Abstract
We report the identification and characterization of the single-stranded DNA-binding protein (SSB) from the mesophile and highly radiation-resistant Deinococcus radiopugnans (DrpSSB). PCR-derived DNA fragment containing the complete structural gene for DrpSSB protein was cloned and expressed in Escherichia coli. The gene consisting of an open reading frame of 900 nucleotides encodes a protein of 300 amino acids with a calculated molecular weight of 32.45 kDa and pI 5.34. The amino acids sequence exhibits 43, 44, 79 and 18% identity with Thermus aquaticus, Thermus thermophilus, Deinococcus radiodurans and E. coli SSBs, respectively. The DrpSSB includes two OB folds per monomer and functions as a homodimer. In fluorescence titrations with poly(dT), DrpSSB bound 24–31 nt depending on the salt concentration, and fluorescence was quenched by about 80%. In a complementation assay in E. coli, DrpSSB took over the in vivo function of EcoSSB. The half-lives of DrpSSB were 120 min at 90°C, 60 min at 95°C and 30 min at 100°C. These results were surprising in the context of half-life of SSB from thermophilic T. aquaticus, which has only 30 s of half-life at 95°C. DrpSSB is the most thermostable SSB-like protein identified to date, offering an attractive alternative for TaqSSB and TthSSB in their applications for molecular biology methods and analytical purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The single-stranded DNA-binding proteins (SSB) play an important role in DNA replication, repair and recombination. They are present in all three Domains of Life and in viruses and share sequences as well as biochemical and structural characteristics. In their soluble form, SSBs are found in different oligomeric states. They are found in different organisms as homodimers (SSBs from bacteriophages, Thermus thermophilus, Thermus aquaticus and Deinococcus radiodurans), heterotrimers (euryarchaeal and eukaryotic SSBs, alias RPAs) and homotetramers (mitochondrial, crenarchaeal and most prokaryotic SSBs) (Williams and Konigsberg 1978; Shamoo et al. 1995; Stassen et al. 1995; Genschel et al. 1996; Webster et al. 1997; Wadsworth and White 2001; Dąbrowski et al. 2002b; Eggington et al. 2004; Kur et al. 2005). Although the sequences of SSB family members are highly variable, two common functional themes have emerged that link this class of proteins across evolution. The first is that SSB proteins use a conserved domain called an oligonucleotide/oligosaccharide-binding (OB) fold to bind ssDNA (Murzin 1993). The second common feature of SSB proteins is obligate oligomerization that brings together four DNA-binding OB folds. Although virtually all bacterial SSB family members act as homotetramers, recent discoveries have shown that SSB proteins from the phylum Deinococcus–Thermus of bacteria adopt a different architecture. They are functional as homodimeric, with each SSB monomer encoding two OB folds linked by a conserved spacer sequence (Dąbrowski et al. 2002a; Bernstein et al. 2004; Eggington et al. 2004; Kur et al. 2005). These homodimers thus mimic the homotetrameric SSBs. The proteins from T. thermophilus (TthSSB) and T. aquaticus (TaqSSB) and their counterpart from D. radiodurans (DraSSB) are the largest bacterial SSBs and consist of 263, 264 and 301 amino acid residues, respectively (Dąbrowski et al. 2002a, b; Eggington et al. 2004; Witte et al. 2005). Homodimeric SSBs, contrary to tetrameric SSB proteins, possess only two C-terminal tails in each active form. Reducing the number of C-terminal tails by half can dramatically affect the function of the two OB-fold containing SSB proteins in vivo (Curth et al. 1996). These SSBs may represent an evolutionary convergence between homotetrameric bacterial/crenarchaeal SSB and eukaryotic/euryarchaeal RPA family members (Bernstein et al. 2004).
At present there are 20 validly described Deinococcus species (Hirsch et al. 2004; de Groot et al. 2005; Omelchenko et al. 2005). With the exception of D. murrayi and D. geothermalis, which have optimum growth temperatures of 45–50°C, all other species have optimum growth temperatures between about 25 and 35°C. The most striking characteristic of the species of this genus is their extreme resistance to UV and gamma radiation. Until now, only D. radiodurans SSB protein has been described in detail (Bernstein et al. 2004; Eggington et al. 2004; Witte et al. 2005).
We report here the identification, purification and characterization of D. radiopugnans SSB and its relationship with other members of this important class of proteins.
