Abstract
The archaea are distinguished by their unique isoprenoid ether lipids, which typically consist of the sn-2,3-diphytanylglycerol diether or sn-2,3-dibiphytanyldiglycerol tetraether core modified with a variety of polar headgroups. However, many hyperthermophilic archaea also synthesize tetraether lipids with up to four pentacyclic rings per 40-carbon chain, presumably to improve membrane thermal stability at temperatures up to∼110 °C. This study aimed to correlate the ratio of tetraether to diether core lipid, as well as the presence of pentacyclic groups in tetraether lipids, with growth temperature for the hyperthermophilic archaeon, Archaeoglobus fulgidus. Analysis of the membrane core lipids of A. fulgidus using APCI–MS analysis revealed that the tetraether-to-diether lipid ratio increases from 0.3 ± 0.1 for cultures grown at 70°C to 0.9 ± 0.1 for cultures grown at 89°C. Thin-layer chromatography (TLC) followed by APCI–MS analysis provided evidence for no more than one pentacycle in the hydrocarbon chains of tetraether lipid from cultures grown at 70°C and up to 2 pentacycles in the tetraether lipid from cultures grown at higher temperatures. Analysis of the polar lipid extract using TLC and negative-ion ESI–MS suggested the presence of diether and tetraether phospholipids with inositol, glycosyl, and ethanolamine headgroup chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
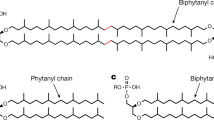
Archaeal membrane lipids have a unique core isoprenoid ether-linked structure, as shown in Fig. 1, which is significantly different than bacterial and eukaryal membrane lipids that generally consist of esters formed by the condensation of fatty acids with glycerol. In contrast, archaeal core lipids typically are composed of C 20 phytanyl or C 40 biphytanyl chains connected to glycerol backbones by more stable ether linkages. The macrocyclic, bipolar tetraether lipids most likely are synthesized by the head-to-head condensation of the chains of two 2,3-di-O-geranylgeranylglyceryl molecules or two diether polar lipids (Langworthy 1985). In addition, one to four pentacyclic groups per C 40 chain also have been reported in tetraether lipids isolated from several hyperthermophilic archaea, presumably to increase thermostability of the cell membrane. Finally, the glycerol basis of archaeal lipid headgroups exhibits the S stereochemistry, enantiomeric to the standard R configuration of typical bacterial and eukaryal lipids (Kates 1992).
Polar headgroups attached to the diether and tetraether core lipids provide diversity to lipid structures among the various species of archaea. Several types of polar lipids exist including phospholipids, glycolipids, glycophospholipids, sulfolipids, and aminolipids. The glycolipids of archaea consist of various sugar residues including glucose, mannose, and galactose, and can vary in glycosidic linkage chemistry. Tetraether lipids are either based on diglycerol or glycerolcalditol structures (Koga and Morii 2005), with headgroups including glucose, glycerophosphate, a glucosylgalactosyl disaccharide, phosphorylinositol, or sulfate (Langworthy 1985). The diether lipids of halophiles have four basic polar lipid structures with headgroups of phosphatidylglycerol, phosphatidylglycerophosphate, phosphatidylglycerosulfate, and a triglycosyl diether glycolipid with a terminal sulfate radical (Langworthy 1985). Methanogens have diether glycolipids as well as phospholipids linked to other groups such as ethanolamine, serine, glycerol, and inositol (Koga and Morii 2005). Archaeal tetraether lipids have been found to exhibit asymmetric headgroup chemistry, which may prescribe a preferred orientation relative to the cell interior and exterior (Kates 1992; Morii and Koga 1994).
