Abstract
The accumulation of organic solutes was investigated in the thermophilic bacteria Persephonella marina and Marinitoga piezophila, two representatives of the deepest lineages in the domain Bacteria. These organisms grow optimally at around 70 °C in medium containing 3 % NaCl. A new disaccharide, accumulating in Persephonella marina, was identified as α(1–6)glucosyl-α(1–2)glucosylglycerate (GGG), by nuclear magnetic resonance. This identification was validated by comparison with the spectra of the compound obtained by chemical synthesis. Besides GGG, the solute pool of Persephonella marina comprised β-glutamate, di-myo-inositol-1,3′-phosphate and 2-O-α-glucosylglycerate. In contrast, amino acids such as α-glutamate, proline and alanine were the dominant components of the solute pool of Marinitoga piezophila and sugar derivatives were absent. The ability of GGG to protect protein structure against heat denaturation was assessed using model proteins. A genomic search for the biosynthetic pathways of known ionic solutes in Aquificales and Thermotogales shows the inability of this analysis to predict the nature of compatible solutes and underlines the need for efficient cultivation techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adaptation of life to extreme environments is a fascinating area that continues to attract the attention of the scientific community as well as the wider public. Besides the scientific curiosity stimulated by the apparent unconventional behaviour of extremophilic microorganisms, they also constitute a field of great biotechnological potential, as sources of new metabolites and robust enzymes able to operate under the harsh conditions often required by industrial processes. Among these metabolites, are new organic solutes from thermophiles and hyperthermophiles (hereafter designated as hyper/thermophiles), which exhibit protein protective properties (Scholz et al. 1992; Nunes et al. 1995; Martins et al. 1996, 1997; Ramos et al. 1997; Silva et al. 1999; Lamosa et al. 2000, 2006; Borges et al. 2002; Jorge et al. 2007). Although belonging broadly to the chemical classes of compatible solutes of mesophilic origin, i.e., polyols, sugars, amino acids and derivatives, osmolytes from hyper/thermophiles clearly diverge insofar as they are generally negatively charged (Müller et al. 2005; Santos et al. 2007). Moreover, solutes from hyper/thermophiles appear to have specialised roles in osmoprotection and thermoprotection. In fact, while mannosylglycerate and diglycerol phosphate are primarily involved in osmoprotection, di-myo-inositol phosphate (DIP) and derivatives are consistently associated with the heat stress response (Martins et al. 1996, 1997; Silva et al. 1999; Lamosa et al. 2006).
Recent research efforts have markedly expanded our knowledge about the nature and accumulation profiles of compatible solutes of hyper/thermophiles (Santos et al. 2011). However, the poor growth yields of such extremophiles and their demand for specialised culture conditions have limited the number of organisms examined, and we are still far from having a comprehensive picture of the chemical diversity and physiological roles of compatible solutes in organisms adapted to high temperatures. Genomic and metagenomic approaches combined with high-throughput technologies have had a tremendous impact on the discovery of novel enzymes, or known enzymes with improved properties, by circumventing the bottleneck associated with organism cultivation. However, to our knowledge, there is no way to avoid the need for culturing microorganisms when the target is the discovery of novel solutes. Therefore, the search for unknown solutes is limited by the success of cultivation methods. As a result of collaboration with many colleagues over the past two decades, we have examined many members of the hyper/thermophilic Archaea and Bacteria and this effort has led to the characterisation of more than a dozen novel organic solutes.
In this study, we set out to examine members of the two deepest bacterial lineages, i.e., the Thermotogales and the Aquificales. There is already some information regarding the nature of compatible solutes in representatives of both lineages, namely in the genera Thermotoga, Thermosipho, Fervidobacteruium and Petrotoga within the Thermotogales (Martins et al. 1996; Jorge et al. 2007; Rodrigues et al. 2009; Fernandes et al. 2010), and in the genus Aquifex in the Aquificales (Lamosa et al. 2006), but members of other genera have not been examined thus far. Therefore, to extend our knowledge on the strategies of osmo- and thermoadaptation in thermophilic bacteria, we decided to study the organic solute pools of Marinitoga piezophila and Persephonella marina, members of the Thermotogales and Aquificales, respectively. Both organisms were isolated from deep-sea vents and display optimal growth temperatures in the range 65–75 °C in medium containing 3 % NaCl (Alain et al. 2002; Götz et al. 2002).
