Abstract
Several researchers have reported that microorganisms can be cultivated only in the presence of other microorganisms. We suggest that a portion of uncultivated microorganisms might be cultivated in the presence of cellular components released from bacteria in their natural environments. In this study, the cell extract of Geobacillus toebii was used to enrich uncultivated thermophiles from compost. In the process of enrichment cultures, cell extract supplementation apparently changed the community composition. This change was monitored by PCR-DGGE targeting 16S rRNA gene. Five novel groups of microorganisms (similarity of 16S rRNA gene to the closest relative <96%) were specifically isolated from enrichment cultures by using cell extract-supplemented culture media. Their growth was found to be dependent on the addition of extract of G. toebii. Putting these findings together, we suggest that the extracts of bacteria could be one of the growth factors in the thermal ecosystem with a possibility of extending other ecological niches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms that are easily isolated by virtue of their ability to grow rapidly into colonies on artificial growth media are estimated to constitute less than 1% of all microbial species (Hugenholtz et al. 1998; Rondon et al. 1999; Hugenholtz 2002). The existence of widespread but uncultivated groups of microorganisms has been suggested by analysis of 16S rRNA gene of the microbial community in natural environments (Amann et al. 1995; Hugenholtz et al. 1998; Zinder and Salyers 2001; Sait et al. 2002; Zengler et al. 2002). A comprehensive understanding of the physiology of these organisms and of their complex biogeochemical processes undoubtedly requires their cultivation, isolation and characterization (Torsvik et al. 2002; Zengler et al. 2002). In order to isolate uncultivated microorganisms in pure culture, various attempts have been made to recover or simulate the physico-chemical conditions of environmental niches (Kaeberlein et al. 2002; Rappe et al. 2002; Torsvik et al. 2002). However, a few species of bacteria failed to grow on artificial media alone, and they formed colonies in the presence of other microorganisms, where the interactions between microorganisms might have been a factor affecting cultivation (de Caralt et al. 2003; Hoffmeister and Martin 2003; Parker 2003; Xu and Gordon 2003). A two-membered mixed culture was reproduced by splitting each growth medium with a diffusible membrane (Ohno et al. 2000; Huber et al. 2002). Moreover, some experimenters have added filtrated cell extracts to the media to cultivate microbes that do not grow axenically (Wais 1988; Maymo-Gatell et al. 1997; Rhee et al. 2002). From this point of view, it may be argued that uncultivated microorganisms might be isolated into pure culture if they are provided with other cell extracts of their natural environment. In this study, we enriched and isolated uncultivated thermophiles from compost by using the whole-cell extract of a strain of thermophilic Bacillaceae.
Materials and methods
Enrichment condition of anaerobic thermophiles in compost soil
Five hundred grams of compost was collected from ten stock farms in Non-San, Korea. Samples were taken at 45–65°C during its compost piling. Samples from ten sites were mixed equally, and five grams was inoculated to 50 ml of basal media (BM) in a 250 ml Erlenmeyer flask. The BM used for incubation and enrichment of compost was: 3 g of K2HPO4, 1 g of KH2PO4, 0.1 g of MgSO4·7H2O, and 5 g of polypeptone per liter of deionized water (adjust pH to 7.4). Cell extracts from Bacillus subtilisT 168 (DSM10; 30°C), Escherichia coliT K12 (DSM30083; 37°C), and Geobacillus toebiiT SK-1 (DSM14590; 55°C) were added to each enrichment medium, respectively. Cells used for extract preparation were grown on 1 l of Luria Bertani broth at each of optimal temperatures. At the late log phase, cells were harvested and washed twice by phosphate-buffered saline (PBS; 4 g of NaCl, 1 g of KCl, 0.72 g of Na2HPO4, and 0.12 g of KH2PO4 l−1 of deionized water; pH adjusted to 7.4) and resuspended in 10 ml of PBS. Cells were disrupted by ultrasonication with an ultrasonicator (Vibra-cell VCX600, Sonics and Materials Inc., USA) preventing from being heated with ice. After centrifuging at 13,000 rpm, extracts were adjusted to 20 mg of protein per ml of 50 mM potassium phosphate buffer (pH 7.5). Cell extracts were filtered with a 0.20 μm filter (Sartorius, UK) prior to being added to each medium. For preparation of enrichment media, 0.5 ml of extract was added to 49.5 ml of the autoclaved BM. For the negative control, yeast extract was added after adjusting same protein concentration (20 mg/ml). Thermophiles from compost were enriched anaerobically for 4 days at 60°C in anaerobic chamber (Bactron anaerobic/environmental chamber, Shellab, USA).
