1 transporter protein was expressed on the basolateral membrane of hepatocytes in PBC patients. c Correlations between hepatic ATP-binding cassette transporter A1 (ABCA1) and liver X receptor α (LXRβ) mRNA levels in primary biliary cirrhosis (PBC) patients. Hepatic LXRβ mRNA levels were correlated with hepatic ABCA1 mRNA levels in PBC patients (r = 0.64). d Southwestern histochemistry (SWH) is used to localize transcription regulatory factors that bind to specific sequences of DNA and regulate the transcriptional activity of the genes using a digoxigenin (DIG)-labeled double-stranded DNA probe14. SWH: a specific consensus sequence that binds to the ATP-binding cassette transporter A1 (ABCA1) transcription factor; the liver X receptor α (LXR)/retinoid X receptor (RXR) direct repeat 4 (DR4) elements and nuclear-binding protein complex in the hepatocyte nucleus of a primary biliary cholangitis (PBC) patient (arrows)
Southwestern histochemistry
SWH showed DNA and its binding nuclear protein interactions; binding protein with the LXR/RXR DR4 elements on hepatic ABCA1 gene localized on the nucleus in PBC patients (Fig. 3d). In SWH, the expression of DNA and its binding nuclear protein complex was localized on the nucleus in each PBC stage. LXR/RXR DR4 element, a transcriptional factor of ABCA1, was activated by cholesterol, which leads to ABCA1 transporter expression.
Discussion
This study showed that hypercholesterolemia in humans and cholestatic rats results from upregulated hepatic ABCA1 transporter with a defense mechanism by which liver cells avoid bile acid and bilirubin accumulation during cholestasis (Fig. 4). Homeostasis of bile acids and cholesterol in cholestatic disorders occurs first with an increased accumulation of bile acids within hepatocytes. Bile acids stimulate FTF, which induces MRP3. Serum bile acid concentrations were higher, because bile acids were exported from hepatocytes to sinusoidal blood via upregulated MRP3 transporter. Bile acids also stimulate the FXR-small heterodimer partner (SHP) signal route, and thus, the LXR element on CYP7A1 was inhibited by SHP. There was a reduction in hepatic CYP7A1 mRNA in BDL rats, which equates to a reduction in biosynthesis of bile acids [33]. The expression of CYP7A1 mRNA was decreased in PBC patients as previously described [20]. In this way, bile acids effectively downregulate their own synthesis. Hepatic bile acid and other transporters undergo adaptive regulation in the liver in response to cholestatic disorders. Elevated cholesterol results in the formation of oxysterols that bind LXRβ. and activates LXR/RXR DR4 element on ABCA1 transcription [34]. Upregulation of ABCA1 results in subsequent excretion of cholesterol. Hence, hepatic ABCA1 may regulate transport and prevent overaccumulation of cholesterol in the hepatocytes of patients with cholestatic disorder.