Materials and methods
Bacterial strains and growth conditions
Two deep-sea thermophilic bacteria Marinitoga piezophila (DSM 14283) and Persephonella marina (DSM 14350) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany. M. piezophila, supplied as an active liquid culture, was cultured on DSMZ medium 945 according to the method of Alain et al. (2002). Growth was investigated in the presence of elemental sulphur (sterilised by tyndallization) and in an alternative medium containing 20 mM maltose. Both media were initially prepared in 30 ml aliquots and filter sterilised into 160 ml serum bottles. The optimal conditions for growth were: pH 6.0, temperature 65 °C, and gassing with oxygen-free nitrogen to 1 bar overpressure. Biomass was produced by batch culture using 3 l of culture medium in 5 l Duran bottles on either the sulphur or maltose-based medium. Cells were harvested by centrifugation (10,000×g, 20 min), transferred to storage tubes and stored frozen below −70 °C until the extraction of organic solutes.
Persephonella marina, supplied as an active liquid culture, was cultured according to methods modified from Götz et al. (2002). Initial cultures were grown in 30 ml aliquots of medium filter sterilised into 160 ml serum bottles. A microaerophilic growth medium was prepared by gassing the basal salts medium with hydrogen/carbon dioxide (80:20) and pressurising to 1 bar overpressure. Oxygen was added to approximately 2 % (vol/vol) by injection of an appropriate volume (~25 ml) of filtered atmospheric air. An anaerobic growth medium was prepared as for the microaerophilic growth medium with the exception of the addition of sodium nitrate, 2 g/l as an electron acceptor. This medium was then gassed with hydrogen/carbon dioxide (80:20) and pressurised to 1 bar overpressure. Cultures were scaled up using the anaerobic growth medium as above. In each case, the volume of medium was kept in proportion to the bottle size, e.g., 800 ml in 5 l Duran bottles fitted with 4-valve PTFE screw-threaded caps (Omnifit).
Biomass samples for solute extraction were produced by preparing 4 l quantities of medium in 20 l polypropylene bottles (Nalgene) modified to permit pH control (set point pH 6.8) and gas exchange. Cultures were grown within a high temperature oven (Sanyo Gallenkamp) at 70 °C and growth was terminated when no further increase in cell or optical density was observed. Cells were harvested by centrifugation (10,000×g, 20 min), transferred to storage tubes and frozen below −70 °C until the extraction of organic solutes.
Extraction and quantification of intracellular solutes and cell protein determination
Organic solutes were extracted twice with boiling 80 % ethanol as described in Santos et al. (2006). Freeze-dried ethanolic extracts were cleansed of lipid components through chloroform addition and subsequent centrifugation of the organic phase. The resulting aqueous extracts were freeze-dried and re-suspended in D2O for NMR analysis. Quantification of organic compounds was performed by 1H-NMR using formate as an internal concentration standard. However, resonance overlapping precluded the use of this method for quantification of amino acid levels, which were determined by high performance liquid chromatography using a Pico-tag amino acid analysis system (Waters, Milford, MA), and the data processed by Waters Millennium 32 System Software. The protein content of the cells was determined by the Bradford assay (Bradford 1976), after treatment with 1 M NaOH (100 °C, 10 min) and neutralisation with 1 M HCl.
Purification of glucosylglucosylglycerate
Cell extracts containing an unidentified compound were combined and applied to an anionic exchange resin (QAE-Sephadex A-25, Pharmacia, Uppsala, Sweden) and developed with a linear gradient of sodium carbonate/bicarbonate buffer (5 mM–1 M, pH 9.8). Sugar-containing fractions, as observed by the colorimetric assay of Dubois et al. (1956), were pooled, freeze-dried, and desalted in an activated cation exchange resin (Dowex 50W-X8, Bio-Rad), eluted with distilled water, after which, samples were degassed under vacuum, and the pH adjusted to 5 with 1 M KOH. The fractions containing the unknown solute, as determined by 1H-NMR, were further purified by gel filtration (G10-Sephadex, Pharmacia, Uppsala, Sweden). A partially purified sample, containing the desired solute, di-myo-inositol-1,3′-phosphate and vestigial amounts of glucosylglycerate (GG) was freeze-dried and re-suspended in D2O for structure characterisation by NMR.
Chemical synthesis of glucosylglycerate and glucosylglucosylglycerate
GG and α(1–6)glucosyl-α(1–2)glucosylglycerate (GGG) were synthesised as described in Lourenço et al. (2009). The compounds were further purified by anionic exchange chromatography with a QAE-Sephadex A25 column (Amersham Pharmacia Biotech), run with sodium carbonate/bicarbonate buffer, pH 9.8 (from 5 mM to 1 M concentration). Fractions containing the pure compounds were pooled, lyophilised and loaded onto an H+-activated Dowex 50W-X8 column (BioRad) eluted with distilled water. Fractions containing the compounds were degassed under vacuum and the pH was adjusted to approximately 5.0 with ultra-pure potassium hydroxide (Fixanal, Riedel-de Haën, AG).
