Abstract
A novel thermophilic, chemolithoautotrophic bacterium, designated as NE1206T, was isolated from a Juan de Fuca Ridge hydrothermal vent sample (tubes of the annelid polychaete Paralvinella sulfincola attached to small pieces of hydrothermal chimney). The cells were rod-shaped (1.2–3.5×0.4–0.7 μm), occurring as single motile rods or forming macroscopic aggregates visible as pinkish to brownish streamers. The new isolate was anaerobic. It grew between 50 and 70 °C (optimum 60–65 °C; doubling time approximately 1 h 15 min at 60 °C), between pH 5.0 and 7.5 (optimum pH around 6.0–6.5) and at sea salts concentrations between 20 and 40 g l−1 (optimum 30 g l−1). Cells grew chemolithoautotrophically in an H2/CO2 atmosphere (80/20, v/v; 200 kPa). Molecular hydrogen was the sole electron donor used by the strain. Nitrate and elemental sulfur served as electron acceptors, yielding ammonia and hydrogen sulfide, respectively (nitrate reduction supported higher growth rates than sulfur reduction). The G+C content of the genomic DNA was 36.7±0.8 mol%. Phylogenetic analyses of the 16S rRNA gene located the strain within the genus Desulfurobacterium. However, the novel isolate possesses physiological and biochemical characteristics that differ from the previously described species of this genus. We propose that the isolate represents a novel species, Desulfurobacterium crinifex sp. nov. The type strain is NE1206T (DSM 15218T, CIP 107649T). An amendment of the genus Desulfurobacterium description is proposed, based on the phenotypic characteristics of the novel species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deepest phylogenetically branching bacterial phyla are, according to 16S rDNA phylogenetic analyses, the Aquificae and the Thermotogae. The phylum Aquificae includes the genera Aquifex (Huber et al. 1992), Thermocrinis (Huber et al. 1998), Hydrogenobacter (Kawasumi et al. 1984), Hydrogenobaculum (Stöhr et al. 2001), Hydrogenothermus (Stöhr et al. 2001), and the very recently described genus Persephonella (Götz et al. 2002). Cultivated representatives of this phylum have been isolated from marine and continental volcanic and geothermal heated springs (Reysenbach 2001). Members of the Aquificae are thermophilic or hyperthermophilic rod-shaped microorganisms. Under some growth conditions and notably under a continuous water flow, many of them form long macroscopic filaments or aggregates forming colored networks of cells (Eder and Huber 2002). With the exception of the strict aerobic heterotroph Hydrogenobacter subterraneus (Takai et al. 2001), almost all Aquificae are strict chemolithoautotrophs able to grow by O2 (under microaerobic conditions) or NO3 − reduction, using CO2 as a carbon source and H2, S2−, S0, or S2O3 2− as an electron donor. Chemoorganoheterotrophic growth on simple organic molecules also occurs within the genus Thermocrinis (Huber et al. 1998). The phylum Thermotogae encompasses the genera Thermotoga (Huber et al. 1986), Petrotoga (Davey et al. 1993), Geotoga (Davey et al. 1993), Fervidobacterium (Patel et al. 1985), Thermosipho (Huber et al. 1989), and Marinitoga (Wery et al. 2001). Almost all cultivated members of this phylum have been isolated from extreme environments such as oil field brines or oil reservoirs, and from terrestrial or submarine volcanic areas (Alain et al. 2002a). Thermotogae are rod-shaped moderate thermophilic or hyperthermophilic bacteria surrounded by a characteristic sheath-like outer structure devoid of cytoplasm called the "toga". To date, all members are obligate anaerobes that derive energy from the fermentation of a broad range of organic compounds (carbohydrates, proteinaceous substrates, or alcohols). In most cases, growth of these microorganisms is enhanced by the addition of thiosulfate or other sulfur compounds (Ravot et al. 1995). In addition, the recently described methanol-fermenting Thermotoga lettingae is able to use ferric iron and anthraquinone-2,6-disulfonate as electron acceptors (Balk et al. 2002). Recently, two new species have been described belonging to two new genera that form a distinct 16S rRNA lineage between the order Aquificales and the order Thermotogales (L'Haridon et al. 1998; Huber et al. 2002). In the latest edition of 'Bergey's Manual of Systematic Bacteriology', the first species, Desulfurobacterium thermolithotrophum (L'Haridon et al. 1998) was placed in the phylum Aquificae but with the designation "genus incertae sedis" (L'Haridon and Jeanthon 2001). Desulfurobacterium thermolithotrophum strain BSAT, isolated from a Mid-Atlantic hydrothermal vent chimney, is a thermophilic strictly anaerobic chemolithoautotrophic bacterium growing by sulfur, thiosulfate, or sulfite reduction with H2 as an electron donor. The second novel isolate, Thermovibrio ruber (Huber et al. 2002), isolated from sandy sediments of a hydrothermal vent off near Papua New Guinea, is a thermophilic, obligate anaerobic chemolithoautotrophic microorganism that reduces nitrate or sulfur in the presence of H2 as an electron donor and CO2 as a carbon source. The authors of both publications underline the fact that D. thermolithotrophum and T. ruber belong to the phylum Aquificae but may, presumably, represent a novel order when further isolates are described (L'Haridon et al. 1998; Huber et al. 2002).