Materials and methods
Bacterial strains, plasmids, enzymes and reagents
Deinococcus radiopugnans DSMZ 12027 strain was purchased from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Germany). E. coli TOP10F′ strain and pBADMycHisA plasmid were purchased from Invitrogen (USA) and used for protein expression. The reagents for PCR and various oligodeoxynucleotides were obtained from DNA-Gdańsk II (Poland). The oligonucleotides 5′-end-labelled with fluorescein were purchased from Metabion (Germany). Restriction enzymes were from Fermentas (Lithuania). The reagents for protein purification and electrophoresis were purchased from USB and Sigma (USA).
Cloning of the ssb-like gene from D. radiopugnans
DNA from D. radiopugnans was isolated using Genomic DNA Prep Kit (A&A Biotechnology, Poland). Based on the known localization of the ssb gene in D. radiodurans (GenBank Accession no. AY293617) and D. geothermalis (GenBank Accession No. AAHE01000002) genomes, where the ssb gene is flanked by the conservative rpsF and rpsR genes encoding the ribosomal proteins S6 and S18, the primers complementary to those genes for PCR amplification were designed and synthesized. The forward primer was 5′-GACAACGTCCGCCGCGTCCTGGTGGT (26 nt) and the reverse primer was 5′-GTGCGGCGGCGGGGAAGAATCTT (23 nt). The reaction solution consisted of 0.2 μg D. radiopugnans DNA, 2 μl (10 μM) of each primer, 5 μl (10 mM) dNTPs, 5 μl of 10 × PCR buffer (200 mM Tris–HCl, pH 8.8, 100 mM KCl, 100 mM (NH4)2SO4, 1% Triton X-100), 5 μl (2 mM) MgSO4 and 2 U Pwo DNA polymerase (DNA-Gdańsk II) and 35 cycles were performed with a temperature profile of 80 s at 94°C, 80 s at 67.4°C and 120 s at 72°C. Specific, about 1,300 bp, PCR product was obtained and then sequenced to confirm the presence of ssb-like gene.
The obtained ssb gene sequence of the D. radiopugnans was used to design gene-specific primers for PCR and for cloning into pBADMycHisA vector. First, PCR was carried out using forward primer, 5′-ATTACC ATG G CCCGAGGCATGAACCA (26 nt, containing NcoI recognition site underlined and ATG start codon italicized), and reverse primer, 5′-ATAGAAGCT TT A GGACGGCAGGTCTTCTTCTTCCG (35 nt, containing HindIII recognition site underlined and UAA stop codon italicized). The boldface parts of primer sequences are complementary to the nucleotide sequences of the ssb gene of D. radiopugnans, whereas 5′ overhanging ends of primers contain recognition sites for restriction endonucleases and are designed to facilitate cloning. The PCR conditions were the same as stated above.
The amplified product (918 bp), digested with NcoI + HindIII, was isolated from an agarose gel band using Gel-Out Kit (A&A Biotechnology). The purified fragment was ligated into pBADMycHisA between NcoI and HindIII sites. E. coli TOP10F′ cells were transformed with the ligation mixture and the obtained colonies were examined for the presence of ssb gene of D. radiopugnans by PCR amplification and restriction analysis. Two independent clones were selected and sequenced to confirm the presence of ssb gene of D. radiopugnans. The constructed plasmid, named pBAD-DrpSSB, was used to transform the E. coli TOP10F′ cells and the recombinant strain obtained was applied for expression and purification procedure.
Protein sequence analysis of DrpSSB
The amino acid sequence of DrpSSB was analysed using standard protein–protein BLAST and RPS-BLAST. Multiple sequence alignment was generated using the program ClustalX. The results were prepared using the editor program Gendoc (© Karl Nicholas).
