Abstract
Microbial proteolytic enzyme is one of the most important industrial enzymes that hydrolyze proteins. The applications of proteases under harsh industrial conditions like alkalinity, salinity, and temperature make them inactive and unstable. This suggests need for search for novel microbial sources for protease production having diverse properties. For this purpose, 54 bacterial strains were isolated from different salt mines of Karak, Pakistan and were investigated for their proteolytic activity on skim milk agar plates. The strain which showed maximum protease activity was characterized by 16S rRNA gene sequence analysis. Furthermore, growth and protease production was optimized for the characterized bacteria under different physical factors, i.e., pH, temperature and salinity. The isolate BLK-1.5 exhibited strong protease production and was identified as Bacillus subtilis based on biochemical characteristics and 16S rRNA gene sequence analysis. Maximum production of protease was recorded at pH 10, 37 °C and 7 % (w/v) NaCl. Molecular weight of proteases was estimated 38 kDa and its optimum activity was observed at pH 10, 50 °C and 2 % (w/v) NaCl. In conclusion, the protease produced by halo-tolerant Bacillus subtilis strain BLK-1.5 has diverse characteristics and could be useful in various industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteases have gained attention from researchers throughout the world because of their extensive role in analytical, physiological and industrial applications. Microbial proteases are preferred due to the relative ease of large scale production of proteases from microbial sources as compared to plant and animal sources and they possess almost all the characteristics required for their industrial applications (Beg et al. 2002). Moreover, microbes require minimum space for cultivation, fast growth, higher productivity with low cost and can be easily genetically manipulated to produce new enzymes with altered properties (Gupta et al. 2002; Rao et al. 1998). Microbial proteases are one of the most important groups of industrial enzymes with broad application including detergents, cheese making, waste water management, silver recovery and de-hairing of leather. The majority of commercial alkaline proteases are produced by bacteria and a number of Bacillus-derived alkaline proteases have been purified and characterized because of their significant proteolytic activity, stability and broad substrate specificity (Haddar et al. 2009). However, these proteases have restricted range of pH, temperature and ionic strength for their activity and the use of these enzymes under harsh industrial conditions make them unstable and inefficient (Jadhav et al. 2013). In view of these restrictions, researchers have focused on isolation and characterization of enzymes from extremophiles. As a result of adaptation to extreme environments, extremophiles have evolved unique properties, which can potentially serve in a variety of industrial applications (Margesin and Schinner 2001). Extreme-halophiles have been proven to be a rich source of biotechnological products such as biopolymers, biosurfactants, anti-tumor drugs, and enzymes produced by halophilic bacteria (Vijay et al. 2010). Extremozymes, the enzymes isolated from extremophiles are now replacing chemical catalysts in many industries and out of the vast pool of extremozymes, halophilic proteases are the most widely exploited enzymes in the processing of food, leather, and detergents. Protease production has been shown in some species of halophiles such as Pseudoalteromonas sp., Halobacterium mediterranei, Bacillus clausii and other halophilic isolates (Jadhav et al. 2013). In all the cases, the growth and protease production and its activity were exclusively observed in the presence of salts.
The remarkable industrial and commercial value of halophilic proteases and the search for new microbial sources for these enzymes is of continuous interest. With this in view, we have isolated a halo-tolerant bacterium (Bacillus subtilis strain BLK-1.5) from salt mines of Karak, Pakistan. Furthermore, we optimized its protease production and stability under different physical factors like pH, temperature and salinity.
Materials and methods
Collection of soil samples and isolation of bacterial isolates
Soil samples were collected from different salt mines and rocks around salt mines of District Karak, in sterile bottles (Fig. 1). The collected samples were serially diluted in autoclaved water and 10−3, 10−6, and 10−9 dilutions were spread over the surface of nutrient agar plates with 5 % (w/v) NaCl and incubated at 37 °C for 48–72 h (Jadhav et al. 2013). All the isolated colonies having different culture characteristics were sub cultured several times by repeated streaking at the same condition to get pure colonies.
Screening of the isolates for extracellular proteases
Fifty-four (54) pure isolates were screened for their proteolytic activities on nutrient agar plates supplemented with 5 % (w/v) NaCl and 1 % (w/v) skim milk. Bacterial isolates were incubated at 37 °C for 48–72 h. Bacterial colonies which showed clear zone of hydrolysis were considered as protease producing bacteria. Among all bacteria, the strain BLK-1.5 exhibited largest zone of hydrolysis which was then selected for further studies.