Enzyme stabilization assays
The effect of GG and GGG on the melting temperature of nuclease A from Staphylococcus aureus (SNase) and mitochondrial malate dehydrogenase (MDH) from pig heart was studied by differential scanning calorimetry (DSC). Recombinant SNase was overproduced and purified as described previously (Faria et al. 2004). MDH was purchased from Roche and used without further purification. Protein stock solutions were prepared by extensive dialysis at 4 °C against 10 mM sodium phosphate buffer pH 7.5. A protein final concentration of approximately 5 μM was typically used for MDH and approximately of 20 μM was used for SNase in the DSC assays. All solutes were used at a final concentration of 0.5 M for both proteins, with the exception of the GGG assay with MDH where a concentration of 0.4 M was used due to the low availability of this compound. DSC assays were performed on a MicroCal VP-DSC Microcalorimeter. After degassing under vacuum for 8 min, samples were heated from 25 to 95 °C with a heating rate of 1 °C/min.
NMR spectroscopy
All spectra were acquired on a Bruker DRX500 spectrometer (Bruker, Rheinstetten, Germany). 13C-NMR spectra were recorded at 125.77 MHz using a 5 mm carbon selective probe head. Typically, spectra were acquired with a repetition delay of 1.5 s and a pulse width of 5 μs corresponding to a 60° flip angle. Proton decoupling was applied during the acquisition time only. Chemical shifts are referenced to the resonance of 3-(trimethylsilyl)propanesulfonic acid designated at 0 ppm.
For quantification purposes, 1H-NMR spectra were acquired with water presaturation, 6 μs pulse width corresponding to a 60° flip angle and a repetition delay of 60 s. Chemical shifts are relative to 3-(trimethylsilyl)propanesulfonic acid (sodium salt) designated at 0.015 ppm. Formate (5 mM) was added as an internal concentration standard.
Two-dimensional (homonuclear COSY, NOESY and TOCSY and heteronuclear 1H-13C HMQC and HMBC) spectra were acquired using standard Bruker pulse programs with presaturation of the solvent water resonance in a phase-sensitive mode. A delay of 3.57 ms was used for evolution of 1 J CH in the heteronuclear multiple quantum coherence spectra (HMQC), while 73.5 ms was used for evolution of long range couplings in the heteronuclear multiple bond connectivity (HMBC) spectrum.
Results
Identification and quantification of organic solutes
The identification of organic solutes in the cell extracts of M. piezophila and P. marina was accomplished by 1H-NMR analysis. Whenever necessary, these identifications were confirmed through the acquisition of 13C-NMR spectra and subsequent comparison of the observed and the published chemical shifts. NMR analysis of cell extracts is a very useful method to identify and quantify organic compounds potentially involved in osmo- or thermoadaptation. These compatible solutes accumulate often to levels above 50 mM and in extreme conditions can reach the molar range of concentration. Their abundance in the cell extract gave rise to strong resonances in the NMR spectra, immediately distinguished from the low intensity signals due to extractable, minor metabolites in the cell.
In M. piezophila extracts, we found α-glutamate, proline and alanine at concentrations of 0.44 ± 0.04, 0.23 ± 0.03 and 0.23 ± 0.06 μmol/mg of protein, respectively, as quantified by HPLC. Growth on maltose as a carbon source or on sulphur as an electron acceptor had little effect on the type or quantity of the accumulated solutes.
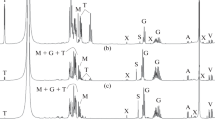
In P. marina, several compounds were readily identified as β-glutamate, di-myo-inositol-1,3′-phosphate, and glucosylglycerate. However, we also found a set of signals in the 1H-NMR spectrum that could not be assigned to a known compound. This set comprised two doublets in the region of the 1H-NMR spectrum where the anomeric resonances of sugars typically occur. Suspecting two possible variants of glucosylglycerate, we carried on to purify these putative compounds following the course of the purification with 1H-NMR analysis of the eluted fractions. We concluded that the resonances were actually due to a single compound, containing two glycosyl moieties. A partially purified sample of this compound was used to acquire two-dimensional NMR correlation spectra. The 1H homonuclear COSY and TOCSY spectra allowed us to identify three spin systems belonging to two glycosyl and one glyceryl moieties, and the position of all the proton frequencies in the compound (Table 1). Also, from the analysis of chemical shifts and coupling constants of the glycosyl signals, these were unequivocally identified as glucosyl residues, (G and G′) (Table 1). The 1 J CH coupling constants of the two anomeric sugar carbon signals (173.4 and 174.9 Hz) proved that the glucose residues had the α-configuration. The resonance at 177.2 ppm in the carbon spectrum (typical of carboxyl groups) confirmed the presence of the glycerate moiety. Therefore, the unknown compound was identified as α-glucosyl-α-glucosylglycerate. To determine the position of the glycosidic bonds, a NOESY spectrum was recorded. This revealed a clear correlation between protons 1 of glucosyl moiety G and proton 2 of the glycerate moiety, thus establishing an α(1–2) glycosidic bond between glucose G and the glycerate group. A correlation was also observed between proton 1 of the glucosyl moiety G′ and a highly complex region of the spectrum containing the signals of protons 6 and 3 of the glucosyl moiety G. The final structure of the molecule was established through a combination of 13C–1H correlation spectra. The 13C–1H correlation spectrum (HMQC) allowed the assignment of all carbon signals belonging to the glycerate and sugar groups (Table 1). This information enabled the interpretation of the 13C–1H multiple bond correlation spectrum (HMBC). In this spectrum, we observed signals correlating carbon 6 of the glucosyl moiety G to proton 1 of the glucosyl moiety G′ and carbon 1 of the glucosyl moiety G′ to protons 6a and 6b of the glucosyl moiety G (Fig. 1). The structure of the new compound was thus established as α(1–6)glucosyl-α(1–2)glucosylglycerate (GGG) (Fig. 2). This conclusion was firmly confirmed by spiking the natural compound with an authentic sample produced by chemical synthesis.