Expression and purification of the recombinant DrpSSB
Escherichia coli TOP10F′ strain transformed with pBAD-DrpSSB was grown at 37°C in 500 ml LB containing 100 μg/ml ampicillin to an A 600 of 0.5. Arabinose was then added to a final concentration of 0.2%. After 4 h, the cells were harvested by centrifugation (about 4 g E. coli TOP10F′ pBAD-DrpSSB wet cell mass) and the pellet was resuspended in 30 ml buffer A (10 mM Tris–HCl, pH 9, 10 mM EDTA, 10 mM CaCl2, 0.5 mg/ml lysozyme). The purification procedure was analogous to the previously published purification scheme for the SSB from calf thymus (Atrazhev et al. 1992) and that for thermostable SSB-like proteins (Dąbrowski et al. 2002a), with some modifications. The cells were disrupted by sonication and the insoluble debris was removed by centrifugation. The supernatant (about 25 ml) was heat-treated at 65°C for 10 min and denatured host proteins were removed by centrifugation. The clarified supernatant was applied further directly onto QAE-cellulose column (70 ml bed volume, Sigma), pre-equilibrated with 3 vol. buffer B (20 mM Tris–HCl, pH 9, 50 mM NaCl and 10 mM EDTA). The column was washed with 240 ml buffer B and the SSB protein was eluted with linear gradient (140 ml) of 0.05–2 M NaCl in buffer B. Proteins eluted at about 0.34 M NaCl (Fig. 2, lane 3) were combined, five times diluted with buffer B and loaded onto a ssDNA-cellulose column (3 ml; USB) equilibrated with buffer B. The column was washed with 150 ml of buffer C (50 mM Tris–HCl, pH 9, 0.3 M NaCl, 10 mM EDTA, 10% glycerol), then with 150 ml of buffer D (50 mM Tris–HCl, pH 9, 0.8 M NaCl, 10 mM EDTA, 10% glycerol) and finally eluted with 50 ml of buffer E (50 mM Tris–HCl, pH 9, 2 mM NaCl, 10 mM EDTA, 10% glycerol). Protein-containing fractions were combined, desalted, concentrated using Amicon Ultra-15 centrifugal filter device (Millipore) and stored at − 20°C in a buffer containing: 20 mM Tris–HCl, pH 8.3, 50 mM NaCl, 50% glycerol, 10 mM EDTA, 0.05% Igepal, 1 mM DTT, 1 mM β-mercaptoethanol, until use. The purity of protein was examined by SDS-PAGE. The amounts of purified DrpSSB protein were estimated spectrophotometrically using the appropriate absorption coefficient factor and by the optical densitometry on SDS-PAGE gel.
The cloning, expression and purification procedure for the SSB from D. radiodurans (DraSSB) were analogous to the previously published scheme by Eggington et al. (2004).
Estimation of the native molecular mass
The molecular mass of DrpSSB protein was determined by HPLC gel filtration on a Superdex HR 75 column (Amersham Bioscience AB, Sweden). The elution pattern of DrpSSB protein was then compared with those of standard proteins: bovine albumin (66 kDa), ovalbumin (43 kDa), carbon anhydrase (29 kDa) and cytochrome C (12.4 kDa).
Gel mobility shift assays: binding to ss oligonucleotides
A fixed quantity (10 pM) of 5′-end fluorescein-labelled oligonucleotides (dT)35, (dT)60, (dT)70 or (dT)76 was incubated with 0, 10 and 100 pM of DrpSSB in 10 μl of buffer B for 20 min at 25°C. The reaction products were resolved by electrophoresis in 2% agarose gels without ethidium bromide.
Fluorescence titration
Fluorescence titrations were carried out in a Perkin-Elmer LS-5B luminescence spectrometer as described earlier (Dąbrowski and Kur 1999). The binding reaction was assembled in 2 ml buffer (20 mM Tris–HCl, pH 7.5, 10 mM EDTA) containing 2 or 100 mM NaCl and was incubated at 25°C. A constant amount of DrpSSB protein (1 nM) was incubated with varying quantities of (dT)60 oligonucleotide (from 0 to 0.8 nM) (Metabion). The excitation and emission wavelengths were 295 and 348 nm, respectively. Binding curve analysis was carried out using the model of Schwarz and Watanabe (1983).
Thermostability
A fixed quantity (10 pM) of 5′-end fluorescein-labelled oligonucleotide (dT)35 was added to 10 pM of DrpSSB or TaqSSB preincubated for 0, 5, 10, 30, 60, 120 and 180 min at 70, 75, 80, 85, 90, 95 and 100°C in 10 μl reaction mixtures in standard buffer B (100 mM NaCl). The assay conditions for DraSSB were the same as stated above with the exception of different temperatures used for protein preincubation: 50, 50, 60 and 65°C. Protein–DNA complexes after 20 min incubation at 25°C were separated from free DNA by 2% agarose gel electrophoresis, and 50% quantities of protein–(dT)35 complex were evaluated by densitometric analysis using VersaDoc imaging system and QuantityOne software (BioRad).
