Abstract
The gene encoding for a novel cold-adapted enzyme from family II of bacterial classification (GDSL family) was cloned from the genomic DNA of Photobacterium sp. strain J15 in an Escherichia coli system, yielding a recombinant 36 kDa J15 GDSL esterase which was purified in two steps with a final yield and purification of 38.6 and 15.3 respectively. Characterization of the biochemical properties showed the J15 GDSL esterase had maximum activity at 20 °C and pH 8.0, was stable at 10 °C for 3 h and retained 50 % of its activity after a 6 h incubation at 10 °C. The enzyme was activated by Tween-20, -60 and Triton-X100 and inhibited by 1 mM Sodium dodecyl sulphate (SDS), while β-mercaptoethanol and Dithiothreitol (DTT) enhanced activity by 4.3 and 5.4 fold respectively. These results showed the J15 GDSL esterase was a novel cold-adapted enzyme from family II of lipolytic enzymes. A structural model constructed using autotransporter EstA from Pseudomonas aeruginosa as a template revealed the presence of a typical catalytic triad consisting of a serine, aspartate, and histidine which was verified with site directed mutagenesis on active serine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esterases are ubiquitous enzymes playing important physiological and biotechnological roles in agriculture, food, beverage, perfume and pharmaceutical industries (Panda and Gowrishankar 2005). Unlike lipases which display maximal activity toward water-insoluble long chain triglycerides, esterases can synthesize or hydrolyse soluble fatty-acid esters with acyl chain length of less than 10 C atoms (Jaeger et al. 1999; Yang et al. 2013). Therefore, newly-found esterases are potentially useful for industrial processes.
In the classification of lipolytic enzymes, the GDSL enzymes are grouped in family II which have multifunctional properties such as broad substrate specificity and regiospecificity due to their active site flexibility. Due to these multiple functions, GDSL lipases and esterases could be used in the hydrolysis and synthesis of abundant ester compounds of pharmaceutical, food, biochemical and biological interest (Messaoudi et al. 2010). Esterases contain the pentapeptide motif GXSXG with the serine active site situated at the centre of the conserved pentapeptide (Akoh et al. 2004). GDSL esterases containing the active site serine residue located near the N-terminus are grouped in family II (Arpigny and Jaeger 1999), and a subgroup is classified as the SGNH-hydrolase family due to the presence of four conserved residues, Ser, Gly, Asn and His, in four conserved blocks (I-V) (Akoh et al. 2004; Dalrymple et al. 1997; Li et al. 2000; Mølgaard et al. 2000).
GDSL hydrolytic enzymes are widely found in microbes and plant microorganisms. To date, a number of bacterial GDSL family genes have been cloned and characterized from Escherichia coli, Pseudomonas fluorescens, Vibrio mimicus, Salmonella typhimurium, Streptomyces rimosus, and Xanthomonas vesicatoria (Akoh et al. 2004; Ling et al. 2006). Reports on cold-adapted enzymes produced by psychrophilic microorganisms has been significantly rising in the past few years due to increasing interest in their unique properties exploited in a broad range of medical processes, agriculture and biotechnology industry such as, in the detergent and food industries, for the production of fine chemicals and in bioremediation processes (Cavicchioli et al. 2002; Gerday et al. 2000). The main sources for cold adapted enzymes such as lipase, esterase, protease and cellulase are bacteria, fungi (in particular yeasts) and microalgae. Among the bacteria that have been identified, the most commonly reported microorganisms are Pseudomonas spp., Vibrio spp. and Photobacterium spp., Coryneforms spp., Arthrobacter sp. and Micrococcus sp. (D’Amico et al. 2006).
So far, many GDSL enzymes from different pshycrophilic bacteria have been characterized. More so, several esterases with pshychrophilic origins and known amino acid sequences have been biochemically characterized. From reported cold-adapted GDSL esterase, the optimum temperatures of cold adapted GDSL esterases are in the range of 30–45 °C with optimum pH 8 (Cieśliński et al. 2007; Kulakova et al. 2004). Moreover, in the aspect of substrate specificity these esterases show preference for pNP esters with short-chain fatty acids ranging from C2 to C8 (Cieśliński et al. 2007; Suzuki et al. 2003).
In this study, the first reported cold-adapted GDSL esterase from Photobacterium sp. strain J15 was cloned, purified and characterized. Sequence analysis revealed this enzyme was a novel member of the SGNH hydrolase superfamily, exhibiting special catalytic properties such as higher activity at low temperature.
Materials and methods
Bacterial strains and plasmid
Photobacterium sp. strain J15 (UPMC 398, DSM25402) was obtained from a stock culture provided by the Enzyme and Microbial Technology Research Center (UPM) which had been previously isolated from seawater (Johor, Malaysia). The expression vector pET-32b(+) (Merck, Germany) was used to construct the recombinant plasmids. E. coli TOP10, BL21(DE3)pLysS and Rosetta-gami (DE3)pLysS (Novagen, USA) were used as cloning and expression hosts respectively.
