Abstract
A novel halotolerant sulfate-reducing bacterium, Desulfovibrio brasiliensis strain LVform1, was isolated from sediments of a dolomite-forming hypersaline coastal lagoon, Lagoa Vermelha, in the state of Rio de Janeiro, Brazil. The cells are vibrio-shaped and 0.30 to 0.45 μm by 1.0 to 3.5 μm in size. These bacteria mediate the precipitation of dolomite [CaMg(CO3)2] in culture experiments. The strain was identified as a member of the genus Desulfovibrio in the δ-subclass of the Proteobacteria on the basis of its 16S rRNA gene sequence, its physiological and morphological properties. Strain LVform1 is obligate sodium-dependent and grows at NaCl concentrations of up to 15%. The 16S rRNA sequence revealed that this strain is closely related to Desulfovibrio halophilus (96.2% similarity) and to Desulfovibrio oxyclinae (96.8% similarity), which were both isolated from Solar Lake, a hypersaline coastal lake in the Sinai, Egypt. Strain LVform1 is barotolerant, growing under pressures of up to 370 bar (37 MPa). We propose strain LVform1 to be the type strain of a novel species of the genus Desulfovibrio, Desulfovibrio brasiliensis (type strain LVform1 = DSMZ No. 15816 and JCM No. 12178). The GenBank/EMBL accession number for the 16S rDNA sequence of strain LVform1 is AJ544687.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mechanism of sedimentary dolomite formation under low temperature conditions (below ≈ 80 °C) has long been a controversy, because it has proven impossible to precipitate dolomite inorganically due to kinetic inhibition (McKenzie 1991; Land 1998; Arvidson and Mackenzie 1999). Vasconcelos et al. (1995) precipitated micritic crystals of dolomite in the presence of a mixed culture of microbes at low temperature (4°C). Based on these results and the study of Lagoa Vermelha, a hypersaline coastal lake in Brazil in which dolomite formation occurs, Vasconcelos and McKenzie (1997) proposed the microbial dolomite model, which includes microorganisms as a factor to overcome the kinetic barrier to dolomite formation. Dolomite formation is rarely observed in modern environments but was very common in the geologic past where dolomite is an abundant sedimentary mineral (Burns et al. 2000; Warren 2000). To study microbial mediation in dolomite precipitation under controlled conditions in the laboratory, sulfate-reducing bacteria were isolated from Lagoa Vermelha. The process of dolomite formation was simulated with pure cultures in a synthetic medium (Warthmann et al. 2000; van Lith et al. 2003b). One of these strains, LVform1, mediated dolomite and high Mg-calcite formation with formate as the substrate. In the present paper, one of the isolates, stain LVform1, is described in detail. Based on the 16S rRNA gene sequence analysis, this strain groups with representatives of the genus Desulfovibrio. We designate strain LVform1 as the type strain of a new species, Desulfovibrio brasiliensis sp. nov.
Methods
Sampling, isolation and cultivation
Sediment samples were taken from the anoxic sediments of Lagoa Vermelha, a shallow hypersaline coastal lagoon, approximately 100 km east of Rio de Janeiro, Brazil. The ambient water temperature of Lagoa Vermelha is 25–31°C, with an average salinity of 45‰ and a pH of 7.6 (Vasconcelos and McKenzie 1997; van Lith et al. 2002). Detailed chemical and physical descriptions of the habitat are published in van Lith et al. (2002), Vasconcelos and McKenzie (1997) and Höhn et al. (1986). Enrichment cultures were grown with formate as the electron donor in a marine mineral medium with a 1.66-fold increased NaCl salinity compared to standard seawater according to IAPSO (International Association for the Physical Sciences of the Ocean). The medium contained (per liter): KH2PO4 0.5 g, NH4Cl 0.25, CaCl2 · 2 H2O 1.5 g, MgSO4 · 7 H2O 2.46 g, KCl 0.64 g, NaCl, 45 g, MgCl2 · 6 H2O 8.1 g, Na-formate 2.5 g and Na-acetate · 2 H2O, 0.168 g. After autoclaving and cooling under N2, the following compounds were added from sterile stock solutions: 30 mM Na-bicarbonate, 1 mM Na2S, 1.0 ml vitamin solution (Balch et al. 1979), 1 ml trace elements solution SL-10 (Widdel et al. 1983) and 1 ml of a Se+W solution (22.8 μM Na2SeO3 . 6 H2O, 24.3 μM Na2WO4 · 2 H2O). The pH was adjusted to 7.6, at which the saturation index of dolomite SIdol reached a value of +4.78. The medium was dispensed under N2 into sterile 50-ml crimp-top vials with butyl rubber stoppers. For enrichment cultures, the liquid media were supplied with 1.0 g sediment sample and were incubated at 30°C. These enrichment cultures were subsequently inoculated into deep agar dilution series to obtain pure cultures. Selected colonies forming a visible milky precipitate were picked up with glass capillaries for further cultivation. This process was repeated until pure cultures were obtained. Pure cultures were grown in the liquid medium described above.