Complementation analysis
Escherichia coli RDP268 strain, in which the chromosomal ssb gene is replaced by a kanamycin resistance (Δssb::Kan R) and harbouring pRPZ146 (ColEl ori, TcR) plasmid coding wild-type SSB protein (Porter and Black 1991), was lysogenized with λDE3 using λDE3 Lysogenization Kit (Novagen). The resulting strain can be used to express target genes cloned in the pET vectors under the control of the T7 promoter. pRPZ146 is essential for the survival of the cells and can be replaced by another plasmid, only if it contains a gene whose product can take over EcoSSB function in vivo. We used the modified RDP268(DE3) strain to transform the plasmids pET23D(+)DrpSSB or pET23D(+)EcoSSB, which carries the DrpSSB or EcoSSB gene under the control of the T7 promoter and confers ampicillin resistance. Strains were grown in the presence of ampicillin (100 μg/ml) and kanamycin (25 μg/ml). After four consecutive overnight subculturings in 3 ml of 2YT (1.6% tryptone, 1% yeast extract and 0.5% NaCl with or without 1 mM IPTG) containing the same antibiotics, the colonies were patched on 2YT agar plates containing ampicillin alone (100 μg/ml) and on plates containing both tetracycline (25 μg/ml) and ampicillin (100 μg/ml). An AmpR and TcS phenotype shows that the incoming plasmid harbours a gene that complements EcoSSB. On the other hand, maintenance of TcR, AmpR phenotype indicates that the tester plasmid is unable to complement EcoSSB.
Nucleotide sequence accession number
The nucleotide sequence of the ssb gene of D. radiopugnans is available in the GenBank database under accession number DQ379516.
Results
Deinococcus radiopugnans SSB protein is closely related to the D. radiodurans, T. thermophilus and T. aquaticus SSB proteins
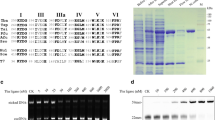
The protein sequence for DrpSSB protein shares 79% identity with the D. radiodurans R1 SSB, 44% identity with the T. thermophilus HB-8 SSB, 43% identity with the T. thermophilus VK-1 SSB and 43% identity with the T. aquaticus YT-1 SSB (Fig. 1). The N-terminal segment of the DrpSSB protein (from amino acids 1 to 124) shares 88% identity with the D. radiodurans R1 and 3% identity with the E. coli SSB protein. The C-terminal segment of the DrpSSB protein (from amino acids 125 to 300) shares 54% identity with the D. radiodurans R1 and 6% identity with the E. coli SSB protein. The DrpSSB protein gene structure is quite similar to that of the T. thermophilus, T. aquaticus and D. radiodurans ssb gene structures and is predicted to contain two OB folds. High similarity of DrpSSB protein sequence to Thermus and D. radiodurans SSB proteins (Fig. 1) suggested that D. radiopugnans SSB protein would also form a homodimer. This prediction was confirmed (see below).
Multiple amino acid sequence alignment of SSB-like proteins. Alignment was performed by dividing amino acids into six similarity groups. Group 1: V, L, I and M; group 2: W, F and Y; group 3: E and D; group 4: K and R; group 5: Q and D; group 6: S and T. Description of similarity: white fonts on black boxes 100% identity; white fonts on grey boxes similarity < 80%; black fonts on grey boxes similarity < 60%. Asterisks indicate conserved amino acids in the EcoSSB sequence that is engaged in stacking interaction with bases of ssDNA (W41, W55, F61 and W89) and stabilization of the tetramer (H56). EcoK12, E. coli K-12; TaqYT1, T. aquaticus strain YT-1; TthHB8, T. thermophilus strain HB-8; TthHB27, T. thermophilus strain HB-27; TthVK1, T. thermophilus strain VK-1; DraR1, D. radiodurans strain R1; Drp, D. radiopugnans strain 12027; -N, N-terminal ssDNA-binding domain, -C, C-terminal ssDNA-binding domain
Expression and purification of the recombinant DrpSSB protein
The nucleotide sequence of the PCR product obtained by amplification of the region between rpsF and rpsR genes revealed a contiguous ORF that encodes a complete SSB protein. Based on this sequence ssb gene was amplified and cloned into an E. coli pBADMycHisA expression vector. Excellent inducible expression of a protein of the predicted size was obtained from the recombinant pBAD-DrpSSB plasmid (Fig. 2, lane 1). DrpSSB protein was expressed in a soluble form in the cytosol. The main advantage of the purification of thermostable SSB-like proteins is the reduction of contamination by the host proteins after heat treatment (Fig. 2, lane 2). The applied overexpression system was efficient, giving about 170 mg of purified DrpSSB protein (from 1 l of induced culture). This protein was purified with about 99% purity (Fig. 2, lane 4). Additionally, the purified DrpSSB protein was free of detectable DNA endo- and exonucleases.