The ether lipid composition of archaea traditionally has been determined by methods based on thin-layer chromatography (TLC), gas-liquid chromatography (GLC), high performance liquid chromatography (HPLC), and fast atom bombardment mass spectrometry (FAB-MS) (De Rosa et al. 1980; Demizu et al. 1992; Nishihara and Koga 1991; Sprott et al. 1994; Tornabene and Langworthy 1979). Recently, the development of atmospheric pressure ionization sources for mass spectrometry has spurred the use of mass spectrometry in the analysis of biomolecules. Atmospheric pressure chemical ionization (APCI) mass spectrometry has been utilized in determining the core ether lipids of Sulfolobus solfataricus and Metallosphaera sedula (Hopmans et al. 2000). Atmospheric pressure chemical ionization mass spectrometry is an accurate technique for analyzing uncharged, less polar molecules of moderate mass range, thus it is especially suitable for lipid analysis (Byrdwell 2001) and determination of the core lipid composition of archaea. It is a mild form of ionization causing little or no fragmentation of molecules. Negative-ion electrospray ionization (ESI) mass spectrometry has also been used to characterize the intact structures of ether lipids of various archaeal microorganisms (Murae et al. 2002; Qui et al. 1998, 2000; Sturt et al. 2003). Electrospray ionization mass spectrometry, also a soft ionization method, allows accurate analysis and sensitive detection of charged and polar compounds (Watson 1997) and is therefore suited for determining the masses of the polar ether lipids of archaea.
This study focuses on the membrane lipids of Archaeoglobus fulgidus VC-16 (DSM4304), a hyperthermophilic neutrophile that was originally isolated from hydrothermal vents in Vulcano, Italy (Stetter et al. 1987). This organism grows optimally under anaerobic conditions at 83°C and is the first sulfur-metabolizing organism to have its genome sequenced (Klenk et al. 1997). In this study, a preliminary characterization of the membrane lipids of A. fulgidus is accomplished using MS and TLC in combination to identify prevalent headgroup chemistries and to determine the change in tetraether-to-diether lipid ratio and in number of pentacyclic groups per tetraether lipid with increasing growth temperature.
The polar lipid composition of A. fulgidus recently has been analyzed using a combination of two-dimensional TLC and GLC. Archaeol (diether) and caldarchaeol (tetraether) were identified as the core lipid structures, along with an unknown core lipid that is suggested to be an H-shaped caldarchaeol due to its similar TLC mobility (Tarui et al. 2007). A recently isolated core lipid, the H-shaped caldarchaeol resembles a modified caldarchaeol with a covalent C-C bridge between the two biphytanyl chains (Morii et al. 1998). The main glycolipid headgroups found were galactose and mannose with minor amounts of glucose, while the phospholipid headgroups found were inositol and ethanolamine (Tarui et al. 2007).
Few reported studies also have focused on changes in archaeal lipid biochemistry as a function of growth temperature. The core tetraether-to-diether lipid ratio of M. jannaschii has been reported to increase with increasing growth temperature (Sprott et al. 1991). In addition, the number of pentacycles along tetraether lipid chains is reported to increase with growth temperature for the core membrane lipids of S. solfataricus (De Rosa et al. 1980), as well as for the lipids of T. acidophilum, T. volcanium, P. oshimae, P. torridus, and F. acidophilum (De Rosa et al. 1980; Uda et al. 2004). The increase in tetraether-to-diether ratio and in the number of pentacyclic groups is suspected to be an adaptive response by thermophilic archaea leading to increased membrane stability at higher temperatures (Gabriel and Chong 2000).
Materials and methods
Organism and culture conditions
Archaeoglobus fulgidus VC-16 (DSM 4304) was grown anaerobically in 8-L cultures on minimal media at pH 7 and at various growth temperatures between 70 and 89°C. Large-scale, 70-L cultures were also grown using a custom-built, 100-L, glass-lined steel fermentor (Pfaudler, Rochester, NY) to obtain a larger quantity of biomass, primarily for headgroup characterization. The cells were harvested when the optical density at 600 nm (OD600) reached 0.5–0.6 (∼36 h, late exponential phase). The cells subsequently were lysed by sonication during three 1-min sessions on ice. Ultracentrifugation at 200,000×g for 1 h was used to isolate the membrane fraction.
Lipid extraction and isolation
Lipids were extracted using an acidified (5% trichloroacetic acid) Bligh and Dyer technique (Nishihara and Koga 1987). Five hundred mg of wet biomass were contacted with 19 ml of chloroform-methanol-5% trichloroacetic acid (1:2:0.8) and stirred for 18 h. Five ml of chloroform followed by 5 ml of water were added to the mixture in a separatory funnel, and the two phases were allowed to separate, with the aid of centrifugation as necessary. The lower chloroform extract was removed and washed with 1.9 volumes of methanol-H2O (1:0.8). The chloroform layer was again removed, diluted 10% with benzene, and evaporated to dryness using a rotary evaporator.