Scheme. Homeostasis of bile acids and cholesterol in cholestatic disorders. This occurs first with an increased accumulation of bile acids within hepatocytes. Bile acids stimulate FTF, which induces MRP3 [19]. Serum bile acid concentrations were higher, because bile acids were exported from hepatocytes to sinusoidal blood via upregulated MRP3 transporter. Bile acids also stimulate the FXR-SHP signal route, and thus, the LXR element on CYP7A1 was inhibited by SHP [37, 38]. There was a reduction in hepatic CYP7A1 mRNA in BDL rats, which equates to a reduction in biosynthesis of bile acids. In this way, bile acids effectively downregulate their own synthesis. Elevated cholesterol results in the formation of oxysterols that bind LXRβ and activates LXR/RXR DR4 element on ABCA1 transcription. Upregulation of ABCA1 results in subsequent excretion of cholesterol. BA bile acid, MRP3 multidrug resistance-associated protein 3, Cho cholesterol, FTF fetoprotein transcription factor, FXR/RXR farnesoid X receptor/retinoid X receptor, SHP1 small heterodimer partner1, LXRE liver X receptor element, LXR/RXR DR4 liver X receptor/retinoid X receptor direct repeat 4, CYP7A1 cholesterol 7α-hydroxylase, BSEP bile salt export pump, MRP2 multidrug resistance-associated protein 2, ABCG5/ABCG8 ATP-binding cassette G5/G8
To analyze the interaction of ABCA1 and its transcriptional region, LXR/RXR DR4 element, we studied LXR/RXR DR4 element and nuclear protein complex using SWH. EMSA is used to detect protein complexes with nucleic acids. It is the core technology underlying a wide range of qualitative and quantitative analyses for the characterization of interacting systems. It is difficult to obtain a sufficient amount of hepatic nuclear protein for EMSA from human liver samples using needle biopsy. SWH is suitable for detection of binding ability with DNA and its binding protein. SWH is not able to quantify amount of these complexes precisely. For the localization of a transcription regulatory protein in tissue sections, SWH, in which a DNA segment, containing a transcription regulatory protein-specific responsive element consensus sequence, is used as a probe.
Bile acids, cholesterol, and phospholipids accumulate in hepatocytes in cholestasis. We have shown increased expressions of mRNA and protein levels of the efflux transporters including MRP3 in cholestatic rats [26]. Another study has demonstrated that a serum total cholesterol level increases and a serum HDL cholesterol level decreases after BDL in mice [35]. In cholestasis, it is a reasonable response to change these transporters alternatively to avoid these compounds from accumulating in hepatocytes.
Cholesterol homeostasis maintenance carried out in many mechanisms. Serum cholesterol is regulated by several factors. Our study revealed that 61% of PBC patients had total plasma cholesterol values above 200 mg/dL. None of the patients had undergone treatment with anti-hyperlipidemic drug including bezafibrate; however, the part of patients had undergone treatment with UDCA. Serum concentrations of cholesterol decreased during UDCA administration [36]. Therefore, it was no correlation between Hepatic ABCA1 mRNA and serum total cholesterol level.
In end-stage cirrhotic patients, including PBC cases, serum cholesterol levels decline. This phenomenon is thought to be accompanied by an impaired ability to synthesize proteins. The amount of hepatic sample obtained by needle biopsy was insufficient for immunoblot analysis of hepatic ABCA1 protein levels (data not shown). Further studies are required, including an investigation of the quantitative levels of ABCA1 protein in PBC patients in liver samples, and this could contribute to the development of a new methodology for protein determination.
Abbreviations
- ABCA1:
-
ATP-binding cassette transporter A1
- HDL:
-
High-density lipoprotein
- GFP:
-
Green fluorescent protein
- BDL:
-
Bile duct ligation
- siRNA:
-
Short interfering RNA
- FTF:
-
Fetoprotein transcriptional factor
- MRP3:
-
Multidrug resistance-associated protein 3
- FRX:
-
Farnesoid X receptor
- CYP7A1:
-
Cholesterol 7-hydroxylase
- LXRα:
-
Liver X receptor α
