Abstract
We systematically investigated the role of HSP genes in the growth and survival of Saccharomyces cerevisiae under high hydrostatic pressure together with analysis of pressure-regulated gene expression. Cells of strain BY4742 were capable of growth at moderate pressure of 25 MPa. When pressure of 25 MPa was applied to the cells, the expression of HSP78, HSP104, and HSP10 was upregulated by about 3- to 4-fold, and that of HSP32, HSP42, and HSP82 was upregulated by about 2- to 2.6-fold. However, the loss of one of the six genes did not markedly affect growth at 25 MPa, while the loss of HSP31 impaired high-pressure growth. These results suggest that Hsp31 plays a role in high-pressure growth but that the six upregulated genes do not. Extremely high pressure of 125 MPa decreased the viability of the wild-type cells to 1% of the control level. Notably, the loss of HSP genes other than HSP31 enhanced the survival rate by about fivefold at 125 MPa, suggesting that the cellular defensive system against high pressure could be strengthened upon the loss of the HSP genes. In this paper, we describe the requirement for and significance of a subset of HSP genes in yeast cell growth at moderate pressure and survival at extremely high pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Living cells display rapid responses when they are exposed to adverse environmental changes. The phenomena are observed in a wide range of organisms from bacteria to higher eukaryotes and are commonly designated the stress response. Molecular responses to heat shock have been extensively analyzed for many years, revealing a set of inducible genes encoding heat-shock proteins (Hsps) (Lindquist and Craig 1988). Hsps have been implicated in growth-related processes such as DNA replication, cell division, transcription, translation, protein folding, membrane protein functions, and protein transport. The expression of many Hsps is regulated by the binding of heat-shock factors to upstream sequences, called heat-shock elements (Mager and Kruijff 1995). Hsps play an essential role in intracellular housekeeping by assisting the correct folding of nascent and stress-accumulated misfolded proteins to prevent aggregation.
While heat shock, cold shock, osmotic pressure shock, and oxidative shock have been widely investigated, hydrostatic pressure shock has received less attention, partly because of experimental difficulties. Hydrostatic pressures greater than 100 MPa generally kill microorganisms, and their survival rates depend on the magnitude and duration of pressure applied. High pressure has been applied instead of heat inactivation in food processing industries to inactivate microorganisms. Iwahashi et al. (1991) reported that short treatment of yeast cells at the sublethal temperature of 42°C dramatically increased the viability of the cells when lethal pressure of 150–180 MPa was applied. In this process, Hsp104 expression is induced and plays an essential role in the unfolding of denatured proteins which occurs at high pressure (Iwahashi et al. 1997). Hsp104 is thought be in the same family as the ClpA and ClpB proteins of Escherichia coli and was confirmed to be essential for thermotolerance by unfolding denatured protein at high temperature (Gottesman et al. 1990). The sensitivity of hsp104Δ cells to pressure of 180 MPa is more significant when the pressure is applied at 35°C than when applied at 4 or 20°C, suggesting that a higher temperature is required for the ATPase activity of Hsp104 (Iwahashi et al. 1997). On the other hand, trehalose has a protective effect on intracellular proteins, irrespective of temperature.
Genome-wide expression in response to high pressure was independently analyzed in two laboratories using DNA microarray hybridization. Iwahashi et al. (2003, 2005) reported that genes categorized as active in energy metabolism, cell defense, and protein metabolism were upregulated after immediate pressure treatment at 180 MPa, whereas those categorized as active in protein metabolism and membrane metabolism were upregulated during growth at 30 MPa. Fernandes et al. (2004) reported that pressure treatment at 200 MPa upregulated genes categorized as active in stress defense and carbohydrate metabolism, while downregulated genes were involved in cell cycle progression and protein synthesis. Some discrepancies are likely to occur in pressure-regulated gene species because of the difference in strain, culture medium, and magnitude of applied pressure used in different laboratories. Meantime, it is still unclear how such upregulated genes play a role in growth and survival at high pressure in yeast.