High-pressure activity
Activity measurements at elevated pressures were conducted by using a self-constructed hydrothermal rig (Institute for Mineralogy& Petrography, ETH Zurich). Pressures up to 70 MPa (700 bar) could be adjusted in nickel containers by a hydraulic pump running with water. Cultures were placed anaerobically in sterilized 3-ml glass tubes closed with relocatable rubber stoppers, transmitting the pressure. As the cultures could not be observed during incubation under pressure, incubation time was set to 72 h. Sulfide concentrations were determined immediately after depressurization by the method of Cline (1969).
16S rRNA analysis
Genomic DNA was extracted and 16S rDNA gene amplified by PCR as described by Rainey et al. (1996). Purified 16S rDNA was sequenced with “Abi Prism dye terminator cycle sequencing ready reaction kits” (Applied Biosystems, Darmstadt, Germany). DNA fragments were separated and analyzed by electrophoresis in a “373A DNA sequencer” (Applied Biosystems).
Phylogenetic analysis
Highest similarities of the 16S rDNA gene sequence of the new strain LVform1 to that of other bacteria were searched using the BLAST tool of GenBank (http://www.ncbi.nlm.nih.gov, Altschul et al. 1997). The new 16S rRNA gene sequence was imported to the ARB database and aligned automatically employing the Fast Aligner V1.03 of the ARB phylogeny software package (Ludwig et al. 1998, 2004; http://www.arb-home.de). The alignment positions were subsequently checked and corrected manually based on secondary structure information. The sequence containing 1,420 unambiguously aligned nucleotides was added to the phylogenetic treeing program implemented in ARB. Distance matrix, maximum-parsimony and maximum-likelihood methods were applied for tree construction as implemented in the ARB software package. Evolutionary distances were calculated by the “FASTA” and “BESTFIT” software (Version 10.3, Accelrys Inc., San Diego, CAUSA) and additionally by the Phylip 3.6 interface (Felsenstein 2000) of the Ribosomal Database Project (Cole et al. 2003; http://rdp.cme.msu.edu/html/analyses.html), by which another phylogenetic tree was calculated. The two phylogenetic trees, calculated with different methods, were almost identical. The 16S rDNA gene sequence of strain LVform1 is available at GenBank/EMBL under the accession number AJ544687.
Results and discussion
Physiological characteristics of strain LVform1
The culture was originally flocculent after it was isolated from Lago Vermelha sediment, and the majority of the biomass was present apparently as 0.5- to 1.0-mm aggregates. Thus, it was not possible to measure the optical density accurately. After repeated transfers, the cultures no longer flocculated, and later, transfers produced homogenously turbid cultures.
Cell morphology
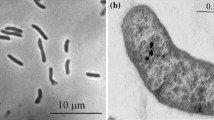
Cells of the Gram-negative LVform1 are vibrio-shaped and 0.30–0.45 μm wide by 2.0–3.5 μm long (Fig. 1a, b). The Cells exhibited one subpolar flagellum as seen in Fig. 1. Some of the cells showed one or two intracelluar inclusions, which may be inorganic material, probably polyphosphate, producing the secondary electron reflection observed in SEM photomicrographs (Fig. 1b). The organism grew in a mineral medium containing 40 mM formate as a substrate for dissimilation and 2 mM acetate as a carbon source. Catalase activity was not observed. The G+C content of genomic DNA of strain LVform1 was 56.3 mol%, analyzed by the Identification Service of the DSMZ, Braunschweig, Germany.