SDS-electrophoresis in 12% polyacrylamide gel of the fractions obtained by expression and purification of the recombinant DrpSSB from E. coli TOP10F′ + pBAD-DrpSSB. Lane M, LMW markers (Fermentas), the molecular weights of proteins are marked; lane 1, soluble protein cell extracts after arabinose induction of protein expression; lane 2 DrpSSB, after heat treatment at 65°C for 10 min; lane 3, DrpSSB after chromatography on QAE-cellulose column; lane 4, DrpSSB after chromatography on ssDNA-cellulose column; lane 5, concentrated DrpSSB protein
DrpSSB protein forms a homodimer
Analysis of the purified proteins by SDS-PAGE revealed single major bands with a molecular mass of 32 kDa (calculated from the amino acid sequences, the molecular mass of DrpSSB is 32.45 kDa). Analysis of purified proteins by gel filtration chromatography revealed single peaks with a molecular mass of about 57.43 kDa. This value is 1.8 times the molecular weight of a DrpSSB monomer. This confirmed our prediction that DrpSSB protein exists as a homodimer.
DNA-binding properties
Whenever two macromolecules interact, the resulting complex will have a larger mass than any of the single components and, thus, it will sediment faster than the single species. Binding SSB proteins to polymeric ssDNA results in a dramatic increase in sedimentation rate. All homotetrameric and homodimeric SSB proteins studied so far show a dramatic decrease of tryptophan fluorescence when binding to ssDNA (Urbanke and Schaper 1990).
To determine the ability of DrpSSB protein to bind to ssDNA, we carried out agarose gel mobility assays with 5′ fluorescein-labelled ss dT-oligonucleotides 35, 60, 70, 76 nucleotides in length (Fig. 3). A single band of reduced mobility was observed, when 10 pM of (dT)35 or (dT)60 were incubated with increasing concentrations of SSB-like proteins. Most of (dT)35 and (dT)60 were shifted after addition of one molar equivalent (10 pM) of DrpSSB (calculated as 60 kDa homodimer), and the mobility of the shifted band remained constant at 10 times higher protein concentrations (100 pM). A band of identical mobility was observed for (dT)70 and (dT)76 at low protein concentrations (10 pM), but a second band with a lower mobility was observed at high protein concentrations (100 pM). These results suggest that DrpSSB protein binds to (dT)35 and (dT)60 as a single protein molecule whereas two SSB molecules bind to (dT)70 and (dT)76. Thus, the ssDNA-binding site for DrpSSB protein is between 30 and 35 nucleotides long.
To further explore the binding properties of the DrpSSB protein, we made use of fluorescence spectroscopy. With an excitation wavelength of 295 nm, the emission spectrum of SSB-like proteins at 25°C had a maximum at 348 nm, consistent with tryptophan fluorescence. On addition of a saturating quantity of ssDNA, the intrinsic fluorescence at 348 nm was quenched by 77% at 2 mM NaCl and 80% at 100 mM NaCl. Thus, at least five of the seven Trp residues of DrpSSB monomer are directly engaged in ssDNA binding. The estimated size of the ssDNA binding site in the presence of 2 mM NaCl for DrpSSB protein is 24 ± 2 nt (Fig. 4). A slight binding-mode transition has been observed when changing the ionic environment from low salt (2 mM NaCl) to high salt (100 mM NaCl); binding-site size increases from 24 ± 2 nt to 31 ± 2 nt per homodimer (Fig. 4a, b).
Thermostability
DrpSSB maintained 100% activity after 180 min incubation at 85°C, with half-lives of 120 min at 90°C, 60 min at 95°C, 30 min at 100°C and 10 min at 105°C (Fig. 5). TaqSSB has a half-life of 10 min at 85°C, 4 min at 90°C and 30–60 s at 95°C (Fig. 5). DraSSB is rather thermally labile protein with half-lives of 30, 20, 10 and 1 min for ssDNA-binding activity at 50, 55, 60 and 65°C, respectively.
DrpSSB can replace EcoSSB in vivo
In the complementation experiments, we attempted to replace the resident plasmid (pRPZ146, ori ColEl, TcR, harbouring a wild-type ssb gene) from E. coli RDP268(DE3) (Δssb::Kan) with the plasmids harbouring test ssb genes (pET23D(+)DrpSSB or pET23D(+)EcoSSB, ori ColEl, AmpR). As SSB is an essential protein, success in replacement of the original TcR plasmid by the incoming AmpR plasmid, resulting in a TcS, AmpR phenotype, shows that the test SSB complements the Δssb strain of E. coli. The pET23D(+)EcoSSB was used as a control to assess the efficacy of the complementation assay. After transformation of modified E. coli cells using pET23D(+)DrpSSB or pET23D(+)EcoSSB, which encodes resistance against ampicillin, and subsequent inoculations, we could isolate clones that showed resistance to ampicillin and kanamycin but not to tetracycline. These clones must have lost the pRPZ146 plasmid encoding for EcoSSB. Additionally, an analysis of plasmid DNA was carried out after plasmid isolation from tested strains. The results confirmed the presence of appropriate plasmids. Interestingly, under the same assay conditions, we obtained better growth of cultures containing pET23D(+)DrpSSB, then with pET23D(+)EcoSSB and the results were independent of IPTG induction of SSB expression.