Identification of the promising protease producing bacterial isolate
Morphological and biochemical characteristics of the selected bacterial isolate (BLK-1.5) was studied and recorded as per Bergey’s Manual of Systematic Bacteriology (Buchanan and Gibbons 1974. The bacterial isolate was further identified through 16S rRNA sequence analysis. The genomic DNA of the isolate was extracted as described by Roohi et al. (Roohi et al. 2012). The 16S rRNA gene of the isolate was amplified using a pair of oligonucleotide primer 9F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1510R (5′-GGCTACCTTGTTACGA-3′) (Roohi et al. 2012). The PCR was performed using optimized PCR program; initial denaturation was carried out at 94 °C for 2 min, annealing temprature was 52 °C for 1 min and extension was at 72 °C for 2 min. The final extension was performed at 72 °C for 10 min and the reaction was repeated for 35 cycles.
The PCR amplified product was sequenced using the commercial services of MACROGEN, Korea (http://dna.macrogen.com/eng/). The sequence was BLAST searched on EZ-Taxon Server to get the exact nomenclature of the isolate (Chun et al. 2007). Phylogenetic tree was constructed with MEGA 6.0 using neighbor-joining method with a bootstrap value of 1000 (Tamura et al. 2013).
Effect of pH, temperature and salinity on the growth and protease production
The strain BLK-1.5 was grown in 250 ml pre-sterilized flasks containing about 50 ml LB culture media with different physical factors, i.e., temperature, pH, and salinity. The effect of pH on bacterial growth and protease production (µg/ml) was determined by growing the isolate in LB culture medium with different pH in the range of 7–11. Effect of temperature was determined by incubating the culture at different temperatures (20, 30, 37, 45, and 50 °C). Similarly, effect of salinity (NaCl) was determined using different concentrations (2, 5, 7, 10, and 12 % (w/v)) of NaCl in the culture media. After 48 h of incubation the bacterial growth was estimated by spectrophotometer at 600 nm (OD) and the production of proteases was measured as per assay procedure.
Assay was performed as described by Yang and Haung (1994) with some modifications. The proteolytic activity of the crude proteases was studied by incubating the reaction mixture of 500 μl of 2 % (w/v) casein solution (as a substrate) in phosphate buffer (pH 7.5) with 200 μl of crude protease at 30 °C for 30 min. The reaction was then terminated via addition of 3 ml of 10 % (w/v) trichloroacetic acid (TCA) and kept at room temperature for about 10 min followed by centrifugation at 17,900 rcf for 5 min to separate the unreacted casein. The supernatant was mixed with 2 ml of 0.4 M Na2CO3 and 1 ml of threefold diluted Follin Ciocalteu’s phenol reagent. The solution was incubated at room temperature for about 30 min and absorbance of the solution was measured at 660 nm using spectrophotometer. One unit of protease activity was defined as the amount of enzyme required to liberate 1 µg of tyrosine per minute per ml under the specific conditions of assay.
Partial purification of proteases
The crude enzyme preparation was subjected to ammonium sulfate precipitation, and the harvested culture was centrifuged at 10,600 rcf for 30 min at 4 °C. Ammonium sulfate was added slowly to the cell-free culture at 70 % saturation to precipitate the protease with continuous shaking. The precipitated protease was separated by centrifugation at 10,600 rcf for 30 min and the resultant pellet was dissolved in 0.5 nmol/L phosphate buffer pH 7 and was used for further characterizations (Suganthi et al. 2013).
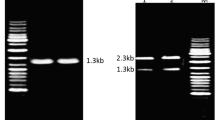
SDS-PAGE (Sodium Dodecyl Polyacrylamide Gel Electrophoresis)
The purity and molecular weight of the protease was estimated using 12 % SDS-PAGE under reducing conditions as described by Laemmli (1970). The partially purified protease enzyme was mixed with loading dye (0.5 M Tris–HCl pH 8.0, 6 % (w/v) 2-β-marcaptoethanol, 10 % (v/v) Glycerol, 6 % (w/v) SDS and 0.3 % (v/v) Bromophenol blue) and heated in water bath for 5 min before use. Protease enzyme was run with Thermo Scientific PageRuler Prestained Protein Ladder (10–170 kDa) at 120 V for 2–2.5 h. After completion of electrophoresis, the gel was stained with Coomessie Brilliant Blue for 30 min following de-stained for about 2.5 h. The picture of clear bands of proteins was captured in camera.