Substrates
Substrates supporting growth with sulfate (10 mM) as electron acceptor were: pyruvate (10 mM), lactate (20 mM), malate (10 mM), fumarate (10 mM), formate (20 mM), casamino acids (3 g/l) and H2 (Table 1). Acetate was produced during growth on lactate. Substrates, which did not support growth in the presence of sulfate, include acetate (10 mM), propionate (10 mM), ethanol (10 mM), methanol (10 mM), benzoate (5 mM), alanine (5 mM), glycine (20 mM), fructose (5 mM) and glucose (5 mM). Growth by fermentation of pyruvate was also observed. Growth occurred when H2 (or formate) was the electron donor, and thiosulfate (10 mM), sulfite (10 mM), elemental sulfur, DMSO (20 mM), FeIII-citrate (10 mM) or fumarate (20 mM) were the electron acceptors. Strain LVform1 did not grow autotrophically with CO2 as carbon source and H2 as electron donor. Growth was not detected with nitrate as an electron acceptor (in absence of sulfide). Fumarate reduction was an alternative electron acceptor with formate as a substrate (fumarate was reduced to succinate). Thiosulfate was disproportionated to sulfide and sulfate and acts as a sole substrate (Table 1).
Salinity, temperature and pH tolerance
D. brasiliensis strain LVform1 had a considerable salinity tolerance. Growth occurred from 1% to 15% NaCl, with an optimum from 3% to 10% NaCl. Growth temperature ranged from 15°C to 45°C with an optimum at approximately 33°C. Growth occurred from pH 6.5–8.4, with an optimum at around 7.6, but the ability to adapt to higher pH was observed by the viability of cultures in the stationary growth phase when pH values of up to 9.3 were observed, as final pH in batch cultures slowly shifted to alkaline.
Growth under high pressure
Strain LVform1 was also tested for its pressure tolerance (Fig. 2) because of its relationship to D. profundus which has an optimum at 15 MPa (Bale et al. 1997). With increasing pressure up to 40 MPa (400 bar), the activity of LVform1 decreased. Cells tended to increase exo-polymer formation and to aggregate when growing under high pressure. LVform1 cells were robust against rapid pressure changes. They tolerated pressure increases to 25 MPa followed by decompression within a minute, as shown by vivid motility of the cells. However, it appears that barotolerance is not a very specific characteristic as discussed by Bartlett (1992).
Phylogenetic position and taxonomy
The results of the 16S rRNA gene analysis of the strain LVform1 clearly showed that the isolate belongs to the genus Desulfovibrio within the δ-subclass of the Proteobacteria. The closest relatives of strain LVform1 on the basis of 16S rDNA gene sequence similarities were D. oxyclinae (96.8%) and D. halophilus (96.2% similarity). It has been suggested that bacteria with 16S rRNA gene sequence similarities higher than 97% are on the boundary value to be assigned to the same species if additional phenotypic and genomic data support species identity (Rosselló-Mora and Amann 2001). Due to its physiological differences, like capacity for fumarate reduction to succinate, thiosulfate disproportionation, the absence of alcohol utilization (Table 1) and a significantly different genomic G+C content, strain LVform1 is sufficiently different from D. halophilus and D. oxyclinae to be considered a distinct species for which the name Desulfovibrio brasiliensis with strain LVform1 as the type strain is proposed. In all phylogenetic trees obtained, the novel 16S rRNA gene sequence of strain LVform1 clustered at the same position. The maximum likelihood tree shown in Fig. 3 is representative of most of the trees produced.
Dolomite precipitation
Inorganic dolomite mineral precipitation is kinetically inhibited at low temperatures (< ~80°C), even when the solutions are oversaturated (Land 1998). In growing cultures of D. brasiliensis, however, dolomite forms in an oversaturated medium in reasonable amounts. From other described species tested (Desulfovibrio profundus, D. halophilus, D. oxyclinae and Deulfonatronovibrio hydrogenovorans), only D. hydrogenovorans mediated dolomite formation in laboratory experiments. Microbial dolomite formation under physiological temperatures and anoxic conditions, mediated by SRB isolated from Lagoa Vermelha, was described by Warthmann et al. (2000) and van Lith et al. (2003a, b). In cultures of strain D. brasiliensis, dolomite precipitated after 4–8 weeks incubation in the range of 25–45°C. The dolomite has a composition of 40–52 mol% Mg and exhibited the typical ordering peaks in the X-ray diffractograms (van Lith et al. 2003b). Sometimes, the mineral occurs with a characteristic dumbbell shape (Fig. 4).