Enrichment monitoring with culture-dependent method
The colony forming units (CFU) were counted after the third transfer of the enrichment cultures. When the enrichment reached just before the stationary phase (about 5 days), 1 ml of each enrichment culture was transferred to a new medium. Culture broths were serially diluted and spread onto BM agar containing the corresponding bacterial extracts. Each plate was incubated for 4 days at 60°C in an anaerobic chamber.
Enrichment monitoring with culture-independent method
At the end of each phase of enrichment, culture samples were collected and immediately frozen at −80°C before the genomic DNA was extracted. Extraction of genomic DNA and PCR-DGGE with the Bacteria-specific primer set (518r and 338f with a GC-clamp) were carried out as described elsewhere (Yeates et al. 1998; Henckel et al. 1999). Major DGGE bands were excised with a razor blade and sequenced to gain information on bacterial composition in enrichment cultures.
Isolation, identification, and polyphasic taxonomic analysis of microorganisms requiring cell extract for their growth
After the fourth enrichment cultures, strains were isolated by spreading the cultures on BM agar containing the corresponding cell extracts. Initially we selected 300 colonies from 30 agar plates containing the cell extract of G. toebii and transferred to new BM agar containing G. toebii extracts. This process was repeated three times to obtain pure cultures. About 40% of isolates vanished away in obtaining pure cultures. Successfully retrieved isolates were tested to prove the indispensability of G. toebii extract for their growth by inoculating each isolate to the medium with and without G. toebii extract.
Polyphasic taxonomic analysis of isolated microorganisms
Genomic DNA was isolated and 16S rRNA gene was PCR-amplified and sequenced according to the method described previously (Yoon et al. 1996). We searched the closest sequences in the GenBank database with BLAST (Altschul et al. 1990). The alignment of 16S rRNA gene and construction of a phylogenetic tree were made by using CLUSTAL X software (Thompson et al. 1997). The partial sequences of representative new isolates determined in this study have been deposited in the GenBank database under accession no. AY466700–AY466717. The DNA–DNA hybridization experiment was carried out according to the method of Ezaki et al. (1989). Biomass for the chemical systematic studies was obtained by cultivating the organism with BM in anaerobic chamber at 60°C for 3 days. The shape, size, and motility of the living and stained cells were determined by light microscopy. The Gram reaction was determined using a Gram Stain Kit according to the manufacturer’s recommended protocol (Difco). To distinguish false negative gram staining, a KOH test was performed in parallel with the gram stain reaction. The KOH test was performed by mixing a visible amount of growth from a colony in a loopful of 3% aqueous KOH on a glass slide (Powers 1995). Most of the physiological tests were carried out using API 20A kits (BioMerieux). Growth in the presence of 0.02% (w/v) sodium azide and 5% (w/v) NaCl was examined according to the method of Gordon et al. (1973). The BM was also used for determination of optimal pH and temperature for growth. The effect of pH on growth was determined on solid BM using four different buffers at a final concentration of 50 mM: citrate-Na2HPO4 buffer, pH range 5.0–7.0; phosphate buffer, pH range 6.0–8.0; Tris buffer, pH range 7.0–9.0; glycine-NaOH buffer, pH range 8.5–10.5. Isolates were cultured on Bacto tryptic soy broth agar (Difco) with Geobacillus cell extract and incubated at 60°C for 48 h, and the cellular fatty acid contents were determined by using the MIDI procedure (MIDI Inc., Newark, DE, USA). G+C content was determined using the method of Tamaoka and Komagata (1984).