Protease activity optimization
Optimization of pH for the activity of proteases was determined using buffered substrate of different pH (7, 8, 9, 10, 11, and 12). The enzyme substrate reaction in the same manner was incubated at varying temperature (20, 30, 40, 50, and 55 °C). Protease activity was also determined at various NaCl concentrations (1, 2, 3, 4, and 5 % (w/v)) and the proteolytic activity was measured as per assay procedure.
Statistical analysis
All assays were performed in triplicate and their means and the standard deviation (SD) among the replicates were calculated by Microsoft Excel Program (2007). Least Statistical Differences (LSD) were obtained using AOV in statistix 9.0 software.
Results and discussion
Isolation and screening of protease producing isolates
In the present study, 54 bacterial strains were isolated and purified on the bases of their cell morphology through repeated streaking from salt mines of Karak, Pakistan. All the 54 bacterial isolates were screened for proteolytic activity on skim milk agar plates in which 23 (42.5 %) bacterial strains showed proteolytic activity ranging from small clear zones to large clear zones around their colonies. The use of skim milk agar media for the screening of proteolytic bacterial isolates has also been reported by some earlier researchers (Nihalani and Satyanarayana 1992; Gessesse and Gashe 1997). Among these, the strain BLK-1.5 exhibited prominent clear zone of hydrolysis around the colony indicating that it secretes significant amounts of protease and was selected for further study.
Identification of the isolate BLK-1.5
The strain BLK-1.5 was rod shaped, spore forming and tentatively identified as Bacillus sp. based on its morphological and biochemical characteristics (Table 1). Subsequently, the comparison of the 16S rRNA gene nucleotide sequence of the strain BLK-1.5 with other 16S rRNA genes sequences of closely related strains by EZ-Taxon server showed that the strain BLK-1.5 has 98 % sequence homology with Bacillus subtilis subsp. Spizizenii strain NRRL B-23049 (Accession No. CP002905). The phylogenetic tree, constructed by the neighbor-joining method indicated that the strain BLK-1.5 is affiliated with the genus Bacillus and closely related to Bacillus subtilis subsp. Spizizenii strain NRRL B-23049 (Fig. 2). The result was in agreement with those of Roohi et al. (2012), who identified different bacterial strains affiliated with the genera Bacillus, Halobacillus and many other genera from salt mines of Karak. Similarly, Rohban et al. (2009) also identified Bacillus species from Howz soltan Lake, Iran. It is known that the majority of the commercially available alkaline proteases are produced by Bacillus species (Jellouli et al. 2009).
Effects of pH, temperature and salinity on the isolate BLK-1.5 growth and protease production
Different pH values showed different effect on protease yield and growth of the B. subtilis strain BLK-1.5 and maximum yield of protease (37.8 µg/ml) was observed at pH 10 while it grows best (OD: 0.43) at pH 9 (Fig. 3a). These findings indicated that there is no direct relation between the production of proteases and growth. The reason could be due to the fact that the protease produced by that isolate is alkaliphilic. Khan et al. (2011) also observed this relationship that, B. tequilensis showed optimal growth at pH 7 whereas maximum enzyme production was achieved at pH 10. The effect of incubation temperature on the growth and production of proteases showed that Bacillus subtilus strain BLK-1.5 achieved maximum growth (OD: 0.32) at 37 °C and it also yielded maximum (32.1 µg/ml) protease at 37 °C (Fig. 3b). These results were in accordance with those of Sharmin et al. (2005), who observed the highest growth and maximum proteolytic activity at 37 °C for Bacillus amovivorus and Shumi et al. (2004), who observed maximum growth and protease production at 37 °C for B. fastidious. Similarly, maximum protease (30.3 µg/ml) was produced at 7 % (w/v) NaCl concentration, same as the highest growth (OD: 0.41) which was also achieved at 7 % (w/v) NaCl concentration by B. subtilis strain BLK-1.5 (Fig. 3c). Nisha and Divakaran (2014) also observed highest yield of proteases at 7 % (w/v) NaCl from Bacillus subtilis isolated from sea water.
Effect of pH (a), temperature (b), and NaCl concentration (c) on protease production and growth of Bacillus subtilis strain BLK-1.5. Each value is an average of three replicates; bars on figures indicate SD among the replicates. Letters on bars represents LSD which differs significantly from each other at P ≤ 0.05, based on AOV in Statistix 9.0 software
Partial purification of proteases
Cell-free alkaline proteases in the supernatant broth produced by Bacillus subtilis strain BLK-1.5 was partially purified by 70 % (w/v) saturation level of ammonium sulfate. SDS-PAGE analysis showed that the protease purified from Bacillus subtilis strain BLK-1.5 was about 38 kDa (Fig. 4). Commonly, the molecular weights of different alkaline proteases produced by different Bacillus species have been reported in the range between 17 to 50 kDa (Tang et al. 2004; Yossan et al. 2006).