Due to the variety and complexity of membrane lipid headgroup chemistry, lipid headgroup cleavage from the core is necessary for efficient analysis of changes in core lipid with growth temperature. For core lipid analysis, the polar headgroups generally are removed by hydrolysis, in this case by acid methanolysis (Kates 1986). Anhydrous 2.5% methanolic-HCl was prepared by adding acetyl chloride to extra-dry methanol. Total lipids were dissolved in 5 ml of 2.5% methanolic-HCl in a screw-cap (Teflon lined) test tube and heated in a block heater at 90°C for 24 h. The tube was then cooled, and 0.5 ml water was added. Core lipids were extracted three times with 5 ml of petroleum ether (b.p. 40–60°C), each time removing the upper petroleum ether phase by pipet. The extracts were pooled and evaporated to dryness.
For headgroup characterization of the lipids, total lipids were extracted from 5 g of wet A. fulgidus biomass grown at 83°C using the acidified Bligh and Dyer technique described above. Total lipids were purified by silica gel chromatography (Kates 1986) to separate them into different lipid classes based on the polarity of their headgroups. Total lipids were loaded onto a glass column packed with silica gel 60 (4 cm dia. × 25 cm ht.). Five hundred ml of chloroform were used to elute neutral lipids, 1,500 ml of acetone were used to elute glycolipids, and 500 ml of methanol were used to elute phospholipids. Each of the fractions was collected, and solvents were evaporated. Neutral lipids were dissolved in chloroform, while glycolipids and phospholipids were dissolved in chloroform-methanol (1:1).
Analysis of lipids by TLC
Core lipids and polar lipids, re-dissolved to 1 mg/ml in chloroform-methanol (1:1), were analyzed by TLC. Lipids were spotted on Whatman TLC plates (K6F silica gel 60). Total lipids from the lipid extract and polar lipids were developed in a solvent mixture containing chloroform-methanol-acetic acid-water (85:30:15:5). Ultraviolet (UV) light, iodine vapor, and sulfuric acid charring were used to visualize the spots (Kates 1986). A variety of stains were sprayed onto the plates to detect various polar headgroup chemistries including molybdenum blue (Alltech, Deerfield, IL) for phosphate (phospholipids), Bial’s orcinol reagent (Alltech) for sugar groups (glycolipids), ninhydrin for amines, and Dragendorff’s reagent for cholines (Kates 1986). Hydrolyzed core lipids were also identified by TLC and preparative TLC using a solvent system of petroleum ether-diethyl ether-acetic acid (70:30:1) (Kates 1986) and bands were visualized with iodine vapor staining or sulfuric acid charring. A diether standard, 1,2-di-O-phytanyl-sn-glycerol (Avanti Polar Lipids, Alabaster, AL), was used as reference.
Analysis of lipids by mass spectrometry
The APCI–MS system was used to analyze the core lipids extracted from biomass. Dried core lipid samples were re-dissolved in chloroform and 100 μl aliquots were injected into a chloroform vapor stream entering an APCI source in positive-ion mode (300 μl/min, nebulizer at 450°C, discharge needle at 4.5 kV, nebulizing gas (3.0 L/min) produced from the vapors of liquid nitrogen) attached to a triple quadrupole mass spectrometer (Perkin-Elmer Sciex, Thornhill, Canada, API III+). The mass spectrometer was tuned and calibrated by flow injection (100 μl/min) of a mixture of polypropylene glycol (PPG) as previously described (Glasgow et al. 1998). The mass spectrometer was scanned from m/z 100 to 2,000 (step size 0.3 Da, scan speed 5.4 s, orifice at 65 V). The spectra collected from the injection of each sample were averaged using manufacturer-supplied software. The diether standard, 1,2-di-O-phytanyl-sn-glycerol, was also analyzed by APCI–MS and used as reference.