Dolomite forms according to the following reactions (simplified):
Reaction 2 (dolomite formation) was, however, not complete in the culture experiments; the observed maximum dolomite yield was about 20% with respect to the organic carbon supplied by the substrate. One critical factor of dolomite precipitation by strain LVform1 and D. hydrogenovorans is probably their ability to be active at high pH at which the medium becomes highly oversaturated in respect to dolomite. Moreover, local chemical gradients formed in cell aggregates and excreted polymers may promote dolomite precipitation, e.g. it was observed that cultures incubated on a shaker did not produce dolomite.
Recent studies have demonstrated that pure cultures of sulfate-reducing bacteria, such as D. brasiliensis, are capable of mediating dolomite formation in a synthetic anoxic hypersaline medium under well-defined conditions (Warthmann et al. 2000; van Lith et al. 2003a, b). The production of microbial dolomite by a pure culture of SRB verifies the microbial dolomite model developed by Vasconcelos and McKenzie (1997) in their study of Lagoa Vermelha, a hypersaline coastal lagoon in Brazil from which D. brasiliensis was isolated. Moreover, the use of pure cultures of SRB confirms the first experiments, in which microbial dolomite was formed in the presence of a mixed culture containing SRB (Vasconcelos et al. 1995).
Geochemical and phylogenetic links
As observed in the phylogenetic tree shown in Fig. 3, the new isolate D. brasiliensis is closely related to the halophile Desulfovibriro halophilus (Caumette et al. 1991) and D. oxyclinae (Krekeler et al. 1997), both isolated from the well-studied Solar Lake in the Sinai, Egypt (Cohen et al. 1977; Krumbein et al. 1997; Jørgensen and Cohen 1977), which is a similar ecosystem to Lagoa Vermelha in Brazil (van Lith et al. 2002; Höhn et al. 1986). Both are coastal lagoons, separated by sand dunes from the open ocean. Due to arid climate with negative hydrologic balance, seawater seepage supplements the evaporated water, leading to hypersaline conditions. Both lagoons exhibit primary dolomite formation in the sediment (for Solar Lake see Lyons et al. 1984), most probably catalyzed or mediated by microbes; however, it has not been proven that stain LVform1, which is capable of producing dolomite in culture experiments is exclusively responsible for dolomite formation in the natural environment. Nevertheless, Lagoa Vermelha and Solar Lake are a showcase example for the correlation of environmental data with microbial function and phylogenetic relationship of the inhabiting organisms.
Description of Desulfovibrio brasiliensis sp. nov.
Desulfovibrio brasiliensis (bra.si.lien’sis. N.L. adj. Brasiliensis, pertaining to the geographic origin of the organism). Vibrioid cells, 0.3–0.45 μm wide and 1.0–3.5 μm long, Gram-negative and motile. One subpolar inserted flagellum is present. Temperature range for growth is from 15°C to 45°C, with an optimum at 33°C. Growth was observed between pH 6.3 and 9.0 and from 10 g NaCl l−1 to 150 g NaCl l−1. Substrates used for anaerobic respiration are lactate, formate and hydrogen and pyruvate, which can be fermented as well. Sulfate, sulfite, thiosulfate, sulfur, dimethyl-sulfoxide, Fe(III)citrate and fumarate serve as electron acceptors. Autotrophic growth with CO2 was not observed. Catalase activity is absent. The G+C content of genomic DNA is 56.3 mol%. The strain was isolated from anoxic, organic carbon-rich sediment of Lagoa Vermelha, a hypersaline coastal lagoon in the state of Rio de Janeiro, Brazil. Type strain: LVform1, deposited as DSMZ No. 15816 with the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany) and the Japan Collection of Microorganisms JCM No. 12178.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arvidson RS, MacKenzie FT (1999) The dolomite problem: control of precipitation kinetics by temperature and saturation state. Am J Sci 299:257−288
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:2602–2696
Bale SJ, Goodman K, Rochelle PA, Marchesi JR, Fry JC, Weightman AJ, Parkes RJ (1997) Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol 47:515–521
Bartlett DH (1992) Microbial life at high pressures. Sci Progress 76:479–496
Burns ST, McKenzie JA, Vasconcelos C (2000) Dolomite formation and biogeochemical cycles in the Phanerozoic. Sedimentology 47(Suppl 1):49–61