Characterization of growth-stimulating factors
In order to identify the stimulating molecule in the Geobacillus extract, a wide range of chemicals including trace minerals (Widdel and Bak 1992), cell wall D-amino acids (D-glutamic acid and D-alanine), vitamin precursors, artificial electron carriers (menadione, trimethylteterazolium, dichlorophenolindophenol, phenazine methosulfate, benzyl viologen, and hydroquinone), biopolymers (L-polyglutamic acid, lipopolysaccharide, lipoteichoic acid, and heparin sulfate), and proteins (chaperonin, catalase, and cytochrome c) were tested as growth factors. All the chemicals were purchased from Sigma–Aldrich Korea Ltd. These chemicals were added to the medium together at the optimal concentration as described previously (Widdel and Bak 1992). Proteinaceous characteristics of extract was also examined by heating to 100°C and treating with proteinase K (Sigma–Aldrich Korea Ltd.); for denaturating the proteins, extract was boiled 10 min and proteinase K was applied to the extract at 37°C for 1 h. Proteinase K treatment buffer contained 10 mM Tris–HCl (pH 7.8), 10 mM MgCl2. Final concentration of 100 μg/ml of proteinase K was treated to cell extract for 4 h at 37°C. Denaturation of proteinase K was not executed. Ultrafiltration with molecular weight cut-off 10,000 cellulose filter (Millipore, MA, USA) was applied to divide the extract in to low (LMW) and high molecular weight compound (HMW). Separated extract was adjusted to 20 mg/ml protein concentration prior to being added to the medium; HMW was diluted with potassium phosphate buffer, and LMW was concentrated with Centricon concentrator (Millipore) with molecular weight cut-off 1000 cellulose filter. Localization of the factors in the cytoplasmic fraction was determined by an ultracentrifugation experiment. The cells of G. toebii were collected and washed once in the buffer by centrifugation. The membrane fraction was extracted by sonication to lyse the cells (Branson Sonicator Cell Disrupter 185, Branson Ultrasonics, Danbury, CT; seven cycles of 30 s with intervening 45 s incubations on ice), low speed centrifugation (5 min, 1500×g) to remove large cell fragments in the pellet, and ultracentrifugation (30 min, 150,000×g) to collect the membrane fraction (the ultracentrifugation pellet). Effect of chemicals, denatured extract, LMW, HMW, and membrane and cytoplasmic fraction of the extract on the growth of isolates was determined by estimating the increased optical density of each medium, which was inoculated in five replicates of each strain in 96 wells. Equal concentrations (finally 0.2 mg/ml of protein) of yeast extract were used for the negative control in all experiments.
Results
Enrichment monitoring by CFU counting
To estimate the effect of adding the bacterial cell extract to enrichment cultures, the number of total CFUs were compared. The CFU in enrichment cultures with the thermophilic Geobacillus cell extract [(12.24±1.3)×108 ml−1] was about four times higher than those without extracts [(3.71±1.6)×108 /,ml] and slightly higher than the cultures with other kinds of extracts [Bacillus: (8.35±2.8)×108 ml−1, Escherichia: (6.75±1.65)×108 ml−1]. This result clearly shows that the extract of cells, especially that of thermophiles (G. toebii), stimulates the enrichment of thermophiles in compost soils.
