Abstract
During bioleaching of Cobalt from waste lithium-ion batteries, the biooxidation activity of acidophilic bacteria is inhibited by a high concentration of Co ion in the liquid phase. However, the mechanism for Co2+ toxicity to acidophilic bacteria has not been fully elucidated. In this study, the effects of Co2+ concentration on the biooxidation activity for Fe2+, intracellular reactive oxygen species (ROS) level and antioxidant defense systems in a mixed-culture of acidophilic bacteria (MCAB) were investigated. The results showed that the biooxidation activity of the MCAB was inhibited by Co2+. Furthermore, it was indicated that the intracellular ROS contents of the MCAB under conditions of 0.4 M and 0.6 M Co2+ were 2.60 and 3.34 times higher than that under the condition of 0 M Co2+. The increase in intracellular malondialdehyde content indicated that the oxidative damage was induced by Co2+. Moreover, the antioxidant systems in MCAB were affected by Co2+. It was observed that the Co2+ exposure increased the catalase and glutathione peroxidase activities while reducing the superoxide dismutase activity and the intracellular glutathione (GSH) content. It was found that an exogenous GSH supplementation eliminated excess intracellular ROS and improved the biooxidation activity of the MCAB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
ROS are unavoidable intermediates of various biochemical reactions in aerobic cellular metabolism. They are mainly composed of radicals such as superoxide anion (\( {\text{O}}_{2}^{ \cdot } \)), hydroxyl radical (·OH) and non-radical oxygen species [e.g., hydrogen peroxide (H2O2)] generated by the partial reduction of oxygen (Ray et al. 2012). Low levels of ROS are essential for cells to perform their biological functions because they are involved in regulations of many important cellular pathways (Wang et al. 2015). Excessive ROS could lead to lipid peroxidation, DNA damage, membrane damage, mutagenesis, cellular aging and apoptosis (Liu et al. 2014).
Organisms have two antioxidant systems for the elimination of ROS, namely enzymatic and non-enzymatic antioxidant systems. The former mainly contains SOD, CAT, thiol/disulfide system and the glutathione system. The latter includes some small-molecule antioxidants such as GSH and phenolics. Normally, a balance between ROS production and clearance is maintained under the synergistic action of antioxidant enzymes and small molecule antioxidants in organisms. However, the ROS induced by heavy metals may destroy the balance and lead to the oxidative damage of organisms (Wang et al. 2015).
Cobalt is an important industrial metal with various applications (Chen et al. 2016). Because of its excellent physical, chemical and mechanical properties, cobalt is used in many different products, such as alloys, magnetic materials and catalysts (Mudd et al. 2013). Cobalt is also widely used in lithium-ion batteries (Chen et al. 2017). With the rapid development of lithium-ion battery market, cobalt consumption is increasing rapidly (Mudd et al. 2013). However, it is quite difficult to obtain cobalt because there are not many Co-dominant mines (Chen et al. 2016). Cobalt usually exists in Cu and Ni ores as an associated metal, and the recovery rate of Co from these ores is usually low (Mudd et al. 2013). Nowadays, a large amount of cobalt is deposited in mine tailings, smelter slags, or electronic wastes. Among them, WLIBs are an important source of cobalt. Therefore, it is desirable to recover cobalt from WLIBs.
Bioleaching is considered a promising technology for cobalt recovery from WLIBs owing to its low costs and environmentally friendliness. Acidophilic bacteria, such as Acidithiobacillus ferrooxidans, Sulfobacillus thermosulfidooxidans, Leptospirillum ferriphilum, Acidithioacillus thiooxodans and Alicyclobacillus acidocaldarius are commonly studied for bioleaching of metals from e-wastes (e.g., WLIBs), metal oxides and sulfide ore (Wu et al. 2018). Mixed cultures have shown better performances in metal recovery than pure cultures (Jiang et al. 2017; Liang et al. 2013). Several researchers have studied the bioleaching of cobalt from WLIBs using these acidophilic bacteria (Xin et al. 2016; Zeng et al. 2013). The low pulp density is one of the most frequently encountered problems in bioleaching of WLIBs (Xin et al. 2016). Many optimization strategies have been tried to resolve the problem, such as the control of pH (Xin et al. 2016), the use of a different energy source (among S, Fe2+ or FeS2) (Mishra et al. 2008) and the addition of a catalyst (Zeng et al. 2012). However, few have focused on the Co2+ stress in acidophilic bacteria. During the bioleaching of cobalt from WLIBs, the biooxidation activity of bacteria is negatively affected by the accumulation of Co2+, and the decreased bacterial activity requires a low pulp density.
Compared with many other heavy metal ions, the toxicity of Co2+ is relatively limited, but exposure to Co2+ can still cause certain damages to organisms (Barras and Fontecave 2011). Exogenous Co2+ in soil stunted the growth of pak choi (Liu et al. 2018). The viability, development and behavior of aquatic species are affected by Co2+ (Zeeshan et al. 2017). The growth of Escherichia coli is stopped by Co2+ at a higher concentration (> 1 mM) (Barras and Fontecave 2011). Co toxicity has been explained using its redox properties and chemical affinity for sulfur atoms (Barras and Fontecave 2011). Because of its redox properties, Co could catalyze the generation of ROS and lead to the oxidative damage of cells. However, few studies have addressed the effect of oxidative stress induced by Co2+ on acidophilic bacteria.
This work investigated the effects of oxidative stress induced by the Co2+ exposure on the MCAB during the bioleaching of WLIBs. The production of ROS and the damage of cells under different concentrations of Co2+ were assessed. The effects of Co2+ on total antioxidant activity (TAA) and reactive oxygen radical scavenging activity (RSA) in the MCAB were studied. In addition, the response of various antioxidant systems in the MCAB, namely enzymatic and non-enzymatic antioxidant systems, were investigated at different concentrations of Co2+. The effect of exogenous GSH on the activity of the MCAB was also studied. This study could provide a novel approach to regulate the bioleaching process of WLIBs.