- EMSA:
-
Electrophoretic mobility shift assay
- RXR:
-
Retinoid X receptor
- DR4:
-
Direct repeat 4
- SWH:
-
Southwestern histochemistry
- PBC:
-
Primary biliary cholangitis
- RT:
-
Reverse transcriptase
- PCR:
-
Polymerase chain reaction
- SHP:
-
Small heterodimer partner
References
Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, Podda M (2002) Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 51:265–269
Brooks-Wilson A, Marcil M, Clee SM, Zhang L-H, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HOF, Loubser O, Ouelette BFF, Fichter K, Ashbourne-Excoffon KJD, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJP, Genest J, Hayden MR (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22:336–345
Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB, Tansey T, Amar MJ, Fruchart-Najib J, Duverger N, Santamarina-Fojo S, Brewer HB Jr (2003) Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res 44:296–302
Neufeld EB, Demosky SJ Jr, Stonik JA, Combs C, Remaley AT, Duverger N, Santamarina-Fojo S, Brewer HB Jr (2002) The ABCA1 transporter functions on the basolateral surface of hepatocytes. Biochem Biophys Res Commun 297:974–979
Knight BL (2004) ATP-binding cassette transporter A1: regulation of cholesterol efflux. Biochem Soc Trans 32:124–127
Oram JF (2002) ATP-binding cassette transporter A1 and cholesterol trafficking. Curr Opin Lipidol 13:373–381
Oram JF, Lawn RM (2001) ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res 42:1173–1179
Wang N, Silver DL, Thiele C, Tall AR (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276:23742–23747
Takeyama Y, Sakisaka S (2012) Hepatobiliary membrane transporters in primary biliary cirrhosis. Hepatol Res 42:120–130
Vaisman BL, Lambert G, Amar M, Joyce C, Ito T, Shamburek RD, Cain WJ, Fruchart-Najib J, Neufeld ED, Remaley AT, Brewer S-F (2001) ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Investig 108:303–309
Groen AK, Bloks VW, Bandsma RH, Ottenhoff R, Chimini G, Kuipers F (2001) Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J Clin Investig 108:843–850
Poernama F, Schreyer SA, Bitgood JJ, Cook ME, Attie AD (1990) Spontaneous high density lipoprotein deficiency syndrome associated with a Z-linked mutation in chickens. J Lipid Res 31:955–963
Attie AD, Hamon Y, Brooks-Wilson AR, Gray-Keller MP, MacDonald ML, Rigot V, Tebon A, Zhang LH, Mulligan JD, Singaraja RR, Bitgood JJ, Cook ME, Kastelein JJ, Chimini G, Hayden MR (2002) Identification and functional analysis of a naturally occurring E89K mutation in the ABCA1 gene of the WHAM chicken. J Lipid Res 43:1610–1617
Schreyer SA, Hart LK, Attie AD (1994) Hypercatabolism of lipoprotein-free apolipoprotein A-I in HDL-deficient mutant chickens. Arterioscler Thromb Vasc Biol 14:2053
Ragozin S, Niemeier A, Laatsch A, Loeffler B, Merkel M, Beisiegel U, Heeren J (2005) Knockdown of hepatic ABCA1 by RNA interference decreases plasma HDL cholesterol levels and influences postprandial lipemia in mice. Arterioscler Thromb Vasc Biol 25:1433–1438
Boyer JL (2005) Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology 129:735–740
Setchell KD, Rodrigues CM, Clerici C, Solinas A, Morelli A, Gartung C, Boyer J (1997) Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology 112:226–235
Takeyama Y, Uehara Y, Inomata S, Morihara D, Nishizawa S, Ueda S, Matsumoto T, Tanaka T, Anan A, Nishimura H, Irie M, Iwata K, Shakado S, Sohda T, Sakisaka S (2009) Alternative transporter pathways in patients with untreated early-stage and late-stage primary biliary cirrhosis. Liver Int 29:406–414
Bohan A, Chen WS, Denson LA, Held MA, Boyer JL (2003) Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem 278:36688–36698
Takeyama Y, Kanegae K, Inomata S, Takata K, Tanaka T, Ueda S, Yokoyama K, Morihara D, Nishizawa S, Anan A, Irie M, Iwata K, Shakado S, Sohda T, Sakisaka S (2010) Sustained upregulation of sodium taurocholate cotransporting polypeptide and bile salt export pump and downregulation of cholesterol 7alpha-hydroxylase in the liver of patients with end-stage primary biliary cirrhosis. Med Mol Morphol 43:134–138
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365