Enrichment monitoring by DGGE
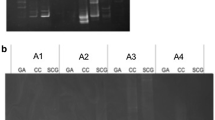
To culture-independently investigate the stimulation effect of cell extracts on microorganisms during enrichment, a 16S rRNA gene-PCR-DGGE analysis was conducted and the dynamics of microbial composition was compared among enrichments. The DGGE patterns observed in the original sample were much more complex than those obtained from the successively transferred enrichment cultures, although enriched cultures contained a subset of the bands present in the original enrichment cultures (Fig. 1a, b). The number of bands decreased, and several prominent bands appeared as the enrichment progressed. However, there was no significant difference between the third and fourth transferred enrichments from each cell extract. As the fourth transfer of enrichment cultures was in progress, a significant difference was observed in the DGGE profiles between enriched samples containing G. toebii extract and other cell extracts. Fewer DGGE bands were observed in cultures with E. coli and B. subtilis extracts and cultures without extract than in cultures with G. toebii extract. This indicates that the microbial composition was altered by the application of the cell extract in this enrichment condition.
DGGE profiles of PCR-amplified 16S rDNA segments (180 bp) from (a) enrichment cultures without cell extract (N1–N4) and with cell extracts from G. toebii (G1–G3), (b) B. subtilis (B1–B3) and E. coli (E1–E3). The number after Roman capital (N, G, B, E) indicates the order of enrichment cultures. The sequence information of bands (g and n series) is presented in Table 1. The bands corresponding to isolated microbial groups are marked with arrows
The DNA sequence analysis of bands on the DGGE gel revealed that enriched bacteria under this growth condition belonged to thermophilic Bacillaceae, Clostridiaceae, Thermoanaerobacteriaceae, and unclassified Actinobacteria, while several bands were not closely related to previously characterized bacteria (less than 90% similarity) (Fig. 1a and Table 1). Interestingly, most of them were related to low G+C Gram-positive bacteria (Firmicutes phylum) despite their low relatedness (84–94%). The partial 16S rRNA gene sequences of the bands observed only in the enrichment with Geobacillus extract were distantly related to previously isolated microorganisms; 84–94% to Anoxybacillus, 90–93% to Clostridium, 94% to Thermoanaerobacter, 94% to Thermoanaerobacterium.
The UPGMA analysis of the DGGE patterns with Dice similarity coefficients also showed that the microbial communities of the bacterial extract-containing cultures were significantly different from control (N series) (Fig. 2). The DGGE profiles of samples containing the same cell extract were grouped together in a clade after several successive transfers. This clearly indicates that each cell extract contains different components capable of affecting growth of specific microorganisms.
UPGMA analysis dendrogram based on DGGE banding patterns of bacterial community in Fig. 1. Dice coefficient was calculated pairwise according to the presence and absence of matching bands using BioNumerics software (BioSystematica, UK)
Isolation of microorganisms requiring cell extract for their growth
To study the characteristics of enriched thermophiles in the presence of cell extract, we isolated microorganisms from enrichment cultures. Agar plates containing the corresponding cell extracts were used to isolate microorganisms from enrichment under anaerobic conditions. Diversity of colony morphology was clearly higher on agar plates containing cell extract than those not containing G. toebii extract (data not shown). Although we used the same agar plates containing the extract that had been used for isolation, some of the colonies (about 40%) could not be successfully transferred. Finally, 184 isolates were successfully retrieved and tested to prove the indispensability of G. toebii extract for their growth. Among the 184 isolates tested, 61 strains required G. toebii extracts for their growth.