Heteronuclear multiple quantum correlation (HMQC) (left) and heteronuclear multiple bond correlation (HMBC) (right) spectra of a partially purified sample of a P. marina extract. Signal arising from the glucosyl moiety which is linked to the glycerate moiety are labelled G, the signals arising from the other glucosyl moiety and from glycerate are labelled as G′ and Y, respectively
The levels of the solutes accumulated by P. marina, a microaerophile that requires hydrogen to grow, were difficult to assess precisely because the organism grows poorly and to relatively low yields (0.1–0.3 g wet weight per litre), displaying long and variable lag times. This growth behaviour makes it difficult to determine the growth phase and to establish reproducible growth conditions, thus influencing the amount of internal compatible solutes at harvesting point. Nevertheless, it was possible to conclude that β-glutamate was always the major compatible solute with values ranging from 0.85 to 1.27 μmol/mg of protein. The levels of the other solutes displayed greater variability. GG was present in the range of 0.04–0.86 μmol/mg of protein, while di-myo-inositol-1,3′-phosphate and the new compound, GGG, were present in amounts up to 0.13 and 0.35 μmol/mg of protein, respectively.
Effect of GG and GGG on the melting temperature of model enzymes
GG (at 0.5 M), produced by chemical synthesis, caused an increase in the melting temperature of SNase and MDH of 8.0 °C and 8.8 °C, respectively, while GGG (at 0.5 M for SNase and 0.4 M for MDH) caused increases of 7.9 °C and 12.5 °C, respectively (Fig. 3). The ability of GG and GGG as stabilisers compares roughly with the stabilization conferred by mannosylglycerate and di-myo-inositol-1,3′-phosphate, while glycerol at the same concentration produced only modest increases (Faria et al. 2008). GGG was by far the best stabilizer of MDH, but when compared with GG and MG, the degree of stabilization per unit mass is not as impressive, meaning that the extra glucosyl unit in GGG brings no clear benefit.
Effect of solutes on the melting temperature of staphylococcal nuclease (SNase) and pig heart malate dehydrogenase (MDH). All solutes were added at 0.5 M concentration, with the exception of GGG, which was added at 0.4 M in the MDH assay. The results for trehalose, α-mannosylglycerate, DIP, glycerol and KCl were collected from Faria et al. (2008). MG α-Mannosylglycerate, DIP di-myo-inositol-1,3′-phosphate, GG glucosylglycerate, GGG α(1–6)glucosyl-α(1–2)glucosylglycerate
Discussion
Despite the long phylogenetic distance between Marinitoga piezophila and Persephonella marina, both organisms were isolated from deep-sea hydrothermal vents around a depth of 2600 m in the Pacific Ocean, and they require similar salinity, temperature and pH conditions for growth (Alain et al. 2002; Götz et al. 2002). However, while M. piezophila is a thermopiezophilic organism, displaying a maximal growth rate at around 40 MPa, the growth of P. marina is not stimulated by pressure (Alain et al. 2002; Götz et al. 2002). Interestingly, these two organisms present very distinct solute pools. While M. piezophila uses mainly amino acids, α-glutamate, proline and alanine, P. marina accumulates a variety of solutes such as DIP, β-glutamate, GG and the newly discovered compound GGG. The amino acids α-glutamate and proline are commonly used in osmoadaptation of mesophilic bacteria (Santos and da Costa 2002; Saum and Müller 2007; Brill et al. 2011), and the accumulation of α-glutamate is also very frequent within hyper/thermophiles. This negatively charged amino acid has been found to accumulate in the two species of Aquifex and in all marine Thermotogales examined thus far: Thermotoga maritima, Thermotoga neapolitana, Petrotoga spp. and Thermosipho africanus (Martins et al. 1996; Lamosa et al. 2006; Jorge et al. 2007; Rodrigues et al. 2009; Fernandes et al. 2010). In contrast, proline is an uncommon solute among hyper/thermophilic organisms and has been detected only in Thermosipho africanus, Palaeococcus ferrophilus and Petrotoga miotherma (Martins et al. 1996; Neves et al. 2005; Jorge et al. 2007). Alanine is an unusual solute encountered in a few bacteria and archaea (e.g., Halobacillus halophilus, Rhizobium UMKL 20 and Palaeococcus ferrophilus) (Hua et al. 1982; Neves et al. 2005; Saum and Müller 2007), but its role as a true compatible solute has been demonstrated only in Methanosarcina mazei Go1 (Saum et al. 2009).