We have been analyzing the effects of hydrostatic pressure at nonlethal levels (Abe 2004). Pressures of 20–50 MPa cause acidification of the cytoplasm and vacuoles in live yeast cells (Abe and Horikoshi 1997, 1998). One of our notable findings is that high pressure significantly impairs the uptake of tryptophan by yeast cells, which is mediated by the high-affinity tryptophan permease Tat2 (Abe and Horikoshi 2000; Abe and Iida 2003). Consequently, hydrostatic pressure restricts the cell growth of tryptophan-auxotrophic (Trp–) strains at moderate pressure of 25 MPa. Overexpression of Tat2 enables Trp– cells to grow at 25 MPa (Abe and Horikoshi 2000). A number of genes involved in the ubiquitin system were identified as responsible for the high-pressure growth of the Trp– strain (Abe and Iida 2003; Miura and Abe 2004; Nagayama et al. 2004). However, the genes responsible for high-pressure growth in Trp+ strains have not yet been identified. To establish the molecular basis for microbial piezoadaptation and piezotolerance, we investigated the expression profile of HSP genes using the DNA microarray and carried out functional analysis using a subset of HSP gene disruptants from a yeast deletion clone library.
Materials and methods
Yeast strains and culture conditions
The strains used in this study were derived from Yeast Deletion Clones (Invitrogen, Carlsbad, CA, USA): BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0; wild type), hsp26Δ (hsp26Δ::kanMX4), hsp31Δ (hsp31Δ::kanMX4), hsp150Δ (hsp150Δ::kanMX4), hsp12Δ (hsp12Δ::kanMX4), hsp30Δ (hsp30Δ::kanMX4), hsp78Δ (hsp78Δ::kanMX4), hsp42Δ (hsp42Δ::kanMX4), hsp104Δ (hsp104Δ::kanMX4), hsp82Δ (hsp82Δ::kanMX4), msn2Δ (msn2Δ::kanMX4), and msn4Δ (msn4Δ::kanMX4). To examine cell growth at high pressure, exponentially growing cells (0.7–1.5×107 cells/ml) in synthetic complete (SC) medium at 24°C were diluted into 1.0×106 cells/ml. Hydrostatic pressure was applied to the cells as described bellow. SC medium was prepared with the following modifications. Tryptophan 40 mg/l, leucine 90 mg/l, lysine 30 mg/l, histidine 20 mg/l, and uracil 20 mg/l were added, and valine was removed. The optical density at 600 nm (OD600) was measured after appropriate dilution of the samples.
High-pressure treatment
For the application of hydrostatic pressure, cells in an exponentially growing culture in SC medium at 24°C were placed in sterilized tubes, sealed with parafilm, and placed in hydrostatic chambers (PV100-360, PV100-500, Teramecs, Kyoto, Japan). Hydrostatic pressure was applied to the samples at 24°C using a hand pump (TP200L, Teramecs). After decompression, OD600 was measured after appropriate dilution of the samples. To determine the survival rate, cells were subjected to high-pressure treatment at 125 MPa for 1 h in SC medium. After decompression, the cells were placed on YPD agar plates and incubated for 3 days at 24°C. The number of colonies was then counted, and the survival rate was expressed as the number of colony-forming units (CFUs).
Isolation of total RNA and DNA microarray analysis
Exponentially growing wild-type cells (5×108 cells) in SC medium at 0.1 MPa were shifted to incubation at 0.1 or 25 MPa for 5 h. After decompression, the cells were collected by centrifugation, washed twice with ice-cold sterilized deionized distilled water (DDW), and resuspended in 10 ml of 50 mM sodium acetate-10 mM EDTA buffer (pH 5.0). The total RNA was extracted using the hot phenol method (Kohrer and Domdey 1991). One milliliter of sodium dodecyl sulfate and 12 ml of preheated Tris-buffered phenol (65°C) were added to the cell suspension and the mixture was maintained at 65°C for 5 min with vortexing every 30 s. After cooling, the mixture was subjected to centrifugation at 2,500 g for 10 min to separate RNA from cell debris. The lower phenol layer was removed and the supernatant was again extracted with an equal volume of phenol. After centrifugation, the supernatant was extracted with an equal volume of chloropane (50% v/v liquid phenol, 50% v/v chloroform, 0.5% 8-hydroxychinoline in 10 mM sodium acetate, 100 mM NaCl, 1 mM EDTA, pH 6.0) and then extracted with an equal volume of chloroform–isoamylalcohol (24:1). Total RNA was precipitated from the resulting supernatant by the addition of ethanol and sodium acetate (pH 5.3) at –20°C for several hours, and then stored in diethylpirocarbonate-treated DDW at –80°C. To average accidental errors in total RNA preparation, we mixed equal amounts of total RNA purified from three independent cultures. Subsequent procedures including the isolation of poly A+-RNA, sample labeling, and hybridization for DNA microarray were performed by NimbleGen Systems Inc. (Madison, WI, USA) and GeneFrontier Inc. (Tokyo, Japan). For the microarray, SuperClean slides of TeleChem International, Inc. (Sunnyvale, CA, USA) were used. Fifteen perfectly matching 60-mer probes for individual genes were used for hybridization. After hybridization, the glass slides were scanned with an Axon 400B scanner (Molecular Devices Corp., Sunnyvale, CA, USA). The images were analyzed using NimbleScan Software (NimbleGen Systems Inc). The expression data from the microarrays were normalized using the Robust Multi-chip Analysis algorithm (Bolstad et al. 2003). Because this analysis yields results with a high confidence level, a 1.5-fold difference between multiple samples generally corresponds to the level of significance (P<0.05) obtained from hybridization signals from 15 perfectly matching probes for each gene.