Chiang JY, Kimmel R, Stroup D (2001) Regulation of cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRalpha). Gene 262:257–265
Plōsch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F (2002) Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem 277:33870–33877
Repa JJ, Turley SD, Lobaccaro JMA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ (2000) Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524
Koji TKK, Nozawa M, Yamada S, Nakane PK (1994) Localization of cyclic adenosine 3′, 5′-monophosphate-responsive element (CRE)-binding proteins by southwestern histochemistry. J Histochem Cytochem 42:1399–1405
Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL (2004) Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol 40:585–591
Miyaso H, Morimoto Y, Ozaki M, Haga S, Shinoura S, Choda Y, Iwagaki H, Tanaka N (2005) Obstructive jaundice increases sensitivity to lipopolysaccharide via TLR4 upregulation: possible involvement in gut-derived hepatocyte growth factor-protection of hepatocytes. J Gastroenterol Hepatol 20:1859–1866
Giacometti A, Cirioni O, Ghiselli R, Mocchegiani F, D’Amato G, Del Prete MS, Orlando F, Kamysz W, Lukasiak J, Saba V, Scalise G (2003) Administration of protegrin peptide IB-367 to prevent endotoxin induced mortality in bile duct ligated rats. Gut 52:874–878
Sewnath ME, Levels HH, Oude Elferink R, van Noorden CJ, ten Kate FJ, van Deventer SJ, Gouma DJ (2000) Endotoxin-induced mortality in bile duct-ligated rats after administration of reconstituted high-density lipoprotein. Hepatology (Baltimore, Md) 32:1289–1299
Working Subgroup for Clinical Practice Guidelines for Primary Biliary C (2014) Guidelines for the management of primary biliary cirrhosis: The Intractable Hepatobiliary Disease Study Group supported by the Ministry of Health, Labour and Welfare of Japan. Hepatol Res 44(Suppl S1):71–90
Sai Y, Nies AT, Arias IM (1999) Bile acid secretion and direct targeting of mdr1-green fluorescent protein from Golgi to the canalicular membrane in polarized WIF-B cells. J Cell Sci 112(Pt 24):4535–4545
Takeyama Y, Tsuchiya N, Kunimoto H, Fukunaga A, Sakurai K, Hirano G, Yokoyama K, Morihara D, Anan A, Irie M, Shakado S, Sohda T, Sakisaka S (2015) Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging as a useful detection method for advanced primary biliary cirrhosis. Hepatol Res 45:E108–E114
Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP (2002) Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Investig 110:109–117
Costet P, Luo Y, Wang N, Tall AR (2000) Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 275:28240–28245
Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M (2005) Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA 102:2063–2068
Poupon RE, Ouguerram K, Chretien Y, Verneau C, Eschwege E, Magot T, Poupon R (1993) Cholesterol-lowering effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology (Baltimore, Md) 17:577–582
Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276:28857–28865
Schaap FG, Trauner M, Jansen PLM (2014) Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 11:55–67
Acknowledgements
We thank Dr. Yoshio Misumi, Ms. Akiko Ono, Ms. Motoko Kawashima, Ms. Kazuko Kanegae, and Ms. Eri Yamauchi for their excellent technical assistance. This work was supported by a Grant from the Clinical Research Foundation and supported partially by the Research Program of Intractable Disease provided by the Ministry of Health, Labor, and Welfare of Japan. The WIF-B9 cells were kindly provided by Professor Ann L. Hubbard (Department of Cell Biology, Johns Hopkins University School of Medicine, Baltimore, MD, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Clinical Research Foundation, the Research Program of Intractable Disease provided by the Ministry of Health, Labor, and Welfare of Japan.
Rights and permissions
About this article
Cite this article
Takeyama, Y., Uehara, Y., Anan, A. et al. Increased hepatic ABCA1 transporter is associated with hypercholesterolemia in a cholestatic rat model and primary biliary cholangitis patients. Med Mol Morphol 50, 227–237 (2017). https://doi.org/10.1007/s00795-017-0166-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-017-0166-7