The role of compatible solutes in adaptation to high pressure remains unclear as the number of studies reported is very limited. Our data on M. piezophila refer to cells grown at low pressure, 0.1 MPa, hence a definitive role for solutes in adaptation to high pressure cannot be ascribed. Interestingly, in the cold adapted piezophilic bacterium Photobacterium profundum strain SS9, the pools of β-hydroxybutyrate and oligomers of β-hydroxybutyrate increased in response to hydrostatic pressure (Martin et al. 2002). Moreover, the level of these solutes also increased with osmolarity, and hence they were called “piezolytes” (Martin et al. 2002). In contrast, in Thermococcus barophilus, the pool of mannosylglycerate appeared to decrease with pressure (Cario et al. 2010). More studies are definitely needed to clarify the role of organic solutes in piezophilic organisms.
In contrast with the amino acid nature of the solutes found in M. piezophila, the solute pool of P. marina comprised primarily sugar derivatives, such as the newly identified solute, α(1–6)glucosyl-α(1–2)glucosylglycerate (GGG), glucosylglycerate (GG) and DIP. The only amino acid present was β-glutamate, which accumulates in the three members of the Aquificales examined thus far and also in Thermotoga spp., in which accumulation occurs in response to osmotic shock (Martins et al. 1996; Lamosa et al. 2006; Rodrigues et al. 2009) (Table 2). β-Glutamate is a relatively rare solute, more commonly found in methanogens with either mesophilic or thermophilic lifestyles (Martin et al. 1999). In most cases, the accumulation of this compound is clearly dependent on the salinity of the medium, which relates to the osmoprotective function usually ascribed to β-glutamate (Robertson et al. 1990, 1992; Martins et al. 1996; Roberts 2005; Lamosa et al. 2006; Rodrigues et al. 2009). The accumulation of DIP was first detected in the hyperthermophile Pyrococcus woesei and is restricted to marine hyper/thermophiles (Scholz et al. 1992; Santos et al. 2011). The organisms known to accumulate DIP are mainly distributed within the domain Archaea with a few exceptions, i.e., the Aquificales, two species of Thermotoga and Rubrobacter xylanophilus (Martins et al. 1996; Rodrigues et al. 2009; Empadinhas et al. 2007) in the domain Bacteria. DIP and derivatives accumulate primarily in response to heat stress, an observation that led to the view that they play a role in cell thermoprotection.
The presence of GG in a thermophilic organism, Persephonella marina, is curious. Although it possesses a strong structural resemblance to mannosylglycerate (often found in hyper/thermophiles), GG is a rare solute, to our knowledge only detected in the archaeon Methanohalophilus portucalensis and some halophilic bacteria, such as Chromohalobacter salexigens, Dickeya dadantii strain 3937, Synechococcus sp. PCC 7002, Prochlorococcus marinus strains SS120 and NATL2A, and Streptomyces caelestis, and never encountered in hyper/thermophiles (Robertson et al. 1992; Cánovas et al. 1999; Kollman et al. 1979; Goude et al. 2004; Pospísl et al. 2007; Klähn et al. 2010). The role of GG as a compatible solute is controversial, since only in a few cases does it respond to salinity fluctuations of the medium. In Dickeya dadantii, GG is used for adaptation at low salinity, while glutamine is the main compatible solute (Goude et al. 2004). In Synechococcus sp. PCC 7002 and Prochlorococcus marinus, GG also responds to increased medium salinity, but the major osmoprotectants are glucosylglycerol and sucrose, respectively (Klähn et al. 2010). Nitrogen limitation appears to be the primary factor that triggers GG accumulation. In fact, the GG level is consistently higher in nitrogen-poor conditions and this behaviour was recently confirmed in Mycobacterium smegmatis (Behrends et al. 2012). In this bacterium, GG accumulates only in nitrogen limiting conditions and its level is unrelated to the medium salinity. These results corroborate the view that GG is a stress metabolite mainly associated with nitrogen deprivation (Behrends et al. 2012).