The diether core lipid corresponds to a monoisotopic peak at m/z 654 [M+H]+ while the tetraether core lipid corresponds to a monoisotopic peak at m/z 1,303 [M+H]+. The individual components of the hydrolyzed lipid mixture were separated first by preparative TLC. Subsequently, the lipid was recovered by identifying the lipid bands with iodine vapor and removing the silica from the plate, followed by extraction with chloroform-methanol (1:1). These lipid samples were analyzed by APCI–MS to detect cyclopentanyl rings present in separated tetraether lipids. For the direct analysis of the hydrolyzed lipid mixture, the ratio of tetraether to diether lipids (TE/DE) was estimated as the ratio of their intensities in mass spectra. Due to slight differences in the mass of tetraether lipid molecules depending on cyclization of the C 40 isoprenoid chain, the intensities for all tetraether lipid peaks at m/z 1,300–1,303 [M+H]+ were summed, while only the single diether lipid peak at m/z 653.8 [M+H]+ was used.
The polar lipid fraction purified by silica gel chromatography was analyzed by negative-ion ESI–MS in order to investigate headgroup chemistry. Dried samples were re-dissolved in chloroform (with 0.1% triethylamine to encourage deprotonation) and 20 μl aliquots were injected into a chloroform vapor stream entering an Articulated Ion Spray™ source attached to a triple quadrupole mass spectrometer (Perkin-Elmer Sciex, Thornhill, Canada, API III+). The mass spectrometer was tuned and calibrated by infusion of a mixture of polypropylene glycol (PPG) as previously described (Glasgow et al. 1998). The mass spectrometer was scanned from m/z 500 to 2,000 (step size 0.3 Da, scan speed 5.0 s, orifice at -75 V). The spectra collected from the injection of each sample were averaged using manufacturer-supplied software.
Results
Core lipid structure and tetraether lipid cyclization
Extracted lipid was hydrolyzed by acid methanolysis to give core lipids, which were analyzed by TLC. These compounds also were separated later on a preparative scale followed by isolation and analysis by mass spectrometry to verify their identity. Diether lipids, including the diether standard, migrated on the plate with an R f value of 0.51, while tetraether lipids migrated with R f values between 0.10 and 0.25 (see Table 1). The R f value for diether core lipid agrees with the value of 0.55 reported by Kates for the same TLC solvent system (Kates 1986).
A respiratory quinone, menaquinone-7 (MK-7H14), was extracted with the membrane lipid, which was discovered and isolated from A. fulgidus by Tindall et al. (Tindall et al. 1989). This menaquinone was separated from core lipids by TLC with an R f value of 0.90 in petroleum ether-diethyl ether-acetic acid (70:30:1) and was identified easily on the plate due to its ability to absorb UV light. The MK-7H14 also was isolated by preparative TLC and extracted from the silica gel with chloroform. Its properties were verified experimentally using spectrophotometry and APCI mass spectrometry. In the spectrophotometric assay (Ocean Optics USB2000, Dunedin, FL), the compound, dissolved in chloroform, absorbed UV light with maxima at 248 and 278 nm, which is characteristic of menaquinones (Collins 1985; Tindall et al. 1989). MK-7H14 has a theoretical exact mass of 662.5, and MS revealed an intense peak at m/z 663.5 [M+H]+.
Atmospheric pressure chemical ionization mass spectrometry easily produced mass spectral data on tetraether and diether core lipids of A. fulgidus. Figure 2 displays an example of a mass spectrum of a total core lipid sample from A. fulgidus grown at 83°C. The diether core lipid corresponds to a monoisotopic peak at m/z 653.8 [M+H]+, while the tetraether core lipid corresponds to a monoisotopic peak at m/z 1303.3 [M+H]+. The identity of other prominent mass peaks observed at m/z 569.5, 773.6, and 792.7 is uncertain, though these peaks consistently appear in the mass spectra of diether core lipids, including the spectrum of the pure diether standard, 1,2-di-O-phytanyl-sn-glycerol.
To investigate the degree of cyclization in the C 40 chains of the tetraether lipid in relation to growth temperature, A. fulgidus membrane lipids from cultures grown at 70, 78, 83, 86, and 89°C were extracted and hydrolyzed prior to core lipid analysis. Preparative TLC was used to separate the core lipid mixture into distinct bands on the plate, indicating the presence of diether lipid (R f 0.51) and tetraether lipids (R f 0.10–0.25). Up to three tetraether lipid bands were detected. However for all samples, the most intense tetraether band corresponded to the major tetraether core lipid at R f 0.25. All R f values are listed in Table 1.