Discussion
We have reported here the identification, purification and characterization of the SSB-like protein of the mesophile bacteria D. radiopugnans. From sequence analysis, it is obvious that two ssDNA-binding domains in one monomer of DrpSSB possess canonical oligonucleotide binding fold, very similar to the homology observed in the structure of the D. radiodurans SSB. This analysis indicates that we may divide DrpSSB monomer protein into two putative domains; a smaller N-terminal domain with a fragment responsible only for binding to ssDNA and a larger C-terminal domain with a second ssDNA-binding sequence, followed by about 50 amino acid long fragment with highly acidic C terminus. So, the functional dimer of DrpSSB protein possesses four ssDNA-binding domains as in other prokaryotes and only two C-terminal regions with a putative role in interactions with other cellular proteins.
Sequence alignment shows a different similarity pattern in the N- and C-terminal ssDNA-binding domains of the DrpSSB monomer relative to critical residues involved in interaction with ssDNA in EcoSSB (Raghunathan et al. 2000). The E. coli SSB base-stacking residues are Trp-40, Trp-54, Phe-60 and Trp-88. Only Trp-40 of EcoSSB is homologous to the Trp-40 (Trp-164 in monomer) residue in the C-terminal domain of DrpSSB and Trp-88 of EcoSSB is homologous to the Trp-88 residue in the N-terminal domain of DrpSSB and DraSSB (Fig. 1). His-55, critical for the formation of the tetrameric form of SSB in E. coli and other prokaryotes (Williams et al. 1983), is homologous to the corresponding His-54 residue present only in the N-terminal domains of the DrpSSB, DraSSB, TaqSSB and TthSSB proteins. These differences could lead to differential ssDNA binding by the individual OB folds in DrpSSB.
The C-terminal, 50 amino acids domain of DrpSSB protein possesses the lowest sequence similarity to EcoSSB. This region in the crystal structure of EcoSSB protrudes from the surface of each subunit as an unstructured fragment due to the high content of Gly and Pro residues (Matsumoto et al. 2000). The function of this region, in its interaction with other proteins, is dependent on its acidic character, as shown by the construction of chimeric proteins (Curth et al. 1996; Handa et al. 2001). In DrpSSB protein, as for TaqSSB, TthSSB and DraSSB, this region possesses conserved acidic character and a high content of Gly and Pro. Besides, the C terminus of DrpSSB and DraSSB possesses additional Glu and Asp residues, respectively, which are absent in EcoSSB, TaqSSB and TthSSB (Fig. 1).
DrpSSB is the most thermostable SSB-like protein identified to date, which has a half-life of 60 min at 95°C, offering an attractive alternative for TaqSSB and TthSSB in their applications for molecular biology methods and analytical purposes (Kur et al. 2005). These results were surprising in the context of the mesophile D. radiopugnans and in comparison with the SSB from thermophilic T. aquaticus, which has a half-life of 30–60 s at 95°C and with the SSB from mesophilic D. radiodurans, which has a half-life of 60 s at 65°C. DrpSSB does not possess any special features relative to the SSB of E. coli and D. radiodurans mesophiles. There is a high amino acid sequence identity between DrpSSB and DraSSB (79%). Sequence comparisons and percentages of the amino acid content of DrpSSB and DraSSB do not explain fully the cause of a high DrpSSB thermostability (Table 1). Only the analysis of the structure of the examined protein may explain the cause of the stability. Sequence comparisons of the thermophilic and the mesophilic proteins have shown some significant substitutions in thermophilic proteins such as Lys to Arg, Ser to Ala, Gly to Ala, Ser to Thr and Val to Ile (Ladenstein and Antranikian 1998; Scandurra et al. 1998). DrpSSB protein has a content of charged (Asp, Glu, Lys and Arg; 26.7%) residues higher than that of EcoSSB (19.1%) (Table 1). These residues in the thermophilic proteins may be involved in the stabilization of the interdomain surface by ionic networks (Karshikoff and Ladenstein 2001). The higher content of positively charged residues may also suggest that the thermophilic SSB proteins interact much more strongly with the backbone atoms of ssDNA than does EcoSSB. There is a significant increase of Arg residues relative to EcoSSB (Table 1). In many cases Arg residues stabilize proteins by hydrophobic interaction at the surface (Van den Burg et al. 1994). As an ionic positively charged residue, Arg is better adapted to high temperatures than Lys. Thermostable DrpSSB possesses a higher content of aliphatic hydrophobic residues than EcoSSB; there is a significant increase in the level of Ala (Table 1). There is a preference for a decrease in the Gly content in positions of low structural importance for fold conservation in many thermostable proteins (Matthews et al. 1987; Korolev et al. 1995). DrpSSB has a lower content of Gly residues than EcoSSB (Table 1).