The detection of GG in P. marina becomes even more interesting if we consider its structural relationship to the newly found solute GGG. Since these solutes share the same glucosyl-α(1–2)-glycerate moiety, it is tempting to speculate that GG could serve as a precursor for GGG synthesis. The accumulation of disaccharide heterosides is extremely rare. To our knowledge, only two thermophilic bacteria, Petrotoga miotherma and Petrotoga mobilis, accumulate mannosyl-α(1–2)-glucosyl-α(1–2)-glycerate (MGG), a disaccharide that is involved in low-level osmotic adaptation in Petrotoga miotherma and the response to hyperosmotic conditions and supra-optimal temperatures in Petrotoga mobilis (Jorge et al. 2007; Fernandes et al. 2010). Two pathways were proposed for the synthesis of MGG in Petrotoga mobilis, but the synthesis of GGG remains elusive (Fernandes et al. 2010).
The distinct nature of the solutes accumulating in M. piezophila and P. marina has a genomic background. M. piezophila accumulates amino acids only, and indeed, it has no genes for the synthesis of the polyol and sugar derivatives that are found in P. marina (Table 3). Besides glutamate, the following ionic solutes have been detected in members of the orders Thermotogales and Aquificales: DIP, MDIP, GG, MGG and GGG. With the exception of GGG, the biosynthetic genes for these solutes have been identified (Rodrigues et al. 2007, 2009; Fernandes et al. 2007, 2010). An extended analysis of the genomes available for Thermotogales and Aquificales showed that the presence of these pathways is not a general trait. For example, genes for DIP synthesis are present only in marine Thermotoga spp., Aquifex aeolicus, Hydrogenivirga sp. 128-5-R1-1, and P. marina, suggesting that those genes were acquired by horizontal gene transfer, probably from hyperthermophilic archaea (Gonçalves et al. 2012). Curiously, genes for MDIP synthesis are present only in marine Thermotoga spp. and Aquifex aeolicus, i.e., only in the hyperthermophilic members.
With respect to glycerylglucosides (GG and MGG), the profiles of gene distribution are more interesting since these solutes can be synthesised via two alternative pathways which show very different prevalences. The two-step pathways are rare while the single-step pathways are nearly ubiquitous within the Thermotogales. Interestingly, the presence of the two-step pathways correlates with the accumulation of GG or MGG. In view of this feature, we postulate that the single-step pathway is unrelated to osmolyte synthesis. This is further supported by the prevalence of the single-step pathway in organisms isolated from freshwater habitats, which naturally are less equipped to cope with changes in osmolarity.
Many members of the Aquificales and Thermotogales do not possess biosynthetic genes for ionic solutes such as DIP, MDIP, GG and MGG and this raises the question about the nature of solutes used in thermo- and osmoadaptation by these organisms (Table 3). Also, the genes encoding proteins for the synthesis and/or uptake of typical solutes from mesophiles such as glycine betaine or trehalose are not present in the majority of the Aquificales and Thermotogales genomes available, indicating that those organisms may rely on other compatible solutes and/or strategies to cope with osmotic and heat stress. Therefore, the in silico analysis of genomes is insufficient to provide a comprehensive picture on stress protectors of hyper/thermophiles, thereby leading to the conclusion that microbial fermentation must not be neglected and further efforts should be directed to the development of novel cultivation techniques.