Materials and methods
Bacteria and materials
The MCAB was provided by a gold mine in China and has been domesticated for several years in previous studies (Wu et al. 2018) to achieve a high bacterial biooxidation activity for Fe2+. In this work, the metatranscriptomic analysis was used to determine the original community structure of the MCAB. Briefly, total RNA was extracted from MCAB using Trizol (Invitrogen, Shanghai, China) followed by DNase digestion and RNase column purification. The quality of RNA was detected by 1% agarose gel electrophoresis. Then the rRNA was removed using Ribo-Zero rRNA Removal Kits (Illumina, Shanghai, China). The metratranscriptomic RNA library was obtained by TruSeq™ RNA Sample Prep Kit (Illumina, Shanghai, China), and was amplified using HiSeq 3000/4000 PE Cluster Kit (Illumina, Shanghai, China). The amplified products were sequenced by an Illumina Hiseq 3000 (Illumina, Shanghai, China). The raw data were stored in Sequence Read Archive (SRX5575021). After quality control, all sequences were annotated to indicate taxonomic level using Non-Redundant Protein Sequence Database. The community structure was defined based on the assigned annotation. The result was shown in Fig. 1. The predominant organisms were L. ferriphilum, A. acidocaldarius and S. thermosulfidooxidans. 9 K medium was adopted to culture the MCAB. It contained the following: 3.0 g (NH4)2SO4, 0.1 g KCl, 0.5 g MgSO4·7H2O, 0.5 g K2HPO4, 0.01 g Ca(NO3)2, 44.2 g FeSO4·7H2O and 0.2 g yeast extract in 1 L distilled water. The initial pH was adjusted to 1.20 by sulfuric acid. The MCAB was cultivated in a rotary shaker (TQHZ-2002A, Taicang, Jiangsu, China) at 42 °C and 180 rpm.
Effect of Co2+ on bacterial activity
Tests were carried out in a 250 mL Erlenmeyer flask with 90 mL 9 K medium and 10 mL seed culture of the MCAB. Different amounts of CoSO4·7H2O were added into the 9 K medium to obtain the final concentrations of Co2+ at 0 M, 0.2 M, 0.4 M and 0.6 M. Samples were collected every 12 h to analyze pH, oxidation-reduction potential (ORP), Fe2+ concentration and bacterial number. Samples were collected at 48 h to analyze the relative abundance of communities. Acidophilic bacteria can be used for the bioleaching of cobalt from WLIBs, and the growth of acidophilic bacteria need to obtain energy from the biooxdation of Fe2+. Thus, when the iron-oxidation capability of bacteria decreases, the activity of acidophilic bacteria is likely inhibited. A low activity of acidophilic bacteria leads to a large decline in bioleaching efficiency of Co from WLIBs (Niu et al. 2014). Therefore, it is useful to use the bacterial iron-oxidation capability as a characterization index of bacterial activity.
pH and ORP were measured by a pH and Eh meter (Model FE20, Mettler, China). The concentration of Fe2+ was measured by titration (Wu et al. 2018). The bacterial number was counted under a microscope using a hemocytometer. The relative abundance of communities in the cells was analyzed in the V4 region of 16S rDNA using a high-throughput DNA sequencing technique. Briefly, one milliliter of MCAB culture at 48 h incubation was collected, the cells were harvested by centrifugation at 16,000g for 5 min at room temperature. The DNA was extracted by Power Soil DNA Isolation Kit (MO BIO Inc., USA). Then the V4 region of 16s rDNA was amplified using PCR primers: 338F (5-ACTCCTACGGGAGGCAGCAG-3) and 806R (5-GGACTACHVGGGTWTCTAAT-3). After that, the amplified products were sequenced by Illumina Hiseq 3000 (Illumina, Shanghai, China), and then the sequence data were aligned by BLAST in the Silva database.
Cell extract preparation
Two liters of a MCAB culture in its late exponential growth phase was collected. Bacteria were harvested by centrifugation at 16,000g for 5 min at room temperature. They were then resuspended in 100 mL of fresh 9 K medium as a bacterial concentrate and shaken for 30 min at 42 °C, 180 rpm. After that, the cells were collected by centrifugation and were resuspended again in fresh 9 K medium supplemented with 0 M, 0.2 M, 0.4 M or 0.6 M Co2+. Then, the culture was incubated in a water bath for 1 h at 42 °C. Afterwards, the cells were collected by centrifugation, followed by washing one time with fresh 9 K medium (pH 1.2, without FeSO4·7H2O) and then washing twice with a buffer (30 mM Tris–HCl, 30 mM NaCl, pH 8.0) in an ultrasonic bath. Cell extract was prepared by ultrasonic disruption in the buffer. The protein concentration in the cell extract was measured with the Bradford assay (Bradford 1976).
Determinations of ROS and MDA
The intracellular level of total ROS was measured using the Reactive Oxygen Species Assay Kit (Beyotime, China). Cells were resuspended in fresh 9 K medium supplemented with 0 M, 0.2 M, 0.4 M or 0.6 M Co2+ and cultured for 1 h at 42 °C. The ROS was measured by DCFH-DA assay according to kit instructions. The fluorescence intensity (FL) was determined using a micro-plate reader (VARIOSKAN LUX, Thermo Fisher, Finland). The ratio of FL to protein concentration was defined as the intracellular ROS content (FL/protein).
The content of MDA was measured from the productions of MDA and thiobarbituric acid (TBA) following Draper and Hadley (1990).
Total antioxidant activity and reactive oxygen radical scavenging activity assay
Total antioxidant activity of the MCAB extracts was measured by 2,2′-azino–bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) (ABTS) assay, as described previously (Re et al. 1999). The \( {\text{O}}_{2}^{ \cdot } \) scavenger ability was measured according to Liu et al. (2014). Briefly, the \( {\text{O}}_{2}^{ \cdot } \) scavenger ability was determined by the inhibition ratio between autoxidation and oxidation rate with the addition of MCAB extracts. The ·OH scavenger activity was assessed by the method used by Liu et al. (2014). The assay was conducted by mixing 1.5 mM FeSO4, 6 mM H2O2, 20 mM sodium salicylate and MCAB extracts. After the mixture was incubated in dark for 30 min, the absorbance of the mixture was measured at 562 nm. H2O2 decomposition activity was determined according to Liu et al. (2014) with minor modification. Briefly, the assay was performed by mixing MCAB extracts and 0.1 mM H2O2 with the volume ratio of 1:1. The mixture was incubated for 30 min at 30 °C in the dark, and then 1 M KI was added. After 10 min, the mixture’s absorbance was measured at 390 nm. H2O2 decomposition activity was determined by measuring the reduction of H2O2.