Analysis of J15 GDSL esterase
Homology searches were performed using the Blastp at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple sequence alignments were performed using CLASTALW, which were analysed using alignment shading software (TEXSHADE in Biology WorkBench.3.2, http://www.workbench.sdsc.edu). Phylogenetic analysis was performed by the neighbor joining (NJ) method using the Molecular Evolutionary Genetics Analysis (MEGA, version 5) software (www.megasoftware.net).
Cloning of the J15 GDSL esterase
The cloning of J15 GDSL esterase was obtained by PCR. Based on the J15 GDSL esterase genomic sequence obtained from the Photobacterium sp. J15 strain, the degenerated primers (PJ15-F: 5′-CTCCATGGTTGCAGAATTCGACCGCAT-3′; and PJ15-R: 5′-GATGCTGAGCTCATAATGTCCTTCCGCTTAT-3′) with restriction endonuclease NcoI and Bpu 1102I sites in the forward and reverse primers respectively, were used to amplify the GDSL esterase gene sequence using pfu DNA polymerase. The amplified gene with the expected size was gel-purified, cloned into pET-32b(+) and transformed into E. coli TOP10 for cloning and sub-cloned into BL21(DE3)pLysS and Rosetta-gami (DE3)pLysS for heterologous expression. Expression was optimized by inoculating recombinant E. coli Rosetta-gami (DE3)pLysS harbouring the pET-32b/J15 esterase vector into 200 mL LB broth (in a 1L Schott bottle) supplemented with 50 µg/mL ampicillin and 34 µg/mL chloramphenicol at different temperatures (10–37 °C), inducer concentrations (IPTG) (0–2 mM), and induction times (0–40 h).
Purification of the J15 GDSL esterase
A volume (200 mL) of culture grown at 20 °C for 16 h was harvested by centrifugation at 10,000×g for 10 min at 4 °C and resuspended in 25 mL of binding buffer (20 mM phosphate buffer, 20 mM imidazole, 300 mM NaCl, pH 7.4). The suspended cells were sonicated (Branson 250 sonifier, USA, output 2, duty cycle 30 and 4 min) and cleared by centrifugation (10,000×g, 10 min). The crude cell lysate was loaded onto 5 ml Co2+-Sepharose (HisPur Cobalt Resin, Thermo Scientific, Chicago, IL, USA) in an XK column (XK 16/20, GE Healthcare Life Sciences, USA) previously equilibrated with 5 CV of binding buffer (20 mM phosphate buffer, 20 mM imidazole, 300 mM NaCl, pH 7.4). The unbound proteins were removed by washing the column with 10 CV of binding buffer until no protein was detected and bound J15 GDSL esterase was gradient-eluted with elution buffer (20 mM phosphate buffer, 500 mM imidazole, 300 mM NaCl, pH 7.4). The purified J15 GDSL esterase was incubated with thrombin for 8 h at 20 °C to cleave the Trx- and His-tags from the protein and dialyzed against 50 mM phosphate buffer (pH 8.0). The dialyzed protein was loaded onto a 5 ml Q-Sepharose High Performance (GE Healthcare Life Science, USA) packed in an XK column previously equilibrated with 5 CV of binding buffer (50 mM phosphate buffer, pH, 8.0) and washed with buffer. The bound protein was eluted (50 mM phosphate buffer, 1 M NaCl, pH 8.0) and dialyzed against 50 mM phosphate buffer (pH 8.0). Purity and concentration was analysed by SDS-PAGE (Laemmli 1970) and Bradford assays (Bradford 1976) respectively.
Site-directed mutagenesis
A Ser31Ala mutant was generated using the QuikChange® Lightning Site-Directed Mutagenesis Kit (Stratagene, USA) according to the manufacturer’s instructions using mutagenesis primers (Forward- 5′-AACTTCGGTGACGCACTGTCCGACATC-3′ and Reverse- 5′-GATGTCGGACAGTGCGTCACCGAAGTT-3′, modified codon underlined). Polymerase Chain Reaction was carried out in a reaction mixture containing 5 μL of 10× reaction buffer, template (60 ng), 2.5 μL of oligonucleotide primers (10 mM), 1 μL of dNTP mix, 1.5 μL of QuikSolution reagent and ddH2O added to a final volume of 50 μL. QuikChange Lightning Enzyme (1 μL) was added to the mixture and the plasmid amplified for 20 cycles using the following thermal cycling conditions: initial denaturation at 95 °C for 2 min, 18 cycles with a 20 s denaturation at 95 °C, 10 s annealing and 3 min 30 s extensions at 68 °C and a final extension step at 68 °C for 5 min. After digestion of the parental (non-mutated template) with 2 μL Dpn I, the PCR product was transformed into XL10-Gold ultra-competent cells (Stratagene, USA) and sub-cloned into E. coli Rosetta-gami (DE3)pLysS (Novagen, Germany) for esterase gene expression.