Separated core lipids were extracted from bands on the preparative TLC plates and were analyzed using APCI–MS. The band with R f 0.51 corresponded to a mass spectral peak at m/z 653.6 [M+H]+, which indicates the diether core lipid. The major tetraether band at R f 0.25 for all cultures had a mass peak at m/z 1303.6 [M+H]+ while additional bands for cultures grown at 78, 83, 86, and 89°C with R f 0.16 and R f 0.10 had mass peaks at m/z 1302.1 and 1300.1 [M+H]+. These m/z peaks at∼1,304, 1,302 and 1,300 corresponding to distinct TLC bands are consistent with core tetraether lipid harboring zero, one, or two cyclopentanyl groups, respectively, as each cyclization within an isoprenoid chain results in the loss of two protons m/z 2[H+]. The culture grown at 70°C did not give rise to a TLC band at R f 0.10 corresponding to the tetraether with two cyclopentanes. These results indicate that the tetraether lipid from A. fulgidus grown at 70°C shows less cyclization than that from the archaeon grown at higher temperatures.
Effect of growth temperature on tetraether-to-diether lipid ratio
Tetraether and diether core lipid content in A. fulgidus was examined at various growth temperatures (70, 78, 83, 86, 89°C). Following membrane lipid extraction and acid methanolysis for headgroup cleavage, core lipids were analyzed by APCI mass spectrometry. For each growth temperature, the ratio of the relative intensities of the corresponding tetraether lipid to diether lipid peaks was calculated and found to range from 0.3 ± 0.1 at 70°C to 0.9 ± 0.1 at 89°C (see Fig. 3).
Headgroup chemistry
A survey of A. fulgidus lipid headgroup chemistry was conducted using TLC combined with headgroup-selective sprays. The TLC plates were run in a moderately polar solvent mixture of chloroform-methanol-acetic acid-water (85:30:15:5) producing a total of six lipid spots, with R f values ranging from 0.03 to 0.74 (Table 2). Phospholipids, glycolipids, and glycophospholipids were identified from the total lipid extract using molybdenum blue and Bial’s orcinol reagent. No evidence of amine or choline groups was detected using ninhydrin and Dragendorff’s reagent sprays. Analysis of the polar lipid fraction purified by silica gel chromatography also verified the presence of these headgroups. Spots observed at R f values of 0.03 and 0.74 are unidentified lipids or other compounds also separated by the extraction.
Using negative-ion ESI–MS, mass spectra of the polar lipid fraction revealed prominent peaks at m/z 773.6 [M-H]−, m/z 893.6 [M-H]−, m/z 1058.0 [M-H]−, and m/z 1866.5 [M-H]− (Fig. 4). Based on current knowledge of archaeal lipid chemistry, the data suggested the probable existence of a phosphoethanolamine-diether lipid, a phosphoinositol-diether lipid, a glycosyl phosphoinositol-diether lipid, and a diglycosyl phosphoinositol-tetraether lipid. These proposed lipid structures were assigned to these mass peaks based on molecular weight calculations of the core lipid structures combined with previously observed archaeal headgroup chemistries. However, we were unable to assign structures to other mass peaks observed in the spectrum based on molecular weight calculations alone. These peaks may correspond to unknown phospholipids and glycolipids, as well as other lipophilic compounds separated by the extraction and purification method used.
Negative-ion ESI mass spectrum of A. fulgidus polar lipids. The mass peaks are consistent with the following polar lipid structures: m/z 773.6 [M-H]−, Phosphoethanolamine-diether lipid (1 ethanolamine headgroup); m/z 893.6 [M-H]−, Phosphoinositol-diether lipid (1 inositol/glycosyl headgroup); m/z 1058.0 [M-H]−, Glycosyl phosphoinositol-diether lipid (2 inositol/glycosyl headgroups); m/z 1866.5 [M-H]−, Diglycosyl phosphoinositol-tetraether lipid (3 inositol/glycosyl headgroups)
Discussion
Effect of growth temperature on tetraether lipid cyclization
An increase in the pentacyclic group content of tetraether lipids at elevated temperature has been observed in other thermophilic archaea (De Rosa et al. 1980; Uda et al. 2004). This biochemical response has been suggested to be an adaptation that increases membrane stability at higher temperatures by providing a more tightly packed and less fluid membrane structure (Sprott 1992). We have observed evidence for up to a total of two cyclopentane groups in the tetraether molecules of A. fulgidus biomass grown at 78, 83, 86, and 89°C based on preparative TLC and mass spectrometry data. Tetraether lipids with zero or one pentacyclic group were detected for A. fulgidus grown at 70°C. The major tetraether core lipid molecule at all temperatures with m/z 1303.6 [M+H]+ has no pentacyclic groups. The increase in pentacyclic group content with growth temperature may arise from temperature-dependent control at the transcriptional level or from thermal effects on enzyme activity.