Antioxidant assay
SOD activity was estimated according to Marklund and Marklund (1974). Briefly, 0.1 mL MCAB extracts was added into 2.5 mL Tris–HCl buffer (0.1 M, pH 8.0) at 25 °C. After 0.15 mL pyrogallol (4.5 mmol/L) was added, the changes of absorbance at 320 nm were measured for 3 min. The SOD activity was quantified by the inhibition of pyrogallol self-oxidation. The water was used to determine the self-oxidation rate. The SOD quantity that inhibits the autoxidation of pyrogallol by 50% is defined as one SOD unit. The activity of GSH-Px was measured according to Aydin et al. (2001). Briefly, the reaction mixture contained 50 mmol/L Tris buffer (pH 7.6), 2 mmol/L reduced glutathione, 1 mmol/L Na2EDTA, 4 mmol/L sodium azide, 0.2 mmol/L NADPH and 1000 U glutathione reductase (GR). After mixing and incubating 50 µL MCAB extracts and 950 µL reaction mixture for 5 min at 37 °C, the reaction was initiated with 8.8 mmol/L H2O2. The decrease of absorbance at 340 nm was measured for 2 min. One GSH-Px unit was defined as 1 mmol of GSH oxidized/min. CAT activity was estimated according to Xu et al. (2015). The reaction mixture contained 2.5 mL PBS (50 mM, pH 7.4), 0.5 mL MCAB extracts. The reaction was initiated with 0.1 mL H2O2 (0.1 M). The changes in absorbance at 240 nm were measured for 3 min. The amount of enzyme that decomposed 1 µmol H2O2 in 1 min was defined as one CAT unit. The content of GSH was determined by the method of Ellman (Ellman 1959; Liu et al. 2014). Briefly, the mixture contained 0.5 mL Tris–HCl (0.25 M, pH 8.0), 0.25 mL methanal (3%) and 0.25 mL MCAB extracts. After the mixture was incubated for 20 min, 3 mL 5,5-dithiobis-2-nitrobenzoic acid (1 mM) was added. Then the mixture kept at room temperature for 5 min. The absorbance of the mixture was measured at 412 nm. Total phenolic contents were estimated by Folin–Ciocalteau method according to Cheung et al. (2003).
Effect of exogenous GSH on intracellular ROS content and bacterial growth
The determination of ROS and MDA were similar to Sect. 2.4. The difference was that the cells were resuspended in fresh 9 K medium containing 0.4 M Co2+ with or without 0.30 g/L GSH.
Different concentrations of GSH (0 g/L, 0.1 g/L, 0.3 g/L, 0.5 g/L) was added to the 9 K Medium containing 0.4 M Co2+ with 10% (v/v) inoculum. Incubation was carried out in a rotary shaker at 42 °C and 180 rpm. Samples were withdrawn twice a day to analyze pH, ORP and Fe2+ concentration. Samples were collected at 48 h to analyze the relative abundance of communities.
Different amounts of CoSO4·7H2O were added to the 9 K medium to reach final concentrations of Co2+ at 0 M, 0.2 M, 0.4 M and 0.6 M with and without 0.3 g/L GSH. Samples were withdrawn twice a day to analyze pH, ORP and Fe2+ concentration.
Statistical analysis
In this work, all tests under different conditions of 0 M, 0.2 M, 0.4 M and 0.6 M Co2+ were conducted in three culture replicates and all of the above analytical tests were carried out in triplicates. Mean values with the standard deviations were shown. P < 0.05 was adopted for statistical significance.
Results
Effect of Co2+ on bacterial activity
During the bioleaching of WLIBs, researchers typically kept a low pulp density of WLIBs (few moved above 4.0% w·v−1) even though many optimization strategies, such as the control of pH (Xin et al. 2016) and the addition of a catalyst (Zeng et al. 2012), had been explored. The Co concentration in the pulp density of 4.0% (w·v−1) LiCoO2 (a most studied cathode active material of WLIBs) is about 0.4 M. Accordingly, Co concentrations of 0 M, 0.2 M, 0.4 M and 0.6 M were selected to investigate the toxicity of Co2+ on the MCAB. In this work, Fe2+ was the main energy source for the MCAB, so the energy uptake by the MCAB could be gauged by measuring the change of the Fe2+ concentration. It was demonstrated by Liu et al. (2017) that at low pH, the chemical oxidation rate of Fe2+ was far slower than the biooxidation rate of Fe2+. As shown in Fig. 2a, the pH in the culture medium gradually increased with time, but the pH increase was suppressed by an increased Co2+ concentration.
Changes of pH (a), ORP (b), Fe2+ concentration (c) and bacterial number (d) with time under condition of different Co2+ concentrations in biooxidation for Fe2+. Community composition (e) at 48 h incubation under condition of different Co2+ concentrations. The tests were conducted in three culture replicates and P < 0.05 was adopted for statistical significance
As shown in Eq. (1), the biooxidation of Fe2+ was an acid-consuming process. The more acid consumed in the culture means the higher biooxidation activity of the bacteria. Ballor et al. (2006) demonstrated that ORP can be used to indicate bacterial biooxidation activity. It is shown in Fig. 2b that the ORP under the condition of 0 M Co2+ rose from 398 mV to 671 mV rapidly, but the ORP values under the conditions of 0.4 M and 0.6 M Co2+ were still less than 480 mV after 84 h. It indicated that the biooxidation activity of the MCAB was inhibited by Co2+. These results were confirmed by Fig. 2c, which shows that the consumption of Fe2+ decreased with more Co2+. As shown in Fig. 2d, the MCAB biomass decreased with the increase of Co2+ concentration in the first 48 h. The biomass reached to 11.89, 4.51, 3.12 and 1.89 (× 107 cells/mL) under the conditions of 0 M, 0.2 M, 0.4 M and 0.6 M Co2+, respectively. The result indicated that the growth of the MCAB was inhibited by Co2+. As shown under the conditions of 0 M and 0.2 M Co2+ in Fig. 2c, d, the biomass did not appear to decrease immediately after the Fe2+ was exhausted. This trend might be due to the fact that the Fe2+ was not the sole energy source in the medium, the MCAB could also use yeast extract as an energy source when Fe2+ was exhausted. Xue et al. (2014) reported S. thermosulfidooxidans was a mixotroph which could use both Fe2+ and yeast extract as energy source. But L. ferriphilum was a chemolithoautotroph which could only utilize the Fe2+ as energy source (Zhang et al. 2010). The biooxidation of Fe2+ by S. thermosulfidooxidans could be accelerated by the addition of yeast extract, and S. thermosulfidooxidans could easily switch from autotrophic growth to heterotrophic growth when yeast extract was the sole substrate (Norris et al. 1996). However, high concentration of yeast extract have a toxic effect on chemolithoautotrophic bacteria (Van Hille et al. 2009), so only a small amount of yeast extract (0.2 g/L) was added into the medium in this test. After 48 h, the biomass decreased under the conditions of 0 M and 0.2 M Co2+.