References
Alain K, Marteinsson VT, Miroshnichenko ML, Bonch-Osmolovskaya EA, Prieur D, Birrien JL (2002) Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 52:1331–1339
Behrends V, Williams KJ, Jenkins VA, Robertson BD, Bundy JG (2012) Free glucosylglycerate is a novel marker of nitrogen stress in Mycobacterium smegmatis. J Proteome Res 11:3888–3896
Borges N, Ramos A, Raven NDH, Sharp RJ, Santos H (2002) Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209–216
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brill J, Hoffmann T, Bleisteiner M, Bremer E (2011) Osmotically controlled synthesis of the compatible solute proline is critical for cellular defense of Bacillus subtilis against high osmolarity. J Bacteriol 193:5335–5346
Cánovas D, Borges N, Vargas C, Ventosa A, Nieto JJ, Santos H (1999) Role of Nγ-acetyldiaminobutyrate as an enzyme stabiliser and an intermediate in the biosynthesis of hydroxyectoine. Appl Environ Microbiol 65:3774–3779
Cario A, Jebbar M, Kervarec N, Oger P (2010) Influence of high hydrostatic pressure on the salt and heat stress response in the piezophilic archaeon Thermococcus barophilus. Book of Abstracts of Extremophiles P7:108
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Empadinhas N, Mendes V, Simoes C, Santos MS, Mingote A, Lamosa P, Santos H, da Costa MS (2007) Organic solutes in Rubrobacter xylanophilus: the first example of di-myo-inositol phosphate in a thermophile. Extremophiles 11:667–673
Faria TQ, Lima JC, Bastos M, Macanita AL, Santos H (2004) Protein stabilization by osmolytes from hyperthermophiles: effect of mannosylglycerate on the thermal unfolding of recombinant nuclease a from Staphylococcus aureus studied by picosecond time-resolved fluorescence and calorimetry. J Biol Chem 279:48680–48691
Faria TQ, Mingote A, Siopa F, Ventura R, Maycock C, Santos H (2008) Design of new enzyme stabilizers inspired by glycosides of hyperthermophilic microorganisms. Carbohydr Res 343:3025–3033
Fernandes C, Empadinhas N, da Costa MS (2007) Single-step pathway for synthesis of glucosylglycerate in Persephonella marina. J Bacteriol 189:4014–4019
Fernandes C, Mendes V, Costa J, Empadinhas N, Jorge C, Lamosa P, Santos H, da Costa MS (2010) Two alternative pathways for the synthesis of the rare compatible solute mannosylglucosylglycerate in Petrotoga mobilis. J Bacteriol 192:1624–1633
Gonçalves LG, Borges N, Serra F, Fernandes PL, Dopazo H, Santos H (2012) Evolution of the biosynthesis of di-myo-inositol phosphate, a marker of adaptation to hot marine environments. Environ Microbiol 14:691–701
Götz D, Banta A, Beveridge TJ, Rushdi AI, Simoneit BR, Reysenbach AL (2002) Persephonella marina gen. nov., sp. nov. and Persephonella guaymasensis sp. nov., two novel, thermophilic, hydrogen-oxidizing microaerophiles from deep-sea hydrothermal vents. Int J Syst Evol Microbiol 52:1349–1359
Goude R, Renaud S, Bonnassie S, Bernard T, Blanco C (2004) Glutamine, glutamate, and α-glucosylglycerate are the major osmotic solutes accumulated by Erwinia chrysanthemi starin 3937. Appl Environ Microbiol 70:6535–6541
Hua SS, Tsai VY, Lichens GM, Noma AT (1982) Accumulation of amino acids in Rhizobium sp. Strain WR1001 in response to sodium chloride salinity. Appl Environ Microbiol 44:135–140
Jorge C, Lamosa P, Santos H (2007) α-d-Mannopyranosyl-(1,2)-α-d-glucopyranosyl-(1,2)glycerate in the thermophilic bacterium Petrotoga miotherma: structure, cellular content and function. FEBS J 274:3120–3127
Klähn S, Steglich C, Hess WR, Hagemann M (2010) Glucosylglycerate: a secondary compatible solute common to marine cyanobacteria from nitrogen-poor environments. Environ Microbiol 12:83–94
Kollman VH, Hanner JL, London RE, Adame EG, Walker TE (1979) Photosynthetic preparation and characterization of 13C-labeled carbohydrates in Agmenellum quadruplicatum. Carbohydr Res 73:193–202
Lamosa P, Burke A, Peist R, Huber R, Liu MY, Silva G, Rodrigues-Pousada C, LeGall J, Maycock C, Santos H (2000) Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from the hyperthermophile Archaeoglobus fulgidus. Appl Environ Microbiol 66:1974–1979
Lamosa P, Gonçalves LG, Rodrigues M, Martins LO, Raven N, Santos H (2006) Occurrence of 1-glyceryl-1-myo-inosityl-phosphate in hyperthermophiles. Appl Environ Microbiol 72:6169–6173
Lourenço EC, Maycock CD, Ventura MR (2009) Synthesis of potassium (2R)-2-O-α-d-glucopyranosyl-(1–>6)-alpha-d-glucopyranosyl-2,3-dihydroxypropanoate a natural compatible solute. Carbohydr Res 344:2073–2078
Martin DD, Ciulla RA, Roberts MF (1999) Osmoadaptation in archaea. Appl Environ Microbiol 65:1815–1825
Martin DD, Bartlett DH, Roberts MF (2002) Solute accumulation in the deep-sea bacterium Photobacterium profundum. Extremophiles 6:507–514