Esterase assay
Esterase activity was assayed colorimetrically as described by Zou et al. (2010) with some modifications, using p-nitrophenol butyrate as the substrate, where one unit of esterase activity was defined as 1 µmol of p-nitrophenol liberated per min under standard assay conditions. Substrate specificity was determined by using a variety of pNP esters: pNP acetate (C2), pNP butyrate (C4), pNP caproate (C6), pNP caprylate (C8), pNP decanoate (C10), pNP laurate, (C12), pNP myristate (C14) and pNP paltimate (C16). The assay was carried out in 1 ml reaction mixture containing 50 mM Tris–HCl buffer (pH 7.0), 25 mM substrate dissolved in DMSO and J15 GDSL esterase, incubated for 10 min at 20 °C and liberated p-nitrophenol measured at A 410 and compared to a standard p-nitrophenol curve.
Characterization of the J15 GDSL esterase
The temperature profile of the J15 GDSL esterase was measured at 4 to 45 °C (at 5 °C intervals) for 10 min by using p-nitrophenyl butyrate (C4) as a substrate. Enzyme stability test was conducted by pre-incubating the enzyme for 30 min at various temperatures ranging from 10 to 25 °C for intervals up to 8 h prior to assaying esterase activity at 20 °C (optimum temperature). The effect of pH on esterase activity was assayed at pH 4–12 by measuring the amount of p-nitrophenol liberated. The buffer systems used to assess pH effects were: 50 mM acetate buffer (pH 4–6), 50 mM potassium phosphate buffer (pH 6–8), 50 mM Tris–HCl buffer (pH 8–9), 50 mM glycine-NaOH (pH 9–11) and 50 mM Na2HPO3/NaOH buffer (pH 11–12). pH stability test was performed by pre-incubating the J15 GDSL esterase for 30 min at 10 °C in aforementioned buffers and assessing esterase activity at optimum temperature (as above). J15 GDSL esterase was treated with various salt concentrations ranging from 0 to 4 M at 0.5 M intervals at 10 °C for 30 min and assayed colorimetrically. J15 GDSL esterase activity was also assayed in the presence of various metal ions (Li+, Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Sr2+, Mn2+, Fe3+, Co2+, Ni2+, Zn2+ at 1 mM/5 mM), surfactants (SDS, Tween-20, Tween-60, Tween-80, Triton X-100 at 0.1 %/0.5 %), and inhibitors (Dithiothreito (DTT), Ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), β-mercaptoethanol at 1 mM/5 mM) by pre-incubating for 30 min at 10 °C prior to esterase activity assay.
Structural modelling
Since there were no close structural homologs of the J15 GDSL esterase, modelling was performed based on threading using the Protein Homology/analogY Recognition Engine version 2.0 (Phyre2, London, UK) 3D-structural threading program (http://www.sbg.bio.ic.ac.uk/phyre2/html) and the model assessed by the Yet Another Scientific Artificial Reality Application software (YASARA, Austria). To predict the position and orientation of the p-nitrophenyl acetate and butyrate substrates bound to the J15 GDSL esterase, protein–ligand docking was performed with the YASARA software.
The J15 GDSL esterase nucleotide sequence has been submitted to the GenBank database under the accession number KP730048.
Results
Analysis of the J15 GDSL esterase
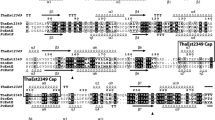
The gene encoding for a putative bacterial esterase was identified by mining the genome of Photobacterium sp. strain J15. Sequence analysis of the J15 GDSL esterase gene showed the open reading frame was 1,044 bp in length which coded for 347 amino acids (Online Resource 1), and the predicted molecular mass and pI were 35.6 kDa and 6.3, respectively. Blast of the J15 GDSL esterase indicated it shared low sequence identity (31 %) with a putative Fischerella sp. JSC-11 GDSL lipolytic family enzyme. Multiple sequence alignments were performed for the J15 GDSL esterase sequence and a selection of SGNH superfamily enzymes derived from various species (Fig. 1). Comparison of the J15 GDSL esterase to other GDSL enzymes revealed the presence of a putative Ser-containing GDSL motif near the N-terminus and four highly-conserved blocks containing serine, glycine, asparagine, and histidine in blocks I, II, III, and V, respectively (Upton and Buckley 1995). Due to the presence of these four conserved residues (Ser, Gly, Asn, and His), the enzyme was further classified as a SGNH hydrolyse (Mølgaard et al. 2000). The critical residues for enzyme activity, assigned by comparison with other SGNH proteins, included the catalytic triad (Ser31, Asp321, and His324) and the oxyanion hole residues (Ser31, Gly124, and Asn180) which appeared in positions similar to those of other GDSL hydrolases in this family. Therefore J15 GDSL esterase can be classified as a novel member of SGNH family hydrolases.