As shown in Fig. 2e, the relative abundances of L. ferriphilum and S. thermosulfidooxidans in the MCAB were much more than others. The high proportion of those two strains (about 99.0%) indicates that L. ferriphilum and S. thermosulfidooxidans were the dominate strains in the MCAB. Moreover, the concentration of Co2+ did not change the species in the MCAB, but rather the relative abundance of the two strains. The relative abundance of L. ferriphilum increased from 38.4% at 0 M Co2+ to 93.9% at 0.6 M Co2+. Apparently, it had a higher cobalt resistance than that of S. thermosulfidooxidans. There might be many reasons for the effect of Co2+ on the biooxidation activity of the MCAB. Co2+ can enhance the generation of ROS, which could be the reason for cobalt toxicity in the MCAB (Kubrak et al. 2012).
Effects of Co2+ on intracellular ROS and MDA
ROS are unavoidable intermediates in the process of microbial growth and metabolism, which can participate in many biochemical reactions. However, excessive ROS have negative effects on bacterial biooxidation activities. As shown in Fig. 3a, the contents of the intracellular ROS in the MCAB under the conditions of 0.4 M and 0.6 M Co2+ were 2.60 and 3.34 times higher than that under the condition of 0 M Co2+. The result proved that the presence of Co2+ did enhance the generation of ROS in the MCAB. Fantino et al. (2010) also found that the content of ROS in E. coli was increased by a high concentration of Co2+. Jomova and Valko (2011) believed that Co2+ would react with oxygen or hydrogen peroxide to produce ROS through Fenton-type reactions.
The intracellular MDA concentration can serve as a biomarker for oxidative stress and lipid peroxidation (Zheng et al. 2018). It is shown in Fig. 3b that the intracellular MDA concentration of the MCAB increased with the concentration of Co2+. This suggests that the presence of Co2+ could cause lipid peroxidation in the MCAB cells, which is in agreement with the findings of several other researchers (Garoui et al. 2013; Lwalaba et al. 2017).
Total antioxidant activity and radical scavenging activity analysis
Under normal conditions, ROS are produced continuously, and the radical scavenging system of microorganisms eliminate these ROS in time so that there is a dynamic balance between ROS production and elimination. Co2+ can disrupt this balance leading to oxidative damage in bacterial cells. Thus, the radical scavenging system of the MCAB can be disrupted by Co2+. In this section, the effect of Co2+ on the total antioxidant activity and the radical scavenging activity of the MCAB were investigated, and the results are shown in Fig. 4.
Total antioxidant activity (a), \( {\text{O}}_{2}^{ \cdot } \) scavenger activity (b), H2O2 decomposition activity (c) and ∙OH scavenger activity (d) of MCAB after incubation for 1 h under condition of different Co2+ concentrations. The tests were conducted in three sample replicates and P < 0.05 was adopted for statistical significance
The total antioxidant activities of the MCAB were evaluated by the ABTS·+ radical method (Liu et al. 2014). As shown in Fig. 4a, the total antioxidant activities of the MCAB decreased with an elevated concentration of Co2+. The result suggests that the radical scavenging system in the MCAB was disrupted by Co2+, which led to the accumulation of ROS. ROS are mainly composed of \( {\text{O}}_{2}^{ \cdot } \), ·OH and H2O2. Radical scavenging activities are also used as indicators of antioxidant activities (Liu et al. 2014). As shown in Fig. 4b, the \( {\text{O}}_{2}^{ \cdot } \) scavenger activity dropped from 32.3% at 0 M Co2+ to 8.6% at 0.6 M Co2+. The result indicates that Co2+ inhibited the \( {\text{O}}_{2}^{ \cdot } \) scavenger activity of the MCAB. As shown in Fig. 4c, Co2+ promoted the H2O2 decomposition activity of the MCAB. The H2O2 decomposition activity increased from 60.6% at 0 M Co2+ to 92.7% at 0.6 M Co2+. This might be because Co treatment could increase the activity of some antioxidant enzymes, such as CAT and GSH-Px. Those enzymes could catalyze H2O2 to produce water and oxygen. Co2+ had little effect on the ·OH scavenger activity of the MCAB as indicated by Fig. 4d.
Effects of Co2+ on antioxidant enzymes and small molecule antioxidants
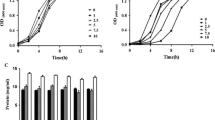
The ability of bacteria to remove ROS is mainly achieved by two major antioxidant systems, namely enzymatic and non-enzymatic antioxidant systems. SOD, CAT and GSH-Px are common antioxidant enzymes in most bacteria, and the effects of Co2+ on those enzymes’ activities of the MCAB are shown in Fig. 5.
SOD is an important antioxidant enzyme which catalyzes the disproportionation of \( {\text{O}}_{2}^{ \cdot } \). It is illustrated by Fig. 5a that the SOD activity showed a rapid decline with the increasing concentration of Co2+. This means that the SOD activity was inhibited by Co2+ aggressively. Kurhaluk and Tkachenko (2016) reported that Co-treatment led to a significant decrease in the SOD activity.
CAT is a terminal oxidase which also widely exists in animals, plants and microorganisms. It can catalyze H2O2 to produce water and oxygen. It is shown in Fig. 5b that the CAT activity under the condition of 0.6 M Co2+ was 2.13 times higher than that under the condition of 0 M Co2+. The result is in agreements with the findings by Lwalaba et al. (2017). Their Co-treatment resulted in the increase of CAT activity.