Caumette P, Cohen Y, Matheron R (1991) Isolation and characterization of Desulfovibrio halophilus sp. nov., a halophilic sulfate-reducing bacterium isolated from Solar Lake (Sinai). System Appl Microbiol 14:33–38
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Cohen Y, Krumbein WE, Shilo M (1977) Solar Lake (Sinai). 2.Distribution of photosynthetic microorganisms and primary production. Limnol Oceanogr 22:609–620
Cohen Y, Krumbein WE, Goldberg M, Shilo M (1977) Solar Lake (Sinai). 1. Physical and chemical limnology. Limnol Oceanogr 22:597−607
Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, Chandra S, McGarrell DM, Schmidt TM, Garrity GM, Tiedje JM (2003) The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res 31:442–443
Felsenstein J (2000) PHYLIP 3.6. Department of Genetics SK-50. University of Washington
Höhn A, Tobschall HJ, Maddock JEL (1986) Biogeochemistry of a hypersaline lagoon east of Rio de Janeiro, Brazil. Sci Total Environ 58:175–185
Jørgensen BB, Cohen Y (1977) Solar Lake (Sinai). 5.The sulfur cycle of the benthic cyanobacterial mats. Limnol Oceanogr 22:657–666
Krekeler D, Sigalevich P, Teske A, Cypionka H, Cohen Y (1997) A sulfate-reducing bacterium from the oxic layer of a microbial mat from Solar Lake (Sinai), Desulfovibrio oxyclinae sp. nov. Arch Microbiol 167:369–375
Krumbein WE, Cohen Y, Shilo M (1977) Solar Lake (Sinai). 4.Distribution of photosynthetic microorganisms and primary production. Limnol Oceanogr 22:609–620
Land LS (1998) Failure to precipitate dolomite at 25° C from dilute solution despite 1000-fold oversaturation after 32 years. Aquat Geochem 4:361–368
van Lith Y, Vasconcelos C, Warthmann R, Martins JCF, McKenzie JA (2002) Bacterial sulfate reduction and salinity: two controls on dolomite precipitation in Lagoa Vermelha and Brejo do Espinho (Brazil). Hydrobiologia 485:254–259
van Lith Y, Warthmann R, Vasconcelos C, McKenzie JA (2003a) Microbial fossilization in carbonate sediments; a result of the bacterial surface involvement in carbonate precipitation. Sedimentology 50:237–245
van Lith Y, Warthmann R, Vasconcelos C, McKenzie JA (2003b) Sulphate-reducing bacteria catalyze anoxic dolomite and high Mg-calcite formation. Geobiology 1:71–79
Ludwig W, Strunk O, Klugbauer S, Klugbauer N, Weizenegger M, Neumaier J, Bachleitner M, Schleifer KH (1998) Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:664–568
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Lyons WB, Long DT, Hines ME, Gaudette HE, Armstrong PB (1984) Calcification of cyanobacterial mats in Solar Lake, Sinai. Geology 12:623–626
McKenzie JA (1991) The dolomite problem:an outstanding controversy. In: Müller DW, McKenzie JA, Weissert H (eds) Controversies in modern geology: evolution of geological theories in sedimentology, earth history and tectonics. Academic, London, pp 37–54
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Rosselló-Mora R, Amann R (2001) The species concept for prokaryotes. FEMS Microbiol Rev 25:39–67
Vasconcelos C, McKenzie JA (1997) Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J Sediment Res 67:378–390
Vasconcelos C, McKenzie JA, Bernasconi S, Grujic D, Tien AJ (1995) Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 377:220–222
Warren J (2000) Dolomite: occurrence, evolution and economically important associations. Earth Sci Rev 52:1–81
Warthmann R, van Lith Y, Vasconcelos C, McKenzie JA, Karpoff AM (2000) Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 28:1091–1094
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov., sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–293
Acknowledgements
We gratefully acknowledge Thomas Horath (University Zürich) for help with sequencing and the phylogenetic tree alignment, Sven Girsperger (ETH Zürich) for conducting the high-pressure experiments and Ernst Wehrli (ETH Zürich) for the SEM micrographs of the bacteria and mineral products. This work was partially supported by Swiss National Science Foundation Grant No. 20-59282 and 20-67620.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Wiegel
Rights and permissions
About this article
Cite this article
Warthmann, R., Vasconcelos, C., Sass, H. et al. Desulfovibrio brasiliensis sp. nov., a moderate halophilic sulfate-reducing bacterium from Lagoa Vermelha (Brazil) mediating dolomite formation. Extremophiles 9, 255–261 (2005). https://doi.org/10.1007/s00792-005-0441-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0441-8