Multiple sequence alignment of J15 GDSL esterase. The sources of GDSL esterases were: Photobacterium johorimaris J15 (J15 GDSL esterase) Rhodoferax ferrireducens T118 (accession no.YP_523580), Fischerella sp. JSC-11 (accession no. WP_009454214), Nostoc punctiforme PCC 73102 (accession no.YP_001869007), Oscillatoria acuminata PCC 6304 (accession no.YP_007086388), Microcoleus vaginatus FGP-2 (accession no.ZP_08494420), Chroococcidiopsis thermalis PCC 7203 (accession no.YP_007091764), Microcystis sp. T1-4 (accession no. ZP_10227436), Beggiatoa alba B18LD (accession no. ZP_10113749), and Nitrococcu smobilis Nb-231 (accession no.ZP_01128151)
Phylogenetic tree of the J15 GDSL esterase
The phylogenetic tree of GDSL esterases was constructed to explore the evolutionary relationships between the GDSL esterase from Photobacterium sp. strain J15 and other GDSL hydrolyses across different genera (Fig. 2). Phylogenetic analysis showed the J15 GDSL esterase was a novel GDSL esterase from family II (GDSL), distantly related to other GDSL hydrolases and could be considered a new SGNH hydrolase.
Phylogenetic analysis GDSL esteraseJ15 from Photobacterium johorimaris strain J15. The phenogram showed the tree view of GDSL hydrolyses across different families. The phylogenetictree was constructed using by the method of Neighbor Joining (NJ) using MEGA5 software. The numbers at the nodes are bootstrap confidencelevels (percentage) from 1000 replicates. The scale bar represents 0.1 substitutions per amino acid position
Expression and purification of the J15 GDSL esterase
The gene encoding for the J15 GDSL esterase was amplified by PCR, cloned into the pET-32b(+) vector and transformed into E. coli BL21(DE3)pLysS for expression, though no expression was observed. Due to unsuccessful expression of the recombinant pET32b/J15 esterase plasmid in BL21(DE3)pLysS, the nucleotide sequence of the J15 GDSL esterase was analysed, which indicated there were five rare codons (three Leu, one Arg, and one Ile) in the sequence. The recombinant plasmid was transformed into an E. coli strain Rosetta-gami(DE3)pLysS expression host to supply the tRNAs for these rare codons and to facilitate high level expression of the gene containing rare E. coli codons. The protein was expressed successfully as a monomeric protein. The expression conditions were optimized and the best expression was found for 0.1 mM IPTG at 20 °C for 16 h. The J15 GDSL esterase was purified using two steps chromatography. Besides the recombinant fusion J15 GDSL esterase, some impurities were also co-eluted from the Co2+-Sepharose affinity column (Fig. 3, lane 2). The enzyme was further purified using Q-Sepharose HP ion exchange chromatography after cleavage of the fusion J15 GDSL esterase with thrombin (Fig. 3, lane 4), a final recovery of 38.6 % and a 15.3-fold of purification were obtained. Approximately 0.915 mg of recombinant J15 GDSL esterase was obtained from 200 ml of bacterial culture.
SDS-PAGE analysis of recombinant J15 GDSL esterase purified using two steps. Lane M, protein molecular weight marker: β-galactosidase (116 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45 kDa), lactate dehydrogenase (35 kDa), restriction endonuclease Bsp 98 l (25 kDa), and β-lactoglobulin (18.4 kDa), lysozyme (14.4 kDa); lane 1, crude protein; lane 2, His-tagged GDSL esterase after affinity chromatography; lane 3, His-tagged J15 GDSL esterase after cleavage with thrombin; lane 4, J15 GDSL esterase after IEX chromatography; lane 5, Western blot analysis of J15 GDSL esterase
Characterization of the J15 GDSL esterase
The substrate specificities of the J15 GDSL esterase were determined at 20 °C using pNPs with different chain lengths (C2–C16) as substrates, where the esterase preferentially hydrolysed short-length acyl chain lengths substrates. The highest specific activity (45.48 U/mg) was detected for pNP butyrate (C4) (P > 0.05), while the specific activity towards pNP butyrate (C4) was approximately twofold higher than that of pNP acetate (C2). However, for substrates with chain lengths longer than C6, no esterase activity was observed. The catalytic activity of the J15 GDSL esterase towards substrates with short-chain acyl groups is a characteristic of esterases (not lipases), a finding consistent with the fact that most GDSL-motif proteins are esterases (Yu Shanshan 2010).