GSH-Px can catalyze the reaction of GSH with H2O2. It can also block the secondary reaction of free radicals induced by lipoperoxide (LOOH) and reduce the damage of LOOH to organisms (Brigelius-Floh et al. 2009). As shown in Fig. 5c, the GSH-Px was increased by the addition of Co2+.
Small molecule antioxidants can directly participate in the reduction of ROS and play an important role in maintaining the balance between antioxidant defense and ROS production in bacteria. The GSH content in the MCAB under different concentrations of Co2+ is shown in Fig. 5d. GSH decreased from 22.7 µmol/L at 0 M Co2+ to 14.2 µmol/L at 0.6 M Co2+, indicating that the production of intracellular GSH was inhibited by Co2+.
Phenolics are also important small molecule antioxidants, which have strong ROS scavenging capacities. Phenolics were detected in the MCAB, but their contents were not affected by Co2+ as shown in Fig. 5e.
Impact of exogenous GSH on intracellular ROS and biooxidation activity of MCAB
GSH can help microorganisms to resist oxidative stress and metal ion toxicity by eliminating intracellular ROS (Muller 2011). Several studies (Goswami et al. 2006; Wang et al. 2016) reported that the activity of bacteria could be improved by adding exogenous GSH. The effect of exogenous GSH on the MCAB which exposure to 0.4 M Co2+ is shown in Fig. 6.
Intracellular levels of ROS (a) and MDA (b) after incubation for 1 h under condition of 0.4 M Co2+ with and without 0.3 g/L GSH. Time period evaluation of ORP (c), Fe2+ concentration (d) and bacterial number (e) under condition of 0.4 M Co2+ with different concentrations of GSH in biooxidation for Fe2+. Community composition (f) at 48 h incubation under 0.4 M Co2+ with different concentrations of GSH. The tests were conducted in three culture replicates and P < 0.05 was adopted for statistical significance
It is illustrated by Fig. 6a that the level of intracellular ROS was decreased by the addition of 0.3 g/L GSH. Moreover, as shown in Fig. 6b, the content of MDA was also reduced by the exogenous GSH. Thus, the exogenous GSH supplementation not only eliminated excess intracellular ROS, but also reduced lipid peroxidation of the MCAB. It is indicated by Fig. 6c, d that the biooxidation activity for Fe2+ by the MCAB was improved by the exogenous GSH supplementation and the optimal activity was attained when 0.3 g/L GSH was added. There was a trend that ORP and the biooxidation of Fe2+ under the condition of 0.5 g/L GSH were less than those of 0.3 g/L GSH. The trend is due to that intracellular ROS are essential for cells to perform their biological functions, so too low levels of ROS have a negative effect on cells. Excessive concentration of GSH may lead to too low levels of ROS which inhibit the biooxidation activity of MCAB. Hu et al. (2004) reported that the bioleaching of sphalerite by Thiobacillus ferrooxidans could be greatly improved at the concentration of l-cysteine less than 0.4 g/L but markedly inhibited at the concentration above 0.6 g/L. As GSH contains l-cysteine, the report indicates that an optimal concentration of exogenous GSH supplementation exists. As shown in Fig. 6d, e, the planktonic cell count in the MCAB did not increase significantly with a higher concentration of exogenous GSH and the microbial community composition of the MCAB shifted slightly as well with the relative abundances of L. ferriphilum in all groups around 80.0%. The biooxidation activities for Fe2+ by the MCAB at different concentrations of Co2+ in the presence 0.3 g/L exogenous GSH are shown in Fig. 7a, b. It demonstrates that the biooxidation activity was improved by 0.2 M and 0.4 M Co2+ concentrations, but not by 0 M and 0.6 M Co2+ concentrations. This might be due to the fact that the oxidative stress on the MCAB was not strong under the condition of 0 M Co2+, so the biooxidation activity of the MCAB was not enhanced by GSH. However, under the condition of 0.6 M Co2+, the oxidative stress on the MCAB was too severe for GSH to be effective. These results are confirmed by Fig. 7c. It shows that the levels of intracellular ROS under the condition of 0 M Co2+ were only 1.63 FL/protein without GSH and 1.53 FL/protein with GSH. However, the level of intracellular ROS under the condition of 0.6 M Co2+ was as high as 5.08 FL/protein even with 0.3 g/L GSH. Moreover, the level of intracellular ROS decreased most significantly under the conditions of 0.2 M and 0.4 M Co2+ concentrations. The results here demonstrate that an exogenous GSH supplementation could improve the bioxidation activity by reducing intracellular ROS if the Co2+ concentration was not too high.
Changes of ORP (a) and Fe2+ concentration (b) with time under condition of different Co2+ concentrations with and without 0.3 g/L GSH in biooxidation for Fe2+. Intracellular levels of ROS (c) after incubation for 1 h under the condition of different Co2+ concentrations with and without 0.3 g/L GSH. The tests were conducted in three culture replicates and P < 0.05 was adopted for statistical significance
Discussion
Cobalt is an important component in lithium-ion batteries used to improve performances. However, cobalt resources are scarce and the price of cobalt is high. With electric vehicles gaining popularity, the demand for cobalt is further expanding. When lithium-ion batteries reach their service life, recovering cobalt from WLIBs can not only reduce environmental pollution but also avoid the waste of cobalt resources. Bioleaching, as a green metal recovery technology, can be used to recover cobalt from WLIBs.
Acidophilic bacteria are commonly used for bioleaching, which live in the environment with the characteristics of high acidity, high pulp concentration and high metal ion concentration. Such an environment can easily stimulate the production of intracellular ROS, so acidophilic bacteria should contain an antioxidant system for the depletion of ROS. Cardenas et al. (2012) demonstrated that almost all bioleaching microorganisms contain at least one copy of a superoxide dismutase-encoding gene (Cu/Zn-SOD, Fe/Mn-SOD or Ni-SOD). However, there are no genes predicted to encode for SOD in Leptospirillum species (Cardenas et al. 2012), but Ferrer et al. (2016) detected the SOD activity when studying the antioxidant activity of Leptospirillum CF-1. They believe that the SOD activity of strain CF-1 came from a non-proteinaceous metabolite. The activity of CAT, SOD and the glutathione reductase system were detected when Zheng et al. (2018) investigated the effects of cadmium exposure on expression of glutathione synthetase system genes in A. ferrooxidans, which was a bacterium in the MCAB in this work. All these studies confirmed that the acidophilic bacteria have antioxidant activities. However, there are very few reports about oxidative stress and antioxidant defenses in acidophilic bacteria in response to a heavy metal ion exposure. Thus, more efforts are required to explore the effects of ROS on acidophilic bacteria.