Activity optimization of protease produced by the isolate BLK-1.5
Some of the fundamental characteristics of the purified proteases produced by Bacillus subtilis strain BLK-1.5 were studied to find out the optimum pH, temperature and NaCl concentration for its activity. The most important feature of partially purified proteases was its activity in a wide range of pHs, 8–12, that showed their alkaline nature and the optimal activity of partially purified proteases was observed at pH 10 (Fig. 5a). The maximum activities of commercially important proteases isolated from microbial sources in the alkaline pH range of 8–12 were also reported by some earlier researchers (Gupta et al. 2002; Rao et al. 1998). The optimum pH 10 for activity of alkaline proteases from a variety of Bacillus species has also been reported by a number of researchers (Uchida et al. 2004; Dodia et al. 2008). The maximum proteolytic activity was observed at 50 °C when tested at their optimum pH for activity using casein as a substrate (Fig. 5b).These results were in conformity with those of Moradian et al. (2006), who reported optimum proteolytic activity of proteases produced by Bacillus strain KR-8102 at 50 °C isolated from soil samples collected from the north and west part of Iran. Similarly, the optimal NaCl concentration for activity of the proteases was evaluated which revealed that the activity of proteases was optimum at 2 % (w/v) NaCl concentration (Fig. 5c). Sivaprakasam et al. (2011) also observed optimum activity of a halo-tolerant protease isolated from Pseudomonas aeruginosa strain BC1 at 2 % (w/v) of NaCl concentration. Similarly, Jadhav et al. (2013) reported that protease was stable and showed activity at 5 % (w/v) NaCl produced by a Bacillus sp. The same results were reported in some previous literature for proteases produced by halo-tolerant bacteria (Sanchez-Porro et al. 2003). These findings indicated that the extracellular proteases produced by Bacillus subtilis strain BLK-1.5 isolated from saline soil samples of District Karak, Pakistan express varying characteristics.
Effect of different pH (a), temperature (b), and NaCl concentrations (c) on partially purified protease activity. Each value is an average of three replicates; bars on figures indicate SD among the replicates. Letters on bars represents LSD which differs significantly from each other at P ≤ 0.05, based on AOV in Statistix 9.0 software
These results showed that Bacillus subtilis strain BLK-1.5 isolated from salt mines of Karak, Pakistan is a good producer of extracellular proteases in an alkaline medium and able to function in broad pH and temperature ranges. This might be an indication that Bacillus sp. reported in this study would produce alkaline proteases which could find applications in industrial and biotechnological research.
Conclusion
In the present study, we have isolated Bacillus subtilis strain BLK-1.5 from salt mines of Karak, Pakistan and investigated the optimal physical parameters for highest protease production. The isolate grew and produced protease in the range of 5–12 % (w/v) NaCl indicating its halo-tolerant nature. Furthermore, the protease was active and stable at 50 °C, pH 10 and 2 % (w/v) NaCl. Therefore, these characteristics of the protease suggest that it can be an interesting candidate for application in different industries like textile, food, pharmaceutical, and detergent.