The effect of temperature on the J15 GDSL esterase activity was determined by using p-nitrophenyl butyrate (C4) as the substrate at 4–45 °C, where the protein was active at 4–30 °C with an optimum temperature of 20 °C, suggesting its marine origin (derived from Photobacterium sp. strain J15 isolated from marine environments at 28–30 °C) (Fig. 4a). By comparison, enzyme activity was 15 % at 37 °C. The stability of the enzyme was tested at 10–25 °C and the half-lives at 10, 15, 20, and 25 °C were 6, 4, 3 h, and 20 min, respectively, while the esterase was completely deactivated after 3 h at 25 °C. Semilog plot of residual activity versus treatment time was linear, indicating that the inactivation is first order over this range (Fig. 4b).
Effect of temperature and pH on J15 GDSL esterase activity and stability. a Effect of temperature on esterase activity. The graph shows the temperature profile of recombinant J15 GDSL esterase. b Rate of heat inactivation of J15 GDSL esterase. The ordinate represents the natural log of relative activity, the ratio of activity (A) to the original activity (A 0) before heat treatment. The thermostability of recombinant enzyme was determined by pre-incubating the enzyme in pH 8.0 buffer at different temperatures 10, 15, 20, 25 °C. c pH profile of J15 GDSL esterase. The enzyme was assayed at various pHs from pH 4 to pH 12 by a colorimetric method. d The effect of pH on the stability of the J15 GDSL esterase. GDSL was assayed at various pHs from pH 4 to pH 12 by a colorimetric method. Symbols used are: (), Sodium acetate buffer; (filled square), Potassium phosphate buffer; (filled triangle), Tris–HCl buffer; (X), Glycine buffer; (filled circle), Na2HPO4/NaOH buffer. e Effect of salt concentration on J15 GDSL esterase activity
The effect of pH on esterase activity was investigated at pH 4.0–12.0 (20 °C). The enzyme was active at pH 6–9, with an optimum of 8.0 (Fig. 4c), which was the same as the cold-adapted GDSL esterase PsEst1 from Pseudomonas sp. B11-1, cold-adapted GDSL esterase from Pseudoalteromonas sp. 643A, cold-adapted AEST esterase from Acinetobacter sp. strain No. 6, cold-adapted PsyEst esterase from Psychrobacter sp. Ant300 (Cieśliński et al. 2007), and GDSL esterase from Geobacillus thermodenitrificans T2 (Yang et al. 2013), suggesting the J15 GDSL esterase was an alkaline esterase. Alkali metal ions, especially Na+ stabilize active sites of the enzyme (Schandl and Pittner 1984) thereby playing important role in the activity of the enzyme, which could probably be present in J15 GDSL esterase and bound to its structure. The enzyme also exhibited high stability in buffers at various pH values, particularly pH 6–8 (Fig. 4d). Li+, Rb+, Cs+, Mg2+, Ca2+, Sr2+, Mn2+ and Zn2+ inhibited J15 GDSL esterase activity 1.3–20.9 %, while Na+, K+, Fe3+, Co2+ and Ni2+ enhanced activity 4–32 % (Table 1).
The effects of surfactant and inhibitors on J15 GDSL esterase activity are shown in the Table. Among surfactants, SDS totally inhibited activity, while Tween-20, Tween-60, Tween-80, and Triton X-100 increased the activity of the enzyme significantly over control (P < 0.05). The enzyme activity was reduced 28.8 and 48.7 % by treatment with EDTA and PMSF, respectively, and higher concentrations further inhibited activity. Treatment with DTT and β-mercaptoethanol increased the activity of the enzyme 4-fivefold. The effect of inhibitors on enzyme activity was statistically significant with difference (P < 0.05) between control and various inhibitors except EDTA.
The effect of various salt concentrations on activity indicated a gradual increase with increasing NaCl concentration from 0.5 to 1.5 M, but activity decreased at higher concentrations (Fig. 4e). The high-salt tolerance of the J15 GDSL esterase suggested the enzyme was halophilic, stable and functional in the presence of salt at high concentrations (1.5 M). The amino acid composition of halophilic enzymes is (in general) characterized by abundant acidic amino acids, leading to high aqueous solubility without aggregation in both native and denatured states (Tokunaga et al. 2008).
Structural modelling
The 3D structural model of the J15 GDSL esterase was determined by threading using the Phyre2 3D-structural threading program, as there are no close structure homologs of the GDSL esterase (Kelley and Sternberg 2009). The GDSL esterase model indicated a large domain with α/ß hydrolase folds (Fig. 5a) which showed structural similarity to the Pseudomonas aeruginosa EstA autotransporter (chain X), a GDSL esterase. Comparing the EstA autotransporter from Pseudomonas aeruginosa (chain X) (van den Berg 2010) and J15 GDSL esterase model, Ser31, Asp321 and His324 were located in close proximity, likely representing the active site of J15 GDSL esterase (Fig. 5b). Structures indicated a root mean square deviation (RMSD) value of 0.452 which indicated that despite having diverse sequences, the structures of EstA and the J15 GDSL esterase were similar. In order to confirm the catalytic residues in the J15 GDSL esterase, the Ser in conserved block I was substituted by site directed mutagenesis. The mutation totally abolished the esterase activity, which confirmed the importance of the S31 residue in catalysis. A similar result was reported after mutation of the catalytic triad (Ser, Asp, and His) in GDSL esterases from Geobacillus thermodenitrificans T2 and Fervidobacterium nodosum Rt17-B1 (Yang et al. 2013; Yu Shanshan 2010).