In the bioleaching of cobalt from WLIBs, a high concentration of Co2+ affects the activity of bacteria. Therefore, in this work, the cobalt sulfate was used to simulate the toxicity of Co2+ to acidophilic bacteria and the results demonstrated that the biooxidation activity of acidophilic bacteria was indeed inhibited by Co2+ (Fig. 2). So far, there are only a few reports about the effect of Co2+ on acidophilic bacteria, and the study on cobalt resistance by acidophilic bacteria is still lacking. Barras and Fontecave (2011) summarized the cobalt resistance mechanism of E. coli and Salmonella enterica, and found that the genes involved in the biosynthesis of Fe-S clusters were related to the Co efflux.
The community structure of MCAB was changed in this work. The original predominate organisms in MCAB were L. ferriphilum, A. acidocaldarius and S. thermosulfidooxidans, but under the condition of 0 M Co2+ at 48 h incubation, L. ferriphilum and S. thermosulfidooxidans were the dominate organisms, and reached to 38.4% and 61.6%, respectively. The addition of 0.2 g/L yeast extract might be the reason for the change of the relative abundances of these species. S. thermosulfidooxidans could use yeast extract as a carbon source and energy source (Xue et al. 2014), but L. ferriphilum could only utilize CO2 as carbon source (Tyson et al. 2004). The yeast extract promotes the growth of S. thermosulfidooxidans. What’s more, with the increased concentration of Co2+, the relative abundances of different species in MCAB were changed. The relative abundance of L. ferriphilum increased from 38.4% at 0 M Co2+ to 93.9% at 0.6 M Co2+, while that of S. thermosulfidooxidans decreased from 61.6% to 5.7%. It is shown that L. ferriphilum has higher cobalt resistance than S. thermosulfidooxidans. Tian et al. (2007) reported that the maximum metal concentrations whereby metabolic activity of L. ferriphilum still occurred were 5–10 mM (Co2+) and 30–40 mM (Ni2+). As there are few reports on cobalt resistance of S. thermosulfidooxidans, nickel resistance is selected to interpret the metal resistance of MCAB because the properties of cobalt and nickel are similar. Nies (1999) demonstrated that nickel toxicity was comparable to that of cobalt. Dopson et al. (2003) found that the maximum metal concentration whereby metabolic activity of S. thermosulfidooxidans still occurred was 5 mM Ni2+. Those reports support that L. ferriphilum could tolerate a higher concentration of Ni2+ and Co2+ than S. thermosulfidooxidans.
It is indicated by Fig. 3 that the oxidative stress and oxidative damage caused by the addition of Co2+ were important factors leading to the inhibition of bacterial biooxidation activity. The oxidative stress caused by Co2+ was also reflected in other organisms (Harrison et al. 2009). The rise of ROS may be due to two factors: (1) The generation of ROS could be catalyzed by Co through Fenton-type reactions (Barras and Fontecave 2011). Leonard et al. (1998) demonstrated that ROS is able to be generated by the reaction of Co2+ with H2O. (2) The antioxidant system in acidophilic bacteria may be disrupted by the addition of Co2+. As a result, the intracellular ROS could not be removed effectively. As shown in Fig. 4a, the total antioxidant activity of the MCAB was inhibited by Co2+. This confirms that the disorder of antioxidant system in acidophilic bacteria in the MCAB was caused by Co2+.
The antioxidant activity of acidophilic bacteria was manifested by antioxidant enzymes and small molecule antioxidants. SOD could catalyze the disproportionation of \( {\text{O}}_{2}^{ \cdot } \) to produce hydrogen peroxide and oxygen. Then the hydrogen peroxide was decomposed to water by the catalysis of CAT and GSH-Px. The decrease of SOD activity indicated that the antioxidant system of acidophilic bacteria in the MCAB was disrupted by the addition of Co2+. The increase of CAT and GSH-Px activity was the response of the antioxidant to prevent cells from oxidative damages.
In addition to antioxidant enzymes, small molecule antioxidants (such as ascorbate, cobalamin and GSH) also play an important role in the resistance of the oxidative stress. Ascorbate is an effective antioxidant which is beneficial control of lipid peroxidation of cellular membranes (Bendich et al. 1986). But ascorbate could be oxidized rapidly (about 6 h) by air in the aqueous solution, and the oxidation of ascorbate could be accelerated by Fe2+ and Cu2+. The 9 K medium used in this work contains a high concentration of Fe2+, so it may be not a good idea to use ascorbate as ROS scavenger in this paper. Cobalamin is a cobalt-coordinated tetrapyrrole which can participate in anti-oxidative protection (Sakultung et al. 2008). Ferrer et al. (2016) found that the exogenous addition of cobalamin in Leptospirillum group II strain CF-1 reduced the level of intracellular ROS and stimulated the growth and survival of cells when the cells were exposed to oxidative stress. Cobalamin is stable at pH 4.5–5.0 and decomposes in strong acid (pH < 2.0). The MCAB used in this work grows under the condition of pH 1.20, so the addition of cobalamin may be not suitable for anti-oxidative protection of MCAB in this paper. However, cobalamin may have an active effect on the cobalt-induced anti-oxidation when the microorganism lives in the condition of pH > 2.0. GSH is one of the most commonly used small-molecule antioxidants. GSH can remove ROS directly by participating in the reduction process of ROS through the transformation of their thiol oxidation–reduction state. It also plays a role in scavenging ROS through glutathione peroxidase. In this work, the content of intracellular GSH was decreased by the addition of Co2+. The reduction of GSH may be due to several reasons. (1) The sulfhydryl group with a high affinity in GSH could form mercaptide complexes with several metals (AS et al. 2007). (2) The redox reaction between GSH and Co-induced free radicals leads to the decrease of GSH (Garoui et al. 2013). Helbig et al. (2008) reported that the removal of GSH led to a strong decrease of heavy metal resistance in Escherichia coli. Several studies (Goswami et al. 2006; Wang et al. 2016) reported that the biooxidation activity of bacteria could be improved by an exogenous GSH supplementation. Begg et al. (2015) found that GSH could improve the growth of Streptococcus pneumoniae and decrease sensitivity to oxidative stress in a high cellular concentration of cadmium (~ 17 mM). In this work, the exogenous GSH supplementation could also eliminate intracellular ROS and MDA contents, and thus increasing the biooxidation activity of the MCAB.