Results and discussion
Expression of a subset of HSP genes in response to high pressure
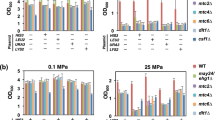
Because strain BY4742 is tryptophan prototrophic (Trp+), the cells are capable of growth at pressure of 25 MPa, in contrast to strain YPH499 (Trp–) (Fig. 1) (Abe and Horikoshi 2000). The expression of a subset of HSP genes and the stress-related genes, MSN1, MSN2, MSN4, and MSN5, was analyzed using DNA microarray hybridization. Table 1 lists the level of HSP gene expression when BY4742 cells were exposed to pressure of 25 MPa for 5 h. Of the 17 genes, HSP104, HSP10, and HSP78 were upregulated by 3- to 4-fold at 25 MPa compared with cells cultured at 0.1 MPa. HSP30, HSP42, and HSP82 were moderately upregulated by 2- to 2.6-fold at 25 MPa, whereas HSP26 and HSP31 were downregulated by about 2-fold. The remainder of the genes investigated were not significantly affected by pressure. Upregulation of HSP104 by pressure is consistent with a previous report (Iwahashi et al. 2005). Hsp104 is known to cooperate with Ydj1 (Hsp40) and Ssa1 (Hsp70) to unfold and reactivate denatured, aggregated proteins (Glover and Lindquist 1998). Hsp10 is a mitochondrial matrix co-chaperonin that inhibits the ATPase activity of Hsp60 and is involved in protein folding and sorting in the mitochondria (Dubaquie et al. 1997). Hsp78 is an oligomeric mitochondrial matrix chaperone that cooperates with Ssc1 in mitochondrial thermotolerance after heat shock (Leonhardt et al. 1993). Accordingly, the loss of mitochondrial functions at high pressure might be compensated for by upregulation of HSP60 and HSP78. Hsp30 is a hydrophobic plasma membrane protein that negatively regulates the H+-ATPase Pma1 (Piper et al. 1997). It is also induced by heat shock, ethanol treatment, weak organic acid, glucose limitation, and entry into the stationary phase (Piper 1995; Seymour and Piper 1999). We have reported that nonlethal levels of hydrostatic pressure cause intracellular acidification in a manner analogous to weak acid treatment (Abe and Horikoshi 1997, 1998). Therefore, intracellular acidification causes HSP30 induction with hydrostatic pressure. Hsp42 is a small, cytosolic, stress-induced chaperone that forms barrel-shaped oligomers and suppresses the aggregation of nonnative proteins (Haslbeck et al. 2004). Hsp82 is a cytoplasmic chaperone required for pheromone signaling and negative regulation of Hsf1 (Erkine et al. 1999; Louvion et al. 1998). Small Hsps such as Hsp26 and Hsp12 are known to be upregulated under various stress conditions (Causton et al. 2001; Ferguson et al. 2005). However, in our study, both genes remained almost unchanged. We speculate that HSP26 and HSP12 are induced at the onset of pressure treatment, e.g., within 30 min, and the expression levels recover to their original levels even during pressure exposure.