For preliminary identification of strains, approximately 600 bp of the nucleotide sequences were determined from the 5′ region of the 16S rRNA gene of the 61 isolates finally selected. Although it is hard to assign a taxonomic position only with 16S rRNA gene sequences (Gest and Favinger 2001), the degree of novelty of the isolates was initially indicated on the basis of 16S rRNA gene sequence similarities. The guidelines of <98% for distinct species (Stackebrandt et al. 1993) and <96% for distinct genera (Everett et al. 1999) of sequence similarity were applied. The 16S rRNA gene-based phylogeny showed that all the 61 isolates were previously undescribed microorganisms consisting of five groups (five novel species belonging to four novel genera). All the groups were closely related to the Bacillus-Clostridium subphylum of Gram-positive bacteria (Fig. 3). In each group, every strain has more than 97% sequence similarity with each other. On the basis of the DNA–DNA hybridization experiment, we found that groups 3 and 4 are two different species in a genus. The two species showed less than 30% of DNA–DNA hybridization ratio (Wayne et al. 1987). Table 2 shows the closest GenBank relative and the percentage of similarity to the closest relative of each group. To clarify their taxonomic positions, phylogenetic tree of each group was constructed with their near full length (more than 1300 bp) of 16S rDNA sequences (Fig. 3). The 16S rDNA sequences from the isolated strains were exactly matched with those of the DGGE bands enriched with G. toebii cell extract as marked in Table 1. All the isolated strains were subjected to polyphasic characterization for phylogenetic investigation. Table 3 summarizes the results of the microscopic analysis for cell sizes and motility, the results of the chemical analysis for production of acid from carbohydrates, and the effect of NaCl concentration, pH and temperature for growth and the profiles of fatty acids of isolates. Interestingly, although the group 2 isolates were negatively stained in gram reactions, they were found to be Gram-positive in a KOH test (Powers 1995). When the FAME profiles of isolates were compared in MIDI database, there were no matches. This polyphasic data supports that the isolates belonged to the undescribed bacterial genera. Phylogenetic comparison of isolated strains to the nearest neighbors is in progress to propose a new genus and species.
Growth-stimulating factors in the extract
In order to understand the function of the extract stimulating bacterial growth, we tested possible mechanisms, in which it could be involved. First, we tested a wide range of chemicals to check if they could replace the function of extracts. As shown in Table 2, no chemicals tested in this experiment could replace the extract of G. toebii. Second, we fractionated the extract to HMW and LMW fractions to narrow down the possibilities. Table 2 shows the effect of the G. toebii extract on the growth of the groups of isolates. Among the isolates, the growth of Group 3 was not dependent on the extract although the growth of strains in Group 3 was enhanced by the cell extract. Strains of Group 3 showed a growth about four times faster in the presence the extract. Strains of groups 4 and 5 could be grown with either LMW or HMW. The LMW had no activity on the growth of strains of groups 1 and 2. Instead, only HMW could support the growth of strains of groups 1 and 2. This indicates that proteins in the extract might play a role as a growth-stimulating factor. Since proteins from G. toebii are stable at 60°C (Sung et al. 2002), the cell extract of thermophilic Geobacillus was capable of showing the most significant effect on the growth of factor-requiring thermophiles in population size and community composition during enrichment as shown in Fig. 2 and Table 1. The following additional results also support this idea. First, the cell extract, in which protein was aggregated and precipitated at pH 4.0, showed no activity of growth stimulation on strains of groups 1 and 2. Second, when the extract was denatured by boiling at 100°C for 10 min, the growth-stimulating activity disappeared. Third, after the extract was treated with proteinase K for 4 h at 37°C, the strains of groups 1 and 2 were unable to grow with the extract. Localization of the factors in the cytoplasmic fraction was determined by an ultracentrifugation experiment. The membrane fraction of G. toebii, which was obtained by ultracentrifugation was unable to support the growth of isolates while cytoplasmic fraction does.
Discussion
Most thermophilic bacteria have been isolated from geothermal environments associated with volcanic activity (Zeikus 1979; Kristjansson and Stetter 1992). However, other thermally heated anaerobic environments including man-made and natural solar-heated environments have also been reported to harbor various thermophilic bacteria (Mathrani and Ahring 1991; Huang et al. 1998). Our study investigated the composting environments that have been assumed to be deep niches for uncultivated microorganisms (Janssen et al. 1997). Based on previous results, we designed media for growing previously uncultivated microorganisms from composts using cell extracts (Rhee et al. 2002). Since growth of extract-requiring bacteria requires death of other cells, it started to grow after long death phase. In that stage, toxic end product also accumulated, which inhibited the growth of symbiotic bacteria requiring other bacteria’s extract. Thus, we reasoned that the extract was added in the initial phase of enrichment for stimulation of extract-requiring microbial communities. However, the effect of adding the bacterial cell extract on CFU of the enrichment cultures of soil microbial communities was small (<1 log unit). This is a strong evidence that microorganisms that can grow only with Geobacillus cell extract are a minority of the soil sample. In DGGE analysis, it was observed that extract-requiring microorganisms were neither diverse nor a major population in the sampled compost.