Structural analysis and Protein–ligand docking of J15 GDSL esterase. a Ribbon diagram shows the overall structure of the GDSL esterase model. The N- and C-terminus are labeled as N and C, respectively. b Ribbon diagrams of superimposed polypeptide backbones of J15 GDSL esterase (yellow) and autotransporter EstA from Pseudomonas aeruginosa (PDB ID 3KVN_X, gray). The proposed active-site residues Ser31, Asp321, and His324 of GDSL esterase are shown in stick representation. The catalytic trial residues (Ser14, Asp286, and His289) of Pseudomonas aeruginosa autotransporter EstA are shown in stick representation and are colored gray. Protein–ligand docking (show surface) with p-nitrophenyl acetate (a) and p-nitrophenyl butyrate (b). The hydrogen bonds between serine and substrates are shown by yellow circle
Protein–ligand docking was performed to predict the structure of the intra-molecular complex formed between the J15 GDSL esterase and p-nitrophenyl acetate or butyrate (Fig. 5c, d), where protein–ligand interaction (after clustering 25 runs) showed binding energies of 5.51 and 5.8 kcal/mol for acetate and butyrate, respectively. The protein–ligand docking revealed the binding energy toward pNP butyrate was higher than pNP acetate, as the esterase formed two hydrogen bounds with butyrate and one with pNP acetate.
Compositional analysis of J15 GDSL esterase
Detailed amino acid and structural analyses of the J15 GDSL esterase revealed the esterase was composed of 51.8 % hydrophobic amino acids located in the core of the protein, where Ala and Leu were most abundant at 11.3 and 8.2 % respectively, serving to stabilize the α-helix (Lyu et al. 1991). Uncharged polar residues accounted for 86 amino acids (26.2 %), and among the polar residues, Thr was the most abundant (8.1 %) and found in beta-pleated sheets. Glycine, often found at the surface of proteins within loops providing high flexibility to these regions, accounted for 9.8 % of the total amino acids encountered. Increased flexibility is proposed as the main structural feature of cold-adapted enzymes explaining high specific activity at lower temperatures (Gerday et al. 2000).
To find out more about the characteristic of J15 GDSL esterase (high specific activity at 20 °C), amino acid composition analysis (Online Resource 2) was performed between psychrophilic J15 GDSL esterase, GDSL esterase from mesophilic Pseudomonas aeruginosa (EstA 3KVN_X) and thermophilic GDSL esterase from Fervidobacterium nodosum Rt17-B1 (FNE) (van den Berg 2010; Yu Shanshan 2010). Compositional analysis indicates that Ala is more abundant in J15 GDSL esterase compared to EstA and esterase Rt17-B1. Increased content of Ala particularly at exposed site in coil regions may enhance surface hydrophobicity. Favorable solvation of non-polar surface at low temperatures should destabilize the entire structure which may increase flexibility (Gianese et al. 2001). Furthermore, amino acid compositional analysis demonstrates that Asn is more frequent in J15 GDSL esterase than in meso/thermophilic enzymes. Asn is a thermolabile residue and its increased frequency in psychrophiles was already noted by Xu et al. (1998) in aspartate carbamoyltransferase. In addition, a common adaptive mechanism of enzymes to low temperature consists of reduction of charged residues (mainly Arg, Glu and Lys) (Online Resource 2) at exposed sites in α-helix or coil regions (Gianese et al. 2001). Therefore, the presence of higher amount of Ala, Asn or non-polar residues at exposed site on α-helix in J15 GDSL esterase, enables the enzyme to catalyse hydrolysis reaction efficiently at 20 °C, thus offers advantages for energy saving industrial processes (Marshall 1997).
Pairwise structural alignment (Online Resource 3) indicates that the structure of J15 GDSL esterase was much similar to the EstA Pseudomonas aeruginosa (3KVN_X) as compared to thermophilic GDSL esterase from Fervidobacterium nodosum Rt17-B1. In addition, Solvent accessible surface area (SASA) analysis pointed out that 49.1 % of the residues of J15 GDSL esterase are considered to be buried in the core. However the percentages of buried residues for EstA and FNE were 48.1 and 44.7 % respectively. Therefore it be may assumed that the characteristics of J15 GDSL esterase such as activity at low temperature has been obtained during evolution by decreasing or weakening intramolecular interactions that stabilize the mesophilic counterparts.