In general, this work confirmed that the increase of the intracellular ROS content caused by Co2+ stress was one of the important reasons for the decrease of bacterial biooxidation activity during the bioleaching of WLIBs. Exogenous small molecule antioxidants could reduce the intracellular ROS and increase the biooxidation activity of acidophilic bacteria.
Conclusion
This work studied the Co2+ toxicity on the MCAB. It was found that the biooxidation activity of the MCAB could be inhibited by Co2+. The balance between ROS production and elimination in the MCAB was disrupted by Co2+ and it led to oxidative damage in cells. The antioxidant systems in the MCAB, namely enzymatic and non-enzymatic antioxidant systems, were affected by Co2+. It was observed that the CAT and GSH-Px activities increased while the SOD activity and the content of intracellular GSH decreased with an elevating concentration of Co2+. Furthermore, it was proven that the exogenous GSH supplementation could effectively reduce the intracellular ROS caused by Co2+ and thus improving the biooxidation activity of the MCAB. These results in this work are useful in developing operational strategies for the bioleaching of WLIBs with a high pulp density loading.
Abbreviations
- ABTS:
-
2,2′-Azino–bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt)
- CAT:
-
Catalase
- Co:
-
Cobalt
- FL:
-
Fluorescence intensity
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- H2O2 :
-
Hydrogen peroxide
- ·OH:
-
Hydroxyl radical
- LOOH:
-
Lipoperoxide
- MCAB:
-
Mixed-culture of acidophilic bacteria
- MDA:
-
Malondialdehyde
- ORP:
-
Oxidation–reduction potential
- ROS:
-
Reactive oxygen species
- \( {\text{O}}_{2}^{ \cdot } \) :
-
Superoxide anion
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- WLIBs:
-
Waste lithium-ion batteries
References
Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Işimer A (2001) Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin Biochem 34:65–70
Ballor NR, Nesbitt CC, Lueking DR (2006) Recovery of scrap iron metal value using biogenerated ferric iron. Biotechnol Bioeng 93:1089–1094. https://doi.org/10.1002/bit.20821
Barras F, Fontecave M (2011) Cobalt stress in Escherichia coli and Salmonella enterica: molecular bases for toxicity and resistance. Metallom Integr Biometal Sci 3:1130–1134
Begg SL et al (2015) Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in Streptococcus pneumoniae Nature communications 6:6418–6419
Bendich A, Machlin LJ, Scandurra O, Burton GW, Wayner DDM (1986) The antioxidant role of vitamin C. Adv Free Radic Biol Med 2:419–444
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brigelius-Floh ER, Kipp A (2009) Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 1790:1555–1568
Cardenas JP, Moya F, Covarrubias P, Shmaryahu A, Levican G, Holmes DS, Quatrini R (2012) Comparative genomics of the oxidative stress response in bioleaching microorganisms. Hydrometallurgy 127–128:162–167. https://doi.org/10.1016/j.hydromet.2012.07.014
Chen G, Yang H, Li H, Tong L (2016) Recovery of cobalt as cobalt oxalate from cobalt tailings using moderately thermophilic bioleaching technology and selective sequential extraction. Minerals 6:67–78. https://doi.org/10.3390/min6030067
Chen X, Ma H, Luo C, Zhou T (2017) Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J Hazard Mater 326:77–86. https://doi.org/10.1016/j.jhazmat.2016.12.021
Cheung LM, Cheung PCK, Ooi VEC (2003) Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem 81:249–255
Dopson M, Bakeraustin C, Koppineedi PR, Bond PL (2003) Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235(3):185–193
Fantino JR, Py B, Fontecave M, Barras F (2010) A genetic analysis of the response of Escherichia coli to cobalt stress. Environ Microbiol 12:2846–2857
Ferrer A et al (2016) Cobalamin protection against oxidative stress in the acidophilic iron-oxidizing bacterium Leptospirillum Group II CF-1. Front Microbiol 7:748–759. https://doi.org/10.3389/fmicb.2016.00748
Garoui E, Ben AI, Driss D, Elwej A, Chaabouni SE, Boudawara T, Zeghal N (2013) Effects of cobalt on membrane ATPases, oxidant, and antioxidant values in the cerebrum and cerebellum of suckling rats. Biol Trace Elem Res 154:387–395
Goswami M, Mangoli SH, Jawali N (2006) Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob Agents Chemother 50:949–954
Harrison JJ et al (2009) Chromosomal antioxidant genes have metal ion-specific roles as determinants of bacterial metal tolerance. Environ Microbiol 11:2491–2509. https://doi.org/10.1111/j.1462-2920.2009.01973.x
Helbig K, Bleuel C, Krauss GJ, Nies DH (2008) Glutathione and transition-metal homeostasis in Escherichia coli. J Bacteriol 190:5431–5438
Hu YH, He ZG, Hu WX, Peng H, Zhong H (2004) Effect of two kinds of amino-acids on bioleaching metal sulfide. Trans Nonferrous Metals Soc China 14:794–797
Jiang LL, Zhou JJ, Quan CS, Xiu ZL (2017) Advances in industrial microbiome based on microbial consortium for biorefinery. Bioresour Bioprocess 4:11–21
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87. https://doi.org/10.1016/j.tox.2011.03.001
Kubrak OI, Rovenko BM, Husak VV, Vasylkiv OY, Storey KB, Storey JM, Lushchak VI (2012) Goldfish exposure to cobalt enhances hemoglobin level and triggers tissue-specific elevation of antioxidant defenses in gills, heart and spleen. Compar Biochem Physiol Toxicol Pharmacol CBP 155:325–332
Kurhaluk N, Tkachenko H (2016) Modulators of KATP channels in the prevention of oxidative stress and antioxidant capacity improvement in the rat heart with different resistance to hypoxia upon cobalt treatment. J Vet Res 60:195–206. https://doi.org/10.1515/jvetres-2016-0029
Leonard S, Gannett PM, Rojanasakul Y, Schwegler-Berry D, Castranova V, Vallyathan V, Shi X (1998) Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J Inorg Biochem 70:239–244