Effect of growth temperature on tetraether-to-diether lipid ratio
The data provide convincing evidence of a qualitative trend of increasing TE/DE ratio with growth temperature, which suggests that this also is an adaptive response of the organism. However, the TE/DE ratio, based on the relative intensities of mass spectral peaks, does not give quantitative information on the actual ether lipid composition of the cell membrane. Without a standard curve of the individual pure compounds, precise quantitative data cannot be obtained by this method. Previous studies indicated that the proportion of tetraether lipid in the cell membrane increased with growth temperature in the archaeon, Methanococcus jannaschii (Sprott et al. 1991). It is expected that tetraether lipid biosynthesis would increase with growth temperature, as increased tetraether lipid content results in increased stability and decreased freedom of motion in the cell membrane leading to improved survivability at elevated temperatures. Increased tetraether lipid biosynthesis also may result from up-regulation of certain genes and/or thermal activation of enzyme activity. DNA microarray studies of A. fulgidus in response to heat shock have been conducted by Rohlin et al. (Rohlin et al. 2005), and results revealing an elevated expression of genes hypothesized to be involved in lipid biosynthesis may help deduce the unknown pathway for tetraether lipid biosynthesis.
Headgroup chemistry
Existing knowledge of archaeal lipid biochemistry aids in identifying the probable structure of A. fulgidus lipids. Previous examination of the lipids of A. fulgidus by Trincone et al. led to the conclusion that its core lipids are based on diphytanylglycerol diether and dibiphytanyldiglycerol tetraether lipid structures (Trincone et al. 1992), also in agreement with our work. In their analysis of the complex lipids using staining tests and TLC, they identified two glycophospholipids, one phospholipid, and one glycolipid as the major lipids. The glycophospholipids identified in this study also are consistent with a diether or tetraether core lipid structure with C 20 phytanyl or C 40 biphytanyl isoprenoid hydrocarbon chains. In addition to a phosphate group at the sn-1 position of glycerol, the glycophospholipid headgroups also consist of either mono- or di-glycosyl groups. Previous studies of archaeal lipids with glycosyl headgroup chemistries indicated that these are commonly hexoses such as the glucose isomers, galactose, and mannose, as seen in the glycolipids of methanogens and related archaeal species (Koga and Morii 2005; Koga et al. 1993). While preparing this paper, Tarui et al. (Tarui et al. 2007) reported lipid headgroup structures from A. fulgidus, similar to those put forward here, including glycosyl headgroups consisting of galactose, mannose, and glucose, and also phospholipid headgroups linked to inositol or ethanolamine.
Figure 5 displays our proposed structures for the phospholipids analyzed by negative-ion ESI–MS. Glycosyl headgroups are drawn with galactosyl moieties, yet the mass spectral data cannot distinguish among inositol and other sugar isomers such as mannose or glucose, all of which have identical molecular mass. The general headgroup chemistry of tetraether lipids with pentacyclic groups could not be identified conclusively. In addition, TLC could not easily separate these molecules due to their similar headgroup chemistry, suggesting that the glycophospholipid spot from TLC could be a mixture of these lipids. Further characterization with other methods such as nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and GLC with lipid derivatization is necessary in order to determine the exact headgroup structure.
The proposed headgroup arrangement for the polar tetraether lipid, similar to a lipid found in M. thermoautotrophicum (Koga et al. 1993), consists of a phosphoryl and three inositol or glycosyl groups, most likely a phosphoinositol group on one end with a diglycosyl group on the other end. The major diether lipids are likely a phosphoinositol-diether lipid and a glycosyl phosphoinositol-diether lipid. Thus far, the proposed headgroup structures of A. fulgidus polar lipids most closely resemble those of other thermoacidophiles (Koga et al. 1993), with the exception of the presence of amine chemistry, previously seen only in methanogens.