Functional analysis of HSP genes responsible for growth at 25 MPa
We attempted to identify the HSP genes responsible for high-pressure growth at 25 MPa. Of the 17 genes, 11 are nonessential and can be disrupted. Cells of the wild type and strains lacking the 11 individual genes were incubated in SC medium at 0.1 or 25 MPa at 24°C with an initial OD600 value of 0.1. The OD600 was measured at 5, 10, and 20 h. The doubling time of the wild-type cells at 0.1 and 25 MPa was 2.4 and 5.8 h, respectively (Fig. 1, Table 2). Upon the loss of HSP31, the doubling time was significantly prolonged to 10 h at pressure of 25 MPa, suggesting that Hsp31 plays a partial role in growth under moderate pressure conditions. The loss of one of the remaining genes, however, did not affect growth significantly but slightly shortened the doubling time (Fig. 1, Table 2). Hsp31 is a 25.5-kDa protein and a possible chaperone and cysteine protease with similarity to E. coli Hsp31 (Malki et al. 2005). The crystal structure of the Hsp31 protein revealed the presence of a putative catalytic triad and a strong resemblance to the E. coli Hsp31 chaperone and the intracellular protease PhPI from Pyrococcus horikoshii (Graille et al. 2004). A large-scale genomic expression analysis indicates that yeast Hsp31 is a stress responsive protein and is proposed to act as a molecular chaperone (de Nobel et al. 2001). In general, pressure of 25 MPa is less severe and does not affect the conformation of soluble proteins as far as investigated in vitro. Therefore, some proteins could be misfolded during de novo protein synthesis in the endoplasmic reticulum at 25 MPa. Such misfolded proteins may be catalyzed by the Hsp31 chaperone.
Functional analysis of HSP genes responsible for survival at 125 MPa
Next, we attempted to identify the HSP genes responsible for survival at high pressure. Cells of the wild type and strains lacking the 11 individual genes were exposed to pressure of 125 MPa for 1 h. After decompression, the cells were placed on YPD agar plates and incubated for 3 days. Table 3 shows the survival rate expressed as the number of CFUs. Pressure of 125 MPa decreased the viability of the wild-type cells to 1.0% of the baseline level, indicating that this pressure is lethal to yeast. Similarly, pressure of 125 MPa decreased the viability of hsp31Δ cells to 0.5%. Unexpectedly, the loss of one of the remaining genes enhanced cell viability by fivefold. This means that the HSP gene disruptants acquired piezotolerance at 125 MPa. The data shown in Table 3 are widely scattered with large error bars even though there are significant differences. This is perhaps because the viability after lethal pressure treatment is influenced by differences in the growth phase of cell cultures or the speed of compression and decompression, which varied slightly in the experiments. A previous report showed that the loss of HSP104 caused hypersensitivity to pressure of 150 MPa against a W303 genetic background, which is inconsistent with our results (Iwahashi et al. 1997). Strain W303 is known to carry defective alleles of the SSD1 gene that encodes multiple functional proteins involved in chromosome maintenance (Uesono et al. 1994), tolerance to Ca2+ (Tsuchiya et al. 1996), or cell morphology (Moriya and Isono 1999). We speculate that, because strain W303 lacks the Ssd1 function, Hsp104 is likely to be more important for the establishment of the cellular stress defense system in strain W303 than in strain BY4742. Hence, the loss of Hsp104 resulted in hypersensitivity to pressure in strain W303. We have confirmed that hsp104Δ cells show hypersensitivity to high-temperature treatment at 50°C for 10 min resulting in decreased viability to about 1.0%, whereas 13% in strain BY4742. Thus, Hsp104 plays an essential role in thermotolerance against the BY4742 genetic background. In our preliminary results, thermotolerance was not acquired upon the loss of any HSP genes investigated in this study (data not shown). Therefore, the acquired piezotolerance could be a unique cellular response to high hydrostatic pressure. It is known that mutations in Hsp70 result in increased expression of Hsps at optimal growth temperature or even at low temperature (Craig and Jacobson 1984; Craig and Gross 1991). In this phenomenon, increased levels of misfolded proteins promote the expression of other Hsps. In an analogous manner, the loss of one of the HSP genes may induce other HSP gene expression, resulting in piezotolerance. Upon the loss of SSA1/2 encoding cytosolic chaperones Hsp70 proteins, genes involved the regulation of proteins synthesis and ubiquitin-dependent proteasomal protein degradation are known to be upregulated by mild-hear shock treatment (Matsumoto et al. 2005). The role of HSP70 subfamily genes, SSA1, SSA2, SSA3, and SSA4, in piezotolerance is under investigation.