The homodimer SSB-like proteins possess only two acidic C-terminal fragments, as compared to four C-terminal fragments in EcoSSB. In the homodimeric SSBs, one monomer protein possesses two ssDNA-binding domains which are reminiscent of the two monomers of EcoSSB. This conjunction in the homodimeric SSB may permit a reduction in the surface to volume ratio and more compactness in the hydrophobic core and in the interface between domains relative to the dimer in EcoSSB (Ladenstein and Antranikian 1998).
Abbreviations
- dsDNA:
-
Double-stranded DNA
- OB fold:
-
Oligonucleotide/oligosaccharide-binding fold
- RPA:
-
Replication protein A
- SSB:
-
Single-stranded DNA binding
References
Atrazhev A, Zhang S, Grosse F (1992) Single-stranded DNA binding protein from calf thymus. Purification, properties, and stimulation of the homologous DNA-polymerase-alpha-primase complex. Eur J Biochem 210(3):855–865
Bernstein DA, Eggington JM, Killoran MP, Misic AM, Cox MM, Keck JL (2004) Crystal structure of the Deinococcus radiodurans single-stranded DNA-binding protein suggests a mechanism for coping with DNA damage. Proc Natl Acad Sci USA 101(23):8575–8580
Curth U, Genschel J, Urbanke C, Greipel J (1996) In vitro and in vivo function of the C-terminus of Escherichia coli single-stranded DNA binding protein. Nucleic Acids Res 24(14):2706–2711
Dąbrowski S, Kur J (1999) Cloning, overexpression, and purification of the recombinant His-tagged SSB protein of Escherichia coli and use in polymerase chain reaction amplification. Protein Expr Purif 16(1):96–102
Dąbrowski S, Olszewski M, Piatek R, Brillowska-Dabrowska A, Konopa G, Kur J (2002a) Identification and characterization of single-stranded-DNA-binding proteins from Thermus thermophilus and Thermus aquaticus—new arrangement of binding domains. Microbiology 148(Pt 10):3307–3315
Dąbrowski S, Olszewski M, Piatek R, Kur J (2002b) Novel thermostable ssDNA-binding proteins from Thermus thermophilus and T. aquaticus—expression and purification. Protein Expr Purif 26(1):131–138
Eggington JM, Haruta N, Wood EA, Cox MM (2004) The single-stranded DNA-binding protein of Deinococcus radiodurans. BMC Microbiol 4:2
Genschel J, Litz L, Thole H, Roemling U, Urbanke C (1996) Isolation, sequencing and overproduction of the single-stranded DNA binding protein from Pseudomonas aeruginosa PAO. Gene 182(1–2):137–143
de Groot A, Chapon V, Servant P, Christen R, Saux MF, Sommer S, Heulin T (2005) Deinococcus deserti sp. nov., a gamma-radiation-tolerant bacterium isolated from the Sahara Desert. Int J Syst Evol Microbiol 55(Pt 6):2441–2446
Handa P, Acharya N, Varshney U (2001) Chimeras between single-stranded DNA-binding proteins from Escherichia coli and Mycobacterium tuberculosis reveal that their C-terminal domains interact with uracil DNA glycosylases. J Biol Chem 276(20):16992–16997
Hirsch P, Gallikowski CA, Siebert J, Peissl K, Kroppenstedt R, Schumann P, Stackebrandt E, Anderson R (2004) Deinococcus frigens sp. nov., Deinococcus saxicola sp. nov., and Deinococcus marmoris sp. nov., low temperature and draught-tolerating, UV-resistant bacteria from continental Antarctica. Syst Appl Microbiol 27(6):636–645
Karshikoff A, Ladenstein R (2001) Ion pairs and the thermotolerance of proteins from hyperthermophiles: a “traffic rule” for hot roads. Trends Biochem Sci 26(9):550–556
Korolev S, Nayal M, Barnes WM, Di Cera E, Waksman G (1995) Crystal structure of the large fragment of Thermus aquaticus DNA polymerase I at 2.5-A resolution: structural basis for thermostability. Proc Natl Acad Sci USA 92(20):9264–9268
Kur J, Olszewski M, Długołęcka A, Filipkowski P (2005) Single-stranded DNA-binding proteins (SSBs)—sources and applications in molecular biology. Acta Biochim Pol 52(3):569–574
Ladenstein R, Antranikian G (1998) Proteins from hyperthermophiles: stability and enzymatic catalysis close to the boiling point of water. Adv Biochem Eng Biotechnol 61:37–85