The asymmetric headgroup structure of the glycophospho-tetraether lipid is not uncommon and may give rise to a preferred orientation in the cell membrane. It has been observed that anionic groups such as the phosphoryl or glycophosphoryl groups of tetraether lipids are oriented to the interior of the membrane, while headgroups solely consisting of glycosyl groups are located preferentially at the exterior membrane surface (Kates 1992; Morii and Koga 1994).
The detected glycophospho-tetraether lipid most likely represents the primary tetraether lipid molecule of the cell membrane. The mass peak was the only significant peak in the high mass range of tetraether molecules. It is likely that the proposed diether and tetraether structures discussed here comprise the majority of lipids in the cell membrane of A. fulgidus.
Abbreviations
- TLC:
-
Thin-layer chromatography
- APCI:
-
Atmospheric pressure chemical ionization
- ESI:
-
Electrospray ionization
- MS:
-
Mass spectrometry
- GLC:
-
Gas–liquid chromatography
- HPLC:
-
High performance liquid chromatography
- TE:
-
Tetraether lipid
- DE:
-
Diether lipid
References
Byrdwell WC (2001) Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids 36:327–346
Collins MD (1985) Analysis of Isoprenoid Quinones. Academic, Orlando
De Rosa M, Esposito E, Gambacorta A, Nicolaus B, Bu’Lock JD (1980) Effects of temperature on ether lipid composition of Caldariella acidophila. Phytochemistry 19:827–831
Demizu K, Ohtsubo S, Kohno S, Miura I, Nishihara M, Koga Y (1992) Quantitative determination of methanogenic cells based on analysis of ether-linked glycerolipids by high-performance liquid chromatography. J Ferment Bioeng 73:135–139
Gabriel JL, Chong PLG (2000) Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem Phys Lipids 105:193–200
Glasgow BJ, Abduragimov AR, Yusifov TN, Gassymov OK, Horwitz J, Hubbell WL, Faull KF (1998) Characterization of the disulfide motif and secondary structure of tear lipocalins. Biochemistry 37:2215–2225
Hopmans EC, Schouten S, Pancost RD, Van der Meer MTJ, Sinninghe Damste JS (2000) Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 14:585–589
Kates M (1986) Techniques of lipidology: isolation, analysis, and identification of lipids. Elsevier, New York
Kates M (1992) Archaebacterial lipids: structure, biosynthesis and function. Biochem Soc Symp 58:51–72
Klenk H-P, Clayton RA, Tomb JF, White O, Nelson KE, Ketchum KA, Dodson RJ, Gwinn M, Hickey EK, Peterson JD, Richardson DL, Kerlavage AR, Graham DE, Kyrpides NC, Fleischmann RD, Quackenbush J, Lee NH, Sutton GG, Gill S, Kirkness EF, Dougherty BA, McKenney K, Adams MD, Loftus B, Peterson S, Reich CI, McNeil LK, Badger JH, Glodek A, Zhou L, Overbeek R, Gocayne JD, Weidman JF, McDonald L, Utterback T, Cotton MD, Spriggs T, Artiach P, Kaine BP, Sykes SM, Sadow PW, D’Andrea KP, Bowman C, Fujii C, Garland SA, Mason TM, Olsen GJ, Fraser CM, Smith HO, Woese CR, Venter JC (1997) The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364–370
Koga Y, Morii H (2005) Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci Biotech Bioch 69:2019–2034
Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M (1993) Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol Rev 57:164–182
Langworthy TA (1985) Lipids of Archaebacteria. In: Woese CR, Wolfe RS (eds) Archaebacteria. Academic, New York, pp 459–497
Morii H, Eguchi T, Nishihara M, Kakinuma K, Konig H, Koga Y (1998) A novel ether core lipid with H-shaped C80-isoprenoid hydrocarbon chain from the hyperthermophilic methanogen Methanothermus fervidus. Biochim Biophys Acta 1390:339–345
Morii H, Koga Y (1994) Asymmetrical topology of diether- and tetraether-type polar lipids in membranes of Methanobacterium thermoautotrophicum cells. J Biol Chem 269:10492–10497
Murae T, Takamatsu Y, Muraoka R, Endoh S, Yamauchi N (2002) Facile distinction of neutral and acidic tetraether lipids in archaea membrane by halogen atom adduct ions in electrospray ionization mass spectrometry. J Mass Spectrom 37:209–215