Perspectives in understanding the effects of hydrostatic pressure in yeast
Hydrostatic pressure exerts complex influences upon the structures of cellular macromolecules, metabolic pathways, transcription, translation, and cell division depending on the magnitude and duration of applied pressure. Our present study of HSP genes demonstrated that upregulated genes are not always responsible for growth under moderate pressure conditions (piezoadaptation) or tolerance against lethal pressure (piezotolerance). This is probably because a number of functionally overlapping genes are involved in piezoadaptation and piezotolerance. In addition, the loss of HSP genes was found to promote the piezotolerance of the yeast cells. Such interplay between the consequence of gene disruption and the phenotype makes the problem more complex. Screening of an entire yeast deletion clone library would yield clues for understanding the requirement of yeast for genes responsible for piezoadaptation and piezotolerance as well as the biological processes employed by organisms inhabiting deep-sea environments.
References
Abe F, Horikoshi K (1997) Vacuolar acidification in Saccharomyces cerevisiae induced by elevated hydrostatic pressure is transient and is mediated by vacuolar H+-ATPase. Extremophiles 1:89–93
Abe F, Horikoshi K (1998) Analysis of intracellular pH in the yeast Saccharomyces cerevisiae under elevated hydrostatic pressure: a study in baro-(piezo-)physiology. Extremophiles 2:223–228
Abe F, Horikoshi K (2000) Tryptophan permease gene TAT2 confers high-pressure growth in Saccharomyces cerevisiae. Mol Cell Biol 20:8093–8102
Abe F, Iida H (2003) Pressure-induced differential regulation of the two tryptophan permeases Tat1 and Tat2 by ubiquitin ligase Rsp5 and its binding proteins, Bul1 and Bul2. Mol Cell Biol 23:7566–7584
Abe F (2004) Piezophysiology of yeast: occurrence and significance. Cell Mol Biol 50:437–445
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193
Causton HC, Ren B, Koh SS, Harbison CT, Kanin E, Jennings EG, Lee TI, True HL, Lander ES, Young RA (2001) Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell 12:323–337
Craig EA, Jacobsen K (1984) Mutations of the heat inducible 70 kilodalton genes of yeast confer temperature sensitive growth. Cell 38:841–849
Craig EA, Gross CA (1991) Is hsp70 the cellular thermometer? Trends Biochem Sci 16:135–140
de Nobel H, Lawrie L, Brul S, Klis F, Davis M, Alloush H, Coote P (2001) Parallel and comparative analysis of the proteome and transcriptome of sorbic acid-stressed Saccharomyces cerevisiae. Yeast 18:1413–1428
Dubaquie Y, Looser R, Rospert S (1997) Significance of chaperonin 10-mediated inhibition of ATP hydrolysis by chaperonin 60. Proc Natl Acad Sci USA 94:9011–9016
Erkine AM, Magrogan SF, Sekinger EA, Gross DS (1999) Cooperative binding of heat shock factor to the yeast HSP82 promoter in vivo and in vitro. Mol Cell Biol 19:1627–1639
Ferguson SB, Anderson ES, Harshaw RB, Thate T, Craig NL, Nelson HC (2005) Protein kinase A regulates constitutive expression of small heat-shock genes in an Msn2/4p-independent and Hsf1p-dependent manner in Saccharomyces cerevisiae. Genetics 169:1203–121
Fernandes PM, Domitrovic T, Kao CM, Kurtenbach E (2004) Genomic expression pattern in Saccharomyces cerevisiae cells in response to high hydrostatic pressure. FEBS Lett 556:153–160
Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73–82
Gottesman S, Squires C, Pichersky E, Carrington M, Hobbs M, Mattick JS, Dalrymple B, Kuramitsu H, Shiroza T, Foster T et al. (1990) Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci USA 87:3513–3517
Graille M, Quevillon-Cheruel S, Leulliot N, Zhou CZ, de la Sierra Gallay IL, Jacquamet L, Ferrer JL, Liger D, Poupon A, Janin J, van Tilbeurgh H (2004) Crystal structure of the YDR533c S. cerevisiae protein, a class II member of the Hsp31 family. Structure 12:839–847