Martins LO, Carreto LS, da Costa MS, Santos H (1996) New compatible solutes related to di-myo-inositol-phosphate in members of the order Thermotogales. J Bacteriol 178:5644–5651
Martins LO, Huber R, Huber H, Stetter KO, da Costa MS, Santos H (1997) Organic solutes in hyperthermophilic Archaea. Appl Environ Microbiol 63:896–902
Müller V, Spanheimer R, Santos H (2005) Stress response by solute accumulation in archaea. Curr Opin Microbiol 8:729–736
Neves C, da Costa MS, Santos H (2005) Compatible solutes of the hyperthermophile Palaeococcus ferrophilus: osmoadaptation and thermoadaptation in the order thermococcales. Appl Environ Microbiol 71:8091–8098
Nunes OC, Manaia CM, da Costa MS, Santos H (1995) Compatible solutes in thermophilic bacteria Rhodothermus marinus and Thermus thermophilus. Appl Environ Microbiol 61:2351–2357
Pospísl S, Halada P, Petrícek M, Sedmera P (2007) Glucosylglycerate is an osmotic solute and an extracellular metabolite produced by Streptomyces caelestis. Folia Microbiol (Praha) 52:451–456
Ramos A, Raven NDH, Sharp RJ, Bartolucci S, Rossi M, Cannio R, Lebbink J, van der Oost J, de Vos WM, Santos H (1997) Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microbiol 63:4020–4025
Roberts MF (2005) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5
Robertson DE, Roberts MF, Belay N, Stetter KO, Boone DR (1990) Occurrence of β-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl Environ Microbiol 56:1504–1508
Robertson DE, Noll D, Roberts MF (1992) Free amino acid dynamics in marine methanogens. J Biol Chem 267:14893–14901
Rodrigues MV, Borges N, Henriques M, Lamosa P, Ventura R, Fernandes C, Empadinhas N, Maycock C, da Costa MS, Santos H (2007) Bifunctional CTP:inositol-1-phosphate cytidylyltransferase/CDP-inositol:inositol-1-phosphate transferase, the key enzyme for di-myo-inositol-phosphate synthesis in several (hyper)thermophiles. J Bacteriol 189:5405–5412
Rodrigues MV, Borges N, Almeida CP, Lamosa P, Santos H (2009) A unique beta-1,2-mannosyltransferase of Thermotoga maritima that uses di-myo-inositol phosphate as the mannosyl acceptor. J Bacteriol 191:6105–6115
Santos H, da Costa MS (2002) Compatible solutes of organisms that live in hot saline environments. Environ Microbiol 4:501–509
Santos H, Lamosa P, Borges N (2006) Characterization and quantification of compatible solutes in (hyper)thermophilic microorganisms. Methods Microbiol 35:173–199
Santos H, Lamosa P, Faria TQ, Borges N, Neves C (2007) The physiological role, biosynthesis and mode of action of compatible solutes from (hyper)thermophiles. In: Gerday C, Glandorff N (eds) Physiology and biochemistry of extremophiles. ASM Publishers, Washington, DC, pp 86–103
Santos H, Lamosa P, Borges N, Gonçalves LG, Pais T, Rodrigues MV (2011) Organic compatible solutes of prokaryotes that thrive in hot environments: the importance of ionic compounds for thermostabilization. In: Horikoshi K (ed) Extremophiles handbook. Springer, Tokyo, pp 497–520
Saum SH, Müller V (2007) Salinity-dependent switching of osmolyte strategies in a moderately halophilic bacterium: glutamate induces proline biosynthesis in Halobacillus halophilus. J Bacteriol 189:6968–6975
Saum R, Mingote A, Santos H, Müller V (2009) A novel limb in the osmoregulatory network of Methanosarcina mazei Gö1: N(epsilon)-acetyl-beta-lysine can be substituted by glutamate and alanine. Environ Microbiol 11:1056–1065
Scholz S, Sonnenbichler J, Schäfer W, Hensel R (1992) Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett 306:239–242
Silva Z, Borges N, Martins LO, Wait R, da Costa MS, Santos H (1999) Combined effect of the growth temperature and salinity of the medium on the accumulation of compatible solutes by Rhodothermus marinus and Rhodothermus obamensis. Extremophiles 3:163–172
Acknowledgments
This work was supported by the European Commission, 6th Framework Programme contract COOP-CT-2003-508644, PRODEP and POCI, Portugal (PTDC/BIO/70806/2006) (POCI/V.5/A0004/2005). Technical assistance by Ana I Mingote is acknowledged. The NMR spectrometers are part of The National NMR Network (REDE/1517/RMN/2005), supported by “Programa Operacional Ciência e Inovação (POCTI) 2010” and Fundação para a Ciência e a Tecnologia (FCT). M.V.R. and L.G.G. received fellowships from FCT (SFRH/BPD/80219/2011 and SFRH/BPD/26905/2006). Data for the amino acid analysis was obtained by the Analytical Laboratory, Analytical Services Unit, Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
P. Lamosa and M. V. Rodrigues contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamosa, P., Rodrigues, M.V., Gonçalves, L.G. et al. Organic solutes in the deepest phylogenetic branches of the Bacteria: identification of α(1–6)glucosyl-α(1–2)glucosylglycerate in Persephonella marina . Extremophiles 17, 137–146 (2013). https://doi.org/10.1007/s00792-012-0500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-012-0500-x