Since G. toebii is a thermophile (Sung et al. 2002), the ingredient of the extract could be stable at enrichment temperature (i.e., 60°C). Since the optimum growth temperature of E. coli and B. subtilis are 37° and 30°C, respectively, this might cause different effects in each extract of the thermophile enrichment, resulting in different numbers of DGGE bands. As expected, most of the strains detected in the DGGE bands failed to develop colonies. Furthermore, during the isolation of strains from enrichment, some of the colonies could not be successfully transferred, although we used agar plate with the corresponding extract. This means there might be other factors generated during enrichment cultures affecting the growth of uncultivated microorganisms.
Our results clearly showed that cell extract is an important factor in stimulating the growth of novel thermophiles, and this newly developed isolation strategy expanded the frontiers of cultivable thermophiles. The components released from dead cells in the natural thermal ecosystem can be an essential interaction factor. Indispensability of the cell extract for the growth of isolates is very similar to that of Symbiobacterium toebii, which grows only in the presence of extract of various prokaryotes (Sung et al. 2003). When the extract dependency of Dehalococcoides ethenogenes in moderate temperature conditions (Huber et al. 2002) and the recovery of halophilic archaebacteria from natural environments (Wais 1988) are taken into consideration, this type of interaction could be ubiquitous in an environment. Presently, the yeast extract is routinely used to cultivate various kinds of microorganisms. We suggest that supplementing the bacterial extract could be one of the alternatives for cultivation of fastidious bacteria defying to be cultivated with the current culture technologies. Consequently, further research is required to identify these factors as they may contribute to a better understanding of the microbial interactions in the ecosystem and to the discovery of other uncultivated microbial groups, ubiquitous, yet still unknown.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
de Caralt S, Agell G, Uriz MJ (2003) Long-term culture of sponge explants: conditions enhancing survival and growth, and assessment of bioactivity. Biomol Eng 20:339–347
Everett KD, Bush RM, Andersen AA (1999) Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol 49:415–440
Ezaki T, Hashimoto Y, Yabuuchi E (1989) Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol 39:224–229
Gest H, Favinger J (2001) Taxonomic ambiguities: a case history. Int J Syst Evol Microbiol 51:707–710
Gordon RE, Haynes WC, Pang CH (1973) The genus Bacillus. USDA, Washington
Henckel T, Friedrich M, Conrad R (1999) Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl Environ Microbiol 65:1980–1990
Hoffmeister M, Martin W (2003) Interspecific evolution: microbial symbiosis, endosymbiosis and gene transfer. Environ Microbiol 5:641–649
Huang CY, Patel BK, Mah RA, Baresi L (1998) Caldicellulosiruptor owensensis sp. nov., an anaerobic, extremely thermophilic, xylanolytic bacterium. Int J Syst Bacteriol 48:91–97
Huber H, Hohn MJ, Rachel R, Fuchs T, Wimmer VC, Stetter KO (2002) A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature 417:63–67
Hugenholtz P, Goebel BM, Pace NR (1998) Impact of culture independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180:4765–4774 (Erratum, 4180:6793)
Hugenholtz P (2002) Exploring prokaryotic diversity in the genomic era. Genome Biol 3:1-8
Janssen PH, Schuhmann A, Morschel E, Rainey FA (1997) Novel anaerobic ultramicrobacteria belonging to the verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol 63:1382–1388
Kaeberlein T, Lewis K, Epstein SS (2002) Isolating uncultivable microorganisms in pure culture in a simulated natural environment. Science 296:1127–1129
Kristjansson JK, Stetter K (1992) Thermophilic bacteria. CRC Press, Boca Raton, FL