Discussion
Multiple sequence alignment between J15 GDSL esterase and selection of SGNH superfamily members showed that J15 GDSL esterase was annotated as a putative SGNH hydrolase. Each of the four residues plays a key role in the catalytic function of the enzyme: the catalytic serine in block I serves as the catalytic nucleophile and proton donor to the oxyanion hole, the glycine in block II and the aspargine in block III serve as two other proton donors to the oxyanion hole and the histidine in block V acts as a base to make the active site serine more nucleophilic by deprotonating the hydroxyl group (Akoh et al. 2004; Ling 2008).
Esterase activity assays proved that J15 GDSL esterase functions as an esterase with preference toward short-chain ester substrates. Similar to other cold-adapted GDSL esterases such as PsEst1 from the psychrotrophic Pseudomonas sp. B11–1 (Suzuki et al. 2003) and EstA psychrophilic Pseudoalteromas sp. strain 643A (Cieśliński et al. 2007), the preferred substrate for the J15 GDSL esterase was pNP butyrate (C4).
The protein structure of cold-adapted enzymes is the key to understanding their function. The flexibility of the enzyme leads to ability of cold-adapted enzymes to function at low temperature. Cold-adapted J15 GDSL esterase had the highest activity at 20 °C which was similar to optimum temperature activity of cold-adapted esterase CHA2 isolated from Antarctic mineral soil metagenomic library (Hu et al. 2012). However, the optimum temperature for the cold-adapted PsyEst esterase from Psychrobacter sp. Ant300 (Kulakova et al. 2004) and EstA from psychrotrophic Pseudoalteromas sp. strain 643A was 35 °C (Cieśliński et al. 2007), while cold-adapted PsEst1 esterase from Pseudomonas sp. B11-1 and AEST esterase from Acinetobacter sp. strain No. 6 were more active at 45 °C (Cieśliński et al. 2007). Moreover, the compositional analysis of J15 GDSL with EstA and esterase Rt17-B1 revealed that all of the active site residues involved in the reaction mechanisms are strictly conserved between enzymes that are adapted to different temperatures. Therefore, the molecular changes responsible for cold adaptation of J15 GDSL esterase have to be found elsewhere in the protein. In addition, the compositional analysis of J15 GDSL esterase with EstA and esterase Rt17-B1 showed that the presence of more non-polar Ala, thermolabile Asn at exposed site on α-helix in J15 GDSL esterase may provide flexibility to J15 GDSL esterase and thus catalysis at lower temperatures.
Conclusion
In this study, the J15 GDSL esterase gene was cloned and overexpressed in a prokaryotic system under the control of the T7 promoter for chemically inducible, high-level expression. It was fused to His-tag to specify and facilitate the purification procedure. The purification condition of J15 GDSL esterase was optimized at two steps purification and the His-tag was removed after second purification step. A study across a wide range of parameters, such as temperature, pH, metal ion, surfactant, substrate, inhibitors, and salt concentration was performed in mature J15 GDSL esterase. The study showed that cold-adapted J15 GDSL esterase had high specific activity at low temperatures (20 °C) and different pH and also buffer compounds had significant effects on J15 GDSL esterase activity. The high-level production and thorough characterization of recombinant cold-adapted GDSL esterase from the locally isolated Photobacterium sp. strain J15 has made a significant contribution in the field of biotechnology as additives in washing machine powder in detergent industry that will work effectively in cold water.
References
Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF (2004) GDSL family of serine esterases/lipases. Prog Lipid Res 43(6):534–552
Arpigny JL, Jaeger KE (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343(Pt 1):177
Bradford MM (1976) Purification and characterization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72(1):248–254
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotechnol 13(3):253–261. doi:10.1016/S0958-1669(02)00317-8
Cieśliński H, Białkowska AM, Długołęcka A, Daroch M, Tkaczuk KL, Kalinowska H, Kur J, Turkiewicz M (2007) A cold-adapted esterase from psychrotrophic Pseudoalteromas sp. strain 643A. Arch Microbiol 188(1):27–36
Dalrymple BP, Cybinski DH, Layton I, McSweeney CS, Xue GP, Swadling YJ, Lowry JB (1997) Three Neocallimastix patriciarum esterases associated with the degradation of complex polysaccharides are members of a new family of hydrolases. Microbiology 143(8):2605
D’Amico S, Collins T, Marx J-C, Feller G, Gerday C (2006) Psychrophilic microorganisms: challenges for life. EMBO Rep 7(4):385–389