Haslbeck M, Braun N, Stromer T, Richter B, Model N, Weinkauf S, Buchner J (2004) Hsp42 is the general small heat shock protein in the cytosol of Saccharomyces cerevisiae. EMBO J 23:638–649
Iwahashi H, Kaul SC, Obuchi K, Komatsu Y (1991) Induction of barotolerance by heat shock treatment in yeast. FEMS Microbiol Lett 64:325–328
Iwahashi H, Obuchi K, Fujii S, Komatsu Y (1997) Effect of temperature on the role of Hsp104 and trehalose in barotolerance of Saccharomyces cerevisiae. FEBS Lett 416:1–5
Iwahashi H, Shimizu H, Odani M, Komatsu Y (2003) Piezophysiology of genome wide gene expression levels in the yeast Saccharomyces cerevisiae. Extremophiles 7:291–298. Erratum in: Extremophiles (2003) 7:433
Iwahashi H, Odani M, Ishidou E, Kitagawa E (2005) Adaptation of Saccharomyces cerevisiae to high hydrostatic pressure causing growth inhibition. FEBS Lett 579:2847–2852
Kohrer K, Domdey H (1991) Preparation of high molecular weight RNA. In: Guthrie C, Fink G (eds) Guide to yeast genetics and molecular biology. Academic, San Diego, pp 398–495
Leonhardt SA, Fearson K, Danese PN, Mason TL (1993) HSP78 encodes a yeast mitochondrial heat shock protein in the Clp family of ATP-dependent proteases. Mol Cell Biol 13:6304–6313
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Louvion JF, Abbas-Terki T, Picard D (1998) Hsp90 is required for pheromone signaling in yeast. Mol Biol Cell 9:3071–3083
Mager WH, De Kruijff AJ (1995) Stress-induced transcriptional activation. Microbiol Rev 59:506–531
Malki A, Caldas T, Abdallah J, Kern R, Eckey V, Kim SJ, Cha SS, Mori H, Richarme G (2005) Peptidase activity of the Escherichia coli Hsp31 chaperone. J Biol Chem 280:14420–14426
Matsumoto R, Akama K, Rakwal R, Iwahashi H (2005) The stress response against denatured proteins in the deletion of cytosolic chaperones SSA1/2 is different from heat-shock response in Saccharomyces cerevisiae. BMC Genomics 6:141
Miura T, Abe F (2004) Multiple ubiquitin-specific protease genes are involved in degradation of yeast tryptophan permease Tat2 at high pressure. FEMS Microbiol Lett 239:171–179
Moriya H, Isono K (1999) Analysis of genetic interactions between DHH1, SSD1 and ELM1 indicates their involvement in cellular morphology determination in Saccharomyces cerevisiae. Yeast 15:481–496
Nagayama A, Kato C, Abe F (2004) The C-terminal mutation stabilizes the yeast tryptophan permease Tat2 under high-pressure and low-temperature conditions. Extremophiles 8:143–149
Piper PW (1995) The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Microbiol Lett 134:121–127
Piper PW, Ortiz-Calderon C, Holyoak C, Coote P, Cole M (1997) Hsp30, the integral plasma membrane heat shock protein of Saccharomyces cerevisiae, is a stress-inducible regulator of plasma membrane H(+)-ATPase. Cell Stress Chaperones 2:12–24
Seymour IJ, Piper PW (1999) Stress induction of HSP30, the plasma membrane heat shock protein gene of Saccharomyces cerevisiae, appears not to use known stress-regulated transcription factors. Microbiology 145:231–239
Tsuchiya E, Matsuzaki G, Kurano K, Fukuchi T, Tsukao A, Miyakawa T (1996) The Saccharomyces cerevisiae SSD1 gene is involved in the tolerance to high concentration of Ca2+ with the participation of HST1/NRC1/BFR1. Gene 176:35–38
Uesono Y, Fujita A, Toh-e A, Kikuchi Y (1994) The MCS1/SSD1/SRK1/SSL1 gene is involved in stable maintenance of the chromosome in yeast. Gene 143:135–138
Acknowledgment
This work was supported in part by a Grant-in-Aid for Young Scientists (B-15780065) from the Japan Society for the Promotion of Science to F. Abe.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Miura, T., Minegishi, H., Usami, R. et al. Systematic analysis of HSP gene expression and effects on cell growth and survival at high hydrostatic pressure in Saccharomyces cerevisiae . Extremophiles 10, 279–284 (2006). https://doi.org/10.1007/s00792-005-0496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0496-6