Mathrani IM, Ahring BK (1991) Isolation and characterization of a strictly xylan-degrading Dictyoglomus from a man-made, thermophilic anaerobic environment. Arch Microbiol 157:13–17
Maymo-Gatell X, Chien Y, Gossett JM, Zinder SH (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568–1571
Ohno M et al (2000) Symbiobacterium thermophilum gen. nov., sp. nov., a symbiotic thermophile that depends on co-culture with a Bacillus strain for growth. Int J Syst Evol Microbiol 50:1829–1832
Parker MA (2003) Genetic markers for analysing symbiotic relationships and lateral gene transfer in Neotropical bradyrhizobia. Mol Ecol 12:2447–2455
Powers EM (1995) Efficacy of the ryu nonstaining KOH technique for rapidly determining gram reactions of food-borne and waterborne bacteria and yeasts. Appl Environ Microbiol 61:3756–3758
Rappe MS, Connon SA, Vergin KL, Giovannoni SJ (2002) Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature 418:630–633
Rhee S-K et al (2002) Characterization of symbiobacterium toebii, an obligate commensal thermophile isolated from compost. Extremophiles 6:57–64
Rondon MR, Goodman RM, Handelsman J (1999) The Earth’s bounty: assessing and accessing soil microbial diversity. Trends Biotechnol 17:403–409
Sait M, Hugenholtz P, Janssen PH (2002) Cultivation of globally distributed soil bacteria from phylogenetic lineages previously only detected in cultivation-independent surveys. Environ Microbiol 4:654–666
Stackebrandt E, Liesack W, Goebel BM (1993) Bacterial diversity in a soil sample from a subtropical Australian environment as determined by 16S rDNA analysis. FASEB J 7:232–236
Sung M-H et al (2002) Geobacillus toebii sp. nov., a novel thermophilic bacterium isolated from hay compost. Int J Syst Evol Microbiol 52:2251–2255
Sung M-H et al (2003) Symbiobacterium toebii sp. nov., commensal thermophile isolated from Korean compost. J Microbiol Biotechnol 13:1013–1017
Tamaoka J, Komagata K (1984) Determination of DNA base composition by reverse-phase high-performance liquid chromatography. FEMS Microbiol Lett 25:125–128
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Torsvik V, Ovreas L, Thingstad TF (2002) Prokaryotic diversity-magnitude, dynamics, and controlling factors. Science 296:1064–1066
Wais AC (1988) Recovery of halophilic archaebacteria from natural environments. FEMS Microbiol Lett 53:211–216
Wayne LG et al (1987) International committee on systematic bacteriology. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Widdel F, Bak F (1992) The prokaryotes. Springer-Verlag, New York
Xu J, Gordon JI (2003) Inaugural article: honor thy symbionts. Proc Natl Acad Sci USA 100:10452–10459
Yeates C, Gillings MR, Davison AD, Altavilla N, Veal DA (1998) Methods for microbial DNA extraction from soil for PCR amplification. Biol Proced Online 1:40–47
Yoon J-H et al (1996) Identification of Saccharomonospora strains by the use of genomic DNA fragments and rRNA gene probes. Int J Syst Bacteriol 46:502–505
Zeikus JG (1979) Thermophilic bacteria: ecology, physiology and technology. Enzyme Microb Technol 1:243–252
Zengler K et al (2002) Cultivating the uncultured. Proc Natl Acad Sci USA 99:15681–15686
Zinder SH, Salyers AA (2001) Microbial ecology-new directions, new importance. Springer-Verlag, New York
Acknowledgements
This work was supported by Grant BDM0200413, Grant NNM0100411 and the NRL research programme (Grant M10104000294–01J000012800) from the Korean Ministry of Science and Technology (MOST).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian
Rights and permissions
About this article
Cite this article
Bae, JW., Rhee, SK., Park, J.R. et al. Isolation of uncultivated anaerobic thermophiles from compost by supplementing cell extract of Geobacillus toebii in enrichment culture medium. Extremophiles 9, 477–485 (2005). https://doi.org/10.1007/s00792-005-0467-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0467-y