Gerday C, Aittaleb M, Bentahir M, Chessa J-P, Claverie P, Collins T, D’Amico S, Dumont J, Garsoux G, Georlette D, Hoyoux A, Lonhienne T, Meuwis M-A, Feller G (2000) Cold-adapted enzymes: from fundamentals to biotechnology. Trends Biotechnol 18(3):103–107. doi:10.1016/S0167-7799(99)01413-4
Gianese G, Argos P, Pascarella S (2001) Structural adaptation of enzymes to low temperatures. Protein Eng 14(3):141–148
Hu XP, Heath C, Taylor MP, Tuffin M, Cowan D (2012) A novel, extremely alkaliphilic and cold-active esterase from Antarctic desert soil. Extremophiles 16(1):79–86
Jaeger K, Dijkstra B, Reetz M (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu Rev Microbiol 53(1):315–351
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protocols 4(3):363–371
Kulakova L, Galkin A, Nakayama T, Nishino T, Esaki N (2004) Cold-active esterase from Psychrobacter sp. Ant300: gene cloning, characterization, and the effects of Gly→Pro substitution near the active site on its catalytic activity and stability. Biochim Biophys Acta (BBA)-Proteins Proteomics 1696(1):59–65
Laemmli U (1970) Most commonly used discontinuous buffer system for SDS electrophoresis. Nature 227:680–685
Li J, Derewenda U, Dauter Z, Smith S, Derewenda ZS (2000) Crystal structure of the Escherichia coli thioesterase II, a homolog of the human Nef binding enzyme. Nat Struct Biol 7(7):555–559
Ling H (2008) Sequence analysis of GDSL lipase gene family in Arabidopsis thaliana. Pak J Biol Sci 11(5):763
Ling H, Zhao J, Zuo K, Qiu C, Yao H, Qin J, Sun X, Tang K (2006) Isolation and expression analysis of a GDSL-like lipase gene from Brassica napus L. J Biochem Mol Biol 39(3):297
Lyu PC, Sherman JC, Chen A, Kallenbach NR (1991) Alpha-helix stabilization by natural and unnatural amino acids with alkyl side chains. Proc Natl Acad Sci 88(12):5317–5320
Marshall CJ (1997) Cold-adapted enzymes. Trends Biotechnol 15(9):359–364
Messaoudi A, Belguith H, Gram I, Hamida JB (2010) Classification of EC 3.1. 1.3 bacterial true lipases using phylogenetic analysis. Afr J Biotechnol 9(48):8243–8247
Mølgaard A, Kauppinen S, Larsen S (2000) Rhamnogalacturonan acetylesterase elucidates the structure and function of a new family of hydrolases. Structure 8(4):373–383
Panda T, Gowrishankar BS (2005) Production and applications of esterases. Appl Microbiol Biotechnol 67(2):160–169. doi:10.1007/s00253-004-1840-y
Schandl A, Pittner F (1984) The role of Na+ and Ca+ ions on the action of pancreatic lipase studied with the help of immobilisation techniques. Eur J Biochem 140(3):547–551
Suzuki T, Nakayama T, Choo DW, Hirano Y, Kurihara T, Nishino T, Esaki N (2003) Cloning, heterologous expression, renaturation, and characterization of a cold-adapted esterase with unique primary structure from a psychrotroph Pseudomonas sp. strain B11-1. Protein Expr Purif 30(2):171–178
Tokunaga H, Arakawa T, Tokunaga M (2008) Engineering of halophilic enzymes: two acidic amino acid residues at the carboxy-terminal region confer halophilic characteristics to Halomonas and Pseudomonas nucleoside diphosphate kinases. Protein Sci 17(9):1603–1610
Upton C, Buckley JT (1995) A new family of lipolytic enzymes? Trends Biochem Sci 20(5):178
van den Berg B (2010) Crystal structure of a full-length autotransporter. J Mol Biol 396(3):627–633. doi:10.1016/j.jmb.2009.12.061
Xu Y, Zhang Y, Liang Z, Van de Casteele M, Legrain C, Glansdorff N (1998) Aspartate carbamoyltransferase from a psychrophilic deep-sea bacterium, Vibrio strain 2693: properties of the enzyme, genetic organization and synthesis in Escherichia coli. Microbiology 144(5):1435–1441
Yang Z, Zhang Y, Shen T, Xie Y, Mao Y, Ji C (2013) Cloning, expression and biochemical characterization of a novel, moderately thermostable GDSL family esterase from Geobacillus thermodenitrificans T2. J J Biosci Bioeng 115(2):133–137
Yu Shanshan ZB, Zhao Xinyu, Feng Yan (2010) Gene cloning and characterization of a novel thermophilic esterase from Fervidobacterium nodosum Rt17-B1. Acta Biochim Biophys Sin: 288–295
Zuo K, Zhang L, Yao H, Wang J (2010) Isolation and functional expression of a novel lipase gene isolated directly from oil-contaminated soil. Acta Biochim Pol 57(3):305
Acknowledgments
This work was supported by Science Fund (02-01-04-SF1321) Ministry of Science Technology and Innovation, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Communicated by H. Atomi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shakiba, M.H., Ali, M.S.M., Rahman, R.N.Z.R.A. et al. Cloning, expression and characterization of a novel cold-adapted GDSL family esterase from Photobacterium sp. strain J15. Extremophiles 20, 45–55 (2016). https://doi.org/10.1007/s00792-015-0796-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0796-4