Liang G, Tang J, Liu W, Zhou Q (2013) Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J Hazard Mater 250–251:238–245
Liu L et al (2014) Inherent antioxidant activity and high yield production of antioxidants in Phanerochaete chrysosporium. Biochem Eng J 90:245–254. https://doi.org/10.1016/j.bej.2014.06.014
Liu J, Wu W, Zhang X, Zhu M, Tan W (2017) Adhesion properties of and factors influencing Leptospirillum ferriphilum in the biooxidation of refractory gold-bearing pyrite. Int J Mineral Process 160:39–46
Liu B, Huang Q, Su Y, Wang M, Ma Y, Kelly RM (2018) Cobalt accumulation and antioxidant system in pakchois under chemical immobilization in fluvo-aquic soil. J Soils Sedim 18:669–679. https://doi.org/10.1007/s11368-017-1804-3
Lwalaba JL et al (2017) Alleviating effects of calcium on cobalt toxicity in two barley genotypes differing in cobalt tolerance. Ecotoxicol Environ Saf 139:488–495
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Mishra D, Kim DJ, Ralph DE, Ahn JG, Rhee YH (2008) Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manage 28:333–338. https://doi.org/10.1016/j.wasman.2007.01.010
Mudd GM, Weng Z, Jowitt SM, Turnbull ID, Graedel TE (2013) Quantifying the recoverable resources of by-product metals: the case of cobalt. Ore Geol Rev 55:87–98. https://doi.org/10.1016/j.oregeorev.2013.04.010
Muller M (2011) Glutathione modulates the toxicity of, but is not a biologically relevant reductant for, the Pseudomonas aeruginosa redox toxin pyocyanin. Free Radic Biol Med 50:971–977
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750. https://doi.org/10.1007/s002530051457
Niu Z, Zou Y, Xin B, Chen S, Liu C, Li Y (2014) Process controls for improving bioleaching performance of both Li and Co from spent lithium ion batteries at high pulp density and its thermodynamics and kinetics exploration. Chemosphere 109:92–98
Norris PR et al (1996) Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide-oxidizing bacteria. Microbiology 142(4):775–783
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990. https://doi.org/10.1016/j.cellsig.2012.01.008
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Sakultung S, Pruksathorn K, Hunsom M (2008) Simultaneous recovery of Ni and Co from scrap mobile phone battery by acid leaching process. Asia-Pac J Chem Eng 3:374–379. https://doi.org/10.1002/apj.158
Tian J, Wu N, Li J, Liu Y, Guo J, Yao B, Fan Y (2007) Nickel-resistant determinant from Leptospirillum ferriphilum. Appl Environ Microbiol 73:2364–2368. https://doi.org/10.1128/AEM.00207-07
Tyson GW et al (2004) Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428:37
Van Hille RP, Bromfield LV, Botha SS, Jones G, van Zyl AW, Harrison STL (2009) The effect of nutrient supplementation on growth and leaching performance of bioleaching bacteria. Adv Mater Res 71–73:413–416
Wang H, Zhang X, Zhu M, Tan W (2015) Effects of dissolved oxygen and carbon dioxide under oxygen-rich conditions on the biooxidation process of refractory gold concentrate and the microbial community. Miner Eng 80:37–44. https://doi.org/10.1016/j.mineng.2015.06.016
Wang T, Xu Z, Lu S, Xin M, Kong J (2016) Effects of glutathione on acid stress resistance and symbiosis between Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. Int Dairy J 61:22–28
Wu W, Liu X, Zhang X, Zhu M, Tan W (2018) Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron-sulfur-oxidizing bacteria. Bioresour Bioprocess 5:10–23. https://doi.org/10.1186/s40643-018-0196-6
Xin Y, Guo X, Chen S, Wang J, Wu F, Xin B (2016) Bioleaching of valuable metals Li Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. J Clean Prod 116:249–258
Xu P et al (2015) Cadmium induced hydrogen peroxide accumulation and responses of enzymatic antioxidants in Phanerochaete chrysosporium. Ecol Eng 75:110–115. https://doi.org/10.1016/j.ecoleng.2014.11.060
Xue G et al (2014) Comparative genome analysis reveals metabolic versatility and environmental adaptations of sulfobacillus thermosulfidooxidans strain ST. PloS One 9:e99417
Zeeshan M, Murugadas A, Ghaskadbi S, Ramaswamy BR, Akbarsha MA (2017) Ecotoxicological assessment of cobalt using Hydra model: ROS, oxidative stress, DNA damage, cell cycle arrest, and apoptosis as mechanisms of toxicity. Environ Pollut 224:54–69. https://doi.org/10.1016/j.envpol.2016.12.042
Zeng G, Deng X, Luo S, Luo X, Zou J (2012) A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries. J Hazard Mater 199–200:164–169. https://doi.org/10.1016/j.jhazmat.2011.10.063
Zeng G, Luo S, Deng X, Li L, Au C (2013) Influence of silver ions on bioleaching of cobalt from spent lithium batteries. Miner Eng 49:40–44. https://doi.org/10.1016/j.mineng.2013.04.021
Zhang RY, Xia JL, Peng JH, Zhang Q, Qiu GZ (2010) A new strain Leptospirillum ferriphilum YTW315 for bioleaching of metal sulfides ores. Trans Nonferrous Metals Soc China 20:135–141
Zheng C et al (2018) Effects of cadmium exposure on expression of glutathione synthetase system genes in Acidithiobacillus ferrooxidans. Extremophiles 22:895–902. https://doi.org/10.1007/s00792-018-1046-3
Acknowledgements
This study was supported by the Open Project Funding of the State Key Laboratory of Bioreactor Engineering of China, the National High Technology Research and Development Program of China (nos. 2007AA060904 and 2012AA061503) and the National Natural Science Foundation of China (NSFC21878083).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by I. Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, W., Li, X., Zhang, X. et al. Characteristics of oxidative stress and antioxidant defenses by a mixed culture of acidophilic bacteria in response to Co2+ exposure. Extremophiles 24, 485–499 (2020). https://doi.org/10.1007/s00792-020-01170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-020-01170-4