Nishihara M, Koga Y (1987) Extraction and composition of polar lipids from the archaebacterium, Methanobacterium thermoautotrophicum: effective extraction of tetraether lipids by an acidified solvent. J Biochem (Tokyo, Jpn) 101:997–1005
Nishihara M, Koga Y (1991) Hydroxyarchaetidylserine and hydroxyarchaetidyl-myo-inositol in Methanosarcina barkeri: polar lipids with a new ether core portion. Biochim Biophys Acta 1082:211–217
Qui D-F, Games MPL, Xiao X-Y, Games DE, Walton TJ (1998) Application of high-performance liquid chromatography/electrospray mass spectrometry for the characterization of membrane lipids in the haloalkaliphilic archaebacterium Natronobacterium magadii. Rapid Commun Mass Sp 12:939–946
Qui D-F, Games MPL, Xiao X-Y, Games DE, Walton TJ (2000) Characterisation of membrane phospholipids and glycolipids from a halophilic archaebacterium by high-performance liquid chromatography/electrospray mass spectrometry. Rapid Commun Mass Sp 14:1586–1591
Rohlin L, Trent JD, Salmon K, Kim U, Gunsalus RP, Liao JC (2005) Heat shock response of Archaeoglobus fulgidus. J Bacteriol 187:6046–6057
Sprott GD (1992) Structures of archaebacterial membrane lipids. J Bioenerg Biomembr 24:555–566
Sprott GD, Dicaire CJ, Patel GB (1994) The ether lipids of Methanosarcina mazei and other Methanosarcina species, compared by fast atom bombardment mass spectrometry. Can J Microbiol 40:837–843
Sprott GD, Meloche M, Richards JC (1991) Proportions of diether, macrocyclic diether, and tetraether lipids in Methanococcus jannaschii grown at different temperatures. J Bacteriol 173:3907–3910
Stetter KO, Lauerer G, Thomm M, Neuner A (1987) Isolation of extremely thermophilic sulfate reducers: evidence for a novel branch of archaebacteria. Science 236:822–824
Sturt HF, Summons RE, Smith K, Elvert M, Hinrichs K-U (2003) Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Commun Mass Sp 18:617–628
Tarui M, Tanaka N, Tomura K, Ohga M, Morii H, Koga Y (2007) Lipid component parts analysis of the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus. J UOEH 29:131–139
Tindall BJ, Stetter KO, Collins MD (1989) A novel, fully saturated menaquinone from the thermophilic, sulphate-reducing archaebacterium Archaeoglobus fulgidus. J Gen Microbiol 135:693–696
Tornabene TG, Langworthy TA (1979) Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science 203:51–53
Trincone A, Nicolaus B, Palmieri G, Rosa MD, Huber R, Huber G, Stetter K, Gambacorta A (1992) Distribution of complex and core lipids within new hyperthermophilic members for the archaea domain. Syst Appl Microbiol 15:11–17
Uda I, Sugai A, Itoh YH, Itoh Y (2004) Variation in molecular species of core lipids from the order Themoplasmales strains depends on the growth temperature. J Oleo Sci 53:399–404
Watson JT (1997) Introduction to Mass Spectrometry. Lippincott-Raven, Philadelphia
Acknowledgments
This work was supported by a NIH Metabolic Engineering Grant (5 R01 GM077627). Technical assistance, use of laboratory facilities and supplies were kindly provided by Dr. Imke Schroeder and Dr. Robert Gunsalus of the Department of Microbiology, Immunology, and Molecular Genetics at UCLA. Help with the mass spectral analyses was kindly provided by Alek Dooley and Dr. Kym Faull in the Pasarow Mass Spectrometry Laboratory at UCLA. Purchase of mass spectrometric instrumentation was made with help from the W.M. Keck Foundation and the Pasarow Family Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Rights and permissions
About this article
Cite this article
Lai, D., Springstead, J.R. & Monbouquette, H.G. Effect of growth temperature on ether lipid biochemistry in Archaeoglobus fulgidus . Extremophiles 12, 271–278 (2008). https://doi.org/10.1007/s00792-007-0126-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0126-6