References
Beg QK, Saxena RK, Gupta R (2002) Kinetic constants determination for an alkaline protease from Bacillus mojavensis using response surface methodology. Biotechnol Bioeng 78:289–295
Buchanan RE, Gibbons NE (1974). In: Bergey’s Manual of Determinative Bacteriology 8th edn. p 15–36
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Dodia MS, Rawal CM, Bhimani HG, Joshi RH, Khare SK, Singh SP (2008) Purification and stability characteristics of an alkaline serine protease from a newly isolated Haloalkaliphilic bacterium sp. AH-6. J Ind Microbiol Biotechnol 35(2):121–131
Gessesse A, Gashe BA (1997) Production of alkaline protease by an alkaliphilic bacteria isolated from an alkaline soda lake. Biotechnol Lett 19:479–481
Gupta R, Beg Q, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Haddar A, Agrebi R, Bougatef A, Hmidet N, SellamiKamoun A, Nasri M (2009) Two detergent stable alkaline serineproteases from Bacillus mojavensis A21: purification, characterization and potential application as a laundry detergent additive. Bioresour Technol 100:3366–3373
Jadhav AG, Jaybhaye AA, Musaddiq M (2013) Salt tolerant protease produced by an aerobic species belonging to the Bacillus genus isolated from Saline Soil. Int J Sci Res Publ 3:478–484
Jellouli K, Bougatef A, Manni L, Agrebi R, Siala R, Younes I, Nasri M (2009) Molecular and biochemical characterization of an extracellular serine-protease from Vibrio metschnikovii J1. J Ind Microbiol Biotechnol 36:939–948
Khan I, Gupta P, Vakhlu J (2011) Thermo-alkaliphilic halo-tolerant detergent compatible protease of Bacillus tequilensis MTCC 9585. Afr J Microbiol Res 5:3968–3975
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Margesin R, Schinner F (2001) Potential of halo-tolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Moradian F, Khajeh K, Naderi-Manesh H, Ahmadvand R, Sajedi RH, Sadeghizadeh M (2006) Thiol-dependent serine alkaline proteases from Bacillus sp. HR-08 and KR-8102. Appl Biochem Biotechnol 134:77–87
Nihalani D, Satyanarayana T (1992) Isolation and characterization of extracellular alkaline enzyme producing bacteria. Indian J Microbiol 32:443–449
Nisha NS, Divakaran J (2014) Optimization of alkaline protease production from Bacillus subtilis NS isolated from sea water. Afr J Biotechnol 13:1707–1713
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Rohban R, Amoozegar MA, Ventosa A (2009) Screening and isolation of halophilic bacteria producing extracellular hydrolyses from Howz Soltan Lake, Iran. J Ind Microbiol Biotechnol 36:333–340
Roohi A, Ahmed I, Iqbal M, Jamil M (2012) Preliminary isolation and characterization of halotolerant and halophilic bacteria from salt mines of Karak, Pakistan. Pak J Bot 44:365–370
Sanchez-Porro C, Mellado E, Bertoldo C, Antranikian G, Ventosa A (2003) Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. strain CP76. Extremophiles 7:221–228
Sharmin S, Hossain MT, Anwar MN (2005) Isolation and characterization of a protease producing bacteria Bacillus amovivorus and optimization of some factors of culture condition for protease production. J Biol Sci 5:358–362
Shumi W, Hossain MT, Anwar MN (2004) Proteolytic activity of a bacterial isolate Bacillus fastidiosus den Dooren de Jong. J Biol Sci 4:370–374
Sivaprakasam S, Dhandapani B, Mahadevan S (2011) Optimization studies on production of a salt-tolerant protease from Pseudomonas aeruginosa strain BC1 and its application on tannery saline wastewater treatment. Braz J Microbiol 42:1506–1515
Suganthi C, Mageswari A, Karthikeyan S, Anbalagan M, Sivakumar A, Gothandam KM (2013) Screening and optimization of protease production from a halotolerant Bacillus licheniformis isolated from saltern sediments. J GenetEng Biotechnol 11:47–52
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tang XM, Lakay FM, Shen W, Shao WL, Fang HY, Prior BA, Zhuge J (2004) Purification and characterisation of an alkaline protease used in tannery industry from Bacillus licheniformis. Biotechnol Lett 26:1421–1424
Uchida H, Kondo D, Yamashita S, Tanaka T, Tran LH, Nagano H, Uwajima T (2004) Purification and properties of a protease produced by Bacillus subtilis CN2 isolated from a Vietnamese fish sauce. World J Microbiol Biotechnol 20:579–582
Vijay AS, Hemapriya J, joseph S, Shegal K (2010) Production and optimization of haloalkaliphilic protease by an extremophile halobacterium sp. Js1 Isolated from Thalassohaline Environment. Glob J Biotechnol Biochem 5:44–49
Yang SS, Huang CI (1994) Protease production by amylolytic fungi in solid state fermentation. J Chin Agric Chem Soc 32:589
Yossan S, Reungsang A, Yasuda M (2006) Purification and characterization of alkaline protease from Bacillus megaterium isolated from Thai fish sauce fermentation process. Sci Asia 32:377–383
Acknowledgments
We are grateful to the Directorate of Science and Technology (DoST) Peshawar, Khyber Pakhtunkhwa for financial support and the Department of Biotechnology and Genetic Engineering, KUST for providing laboratory facilities to carry out the present research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. da Costa.
Rights and permissions
About this article
Cite this article
Ali, N., Ullah, N., Qasim, M. et al. Molecular characterization and growth optimization of halo-tolerant protease producing Bacillus Subtilis Strain BLK-1.5 isolated from salt mines of Karak, Pakistan. Extremophiles 20, 395–402 (2016). https://doi.org/10.1007/s00792-016-0830-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-016-0830-1