Matsumoto T, Morimoto Y, Shibata N, Kinebuchi T, Shimamoto N, Tsukihara T, Yasuoka N (2000) Roles of functional loops and the C-terminal segment of a single-stranded DNA binding protein elucidated by X-ray structure analysis. J Biochem (Tokyo) 127(2):329–335
Matthews BW, Nicholson H, Becktel WJ (1987) Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc Natl Acad Sci USA 84(19):6663–6667
Murzin AG (1993) OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J 12(3):861–867
Omelchenko MV, Wolf YI, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Daly MJ, Koonin EV, Makarova KS (2005) Comparative genomics of Thermus thermophilus and Deinococcus radiodurans: divergent routes of adaptation to thermophily and radiation resistance. BMC Evol Biol 5:57
Porter RD, Black S (1991) The single-stranded-DNA-binding protein encoded by the Escherichia coli F factor can complement a deletion of the chromosomal ssb gene. J Bacteriol 173(8):2720–2723
Raghunathan S, Kozlov AG, Lohman TM, Waksman G (2000) Structure of the DNA binding domain of E. coli SSB bound to ssDNA. Nat Struct Biol 7(8):648–652
Scandurra R, Consalvi V, Chiaraluce R, Politi L, Engel PC (1998) Protein thermostability in extremophiles. Biochimie 80(11):933–941
Schwarz G, Watanabe F (1983) Thermodynamics and kinetics of co-operative protein–nucleic acid binding. I. General aspects of analysis of data. J Mol Biol 163(3):467–484
Shamoo Y, Friedman AM, Parsons MR, Konigsberg WH, Steitz TA (1995) Crystal structure of a replication fork single-stranded DNA binding protein (T4 gp32) complexed to DNA. Nature 376(6538):362–366
Stassen MJ, Bailey D, Nelson S, Chinwalla V, Harte PJ (1995) The Drosophila trithorax proteins contain a novel variant of the nuclear receptor type DNA binding domain and an ancient conserved motif found in other chromosomal proteins. Mech Dev 52(2–3):209–223
Urbanke C, Schaper A (1990) Kinetics of binding of single-stranded DNA binding protein from Escherichia coli to single-stranded nucleic acids. Biochemistry 29(7):1744–1749
Van den Burg B, Dijkstra BW, Vriend G, Van der Vinne B, Venema G, Eijsink VG (1994) Protein stabilization by hydrophobic interactions at the surface. Eur J Biochem 220(3):981–985
Wadsworth RI, White MF (2001) Identification and properties of the crenarchaeal single-stranded DNA binding protein from Sulfolobus solfataricus. Nucleic Acids Res 29(4):914–920
Webster G, Genschel J, Curth U, Urbanke C, Kang C, Hilgenfeld R (1997) A common core for binding single-stranded DNA: structural comparison of the single-stranded DNA-binding proteins (SSB) from E. coli and human mitochondria. FEBS Lett 411(2–3):313–316
Williams KR, Konigsberg W (1978) Structural changes in the T4 gene 32 protein induced by DNA polynucleotides. J Biol Chem 253(7):2463–2470
Williams KR, Spicer EK, LoPresti MB, Guggenheimer RA, Chase JW (1983) Limited proteolysis studies on the Escherichia coli single-stranded DNA binding protein. Evidence for a functionally homologous domain in both the Escherichia coli and T4 DNA binding proteins. J Biol Chem 258(5):3346–3355
Witte G, Urbanke C, Curth U (2005) Single-stranded DNA-binding protein of Deinococcus radiodurans: a biophysical characterization. Nucleic Acids Res 33(5):1662–1670
Acknowledgments
The work was supported by the Gdańsk University of Technology. We thank Dr. M. Olszewski for TaqSSB protein used in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian
Rights and permissions
About this article
Cite this article
Filipkowski, P., Koziatek, M. & Kur, J. A highly thermostable, homodimeric single-stranded DNA-binding protein from Deinococcus radiopugnans . Extremophiles 10, 607–614 (2006). https://doi.org/10.1007/s00792-006-0011-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0011-8