In 2001, samples were collected from a deep-sea hydrothermal vent field on the Juan de Fuca Ridge, in the northeast Pacific ocean. In this paper, we report the isolation and describe the unique physiological and metabolic features of a novel isolate belonging to the genus Desulfurobacterium. In light of the phenotypic properties of this isolate, we describe it as the type strain of a new species and propose an amendment of the genus Desulfurobacterium.
Materials and methods
Collection of samples
Samples were collected at a depth of 1,581 m from a sulfide edifice (T&S edifice) in the CASM vent field (130°01′W, 45°59′N) at Axial Volcano, on the Juan de Fuca Ridge, during the NeMO (New Millennium Observatory) 2001 expedition. Material collected by the remotely operated vehicle (ROV) ROPOS consisted of small fragments of sulfide chimney and adhering tubes of the polychaete worm Paralvinella sulfincola, which colonizes the surface of actively venting sulfide edifices or chimneys. Once aboard ship, mucous tubes still attached to individual P. sulfincola were aseptically placed into sterile tubes containing sterile seawater [3% (v/v) sea salts solution], 20% (v/v) glycerol, and frozen at 20 °C. Samples were transported frozen to the laboratory and thawed just before enrichment cultures.
Culture medium and conditions
Isolate NE1206T was enriched and grown with KA22 medium (Alain et al. 2002b), containing per liter: 30 g sea salts (Sigma), 2.54 g Mg(NO3)2×6H2O (Merck), 1 g NaHCO3, 1.95 g MES buffer (Sigma), 12 g elemental sulfur (Prolabo), 0.3 g KH2PO4 (Sigma), 0.5 ml vitamin mixture (Balch et al. 1979), 10 ml trace element solution (Balch et al. 1979), and 1 mg resazurin (Sigma). The pH was adjusted to 6.5 at room temperature, before autoclaving, and the medium was reduced by 0.5 g l−1 sodium sulfide before inoculation. A mixture of H2/CO2 (80/20, v/v; 200 kPa) was used as the gas phase. Unless indicated otherwise, cultures were incubated at 60 °C with shaking (100 rpm).
Enrichment and purification procedures
Enrichment cultures were carried out anaerobically in liquid KA22 medium inoculated with solid fragments of P. sulfincola tubes, under an atmosphere of H2/CO2 (80/20, v/v; 200 kPa). After 36 h incubation at 60 °C, growth was observed by phase contrast microscopy. Positive cultures were subcultured and then purified using a dilution-to-extinction technique (Baross 1995). Six serial dilutions to extinction were performed. A supplementary purification step was performed by streaking onto KA22 medium solidified with 0.8% (w/v) Phytagel (Sigma) gelling agent. Plates were incubated in anaerobic jars at 60 °C for 10 days under an H2/CO2 (80/20, v/v; 200 kPa) atmosphere.
Observation of the culture and quantification
Cells were observed under a light microscope equipped with a phase-contrast oil immersion objective (×100 magnification). Cells were counted by direct cell counting using a Thoma chamber (depth, 0.02 mm).
Morphology
The Gram type was determined by use of the Ryu non-staining KOH test (Ryu 1940; Buck 1982; Powers 1995). The SpotTest flagella stain (Difco) was used for flagella detection.
For scanning electron microscopy, cells were fixed with 10% formaldehyde (v/v) for 1 h, placed on filters (0.22 μm pore size, Nucleopore), and dried overnight at room temperature. Macroscopic streamers were deposited directly onto filters and fixed overnight in a box under an atmosphere of formaldehyde. Samples were then coated with gold (SCD040; Balzers) and examined using a Philips XL 30-LaB6 scanning electron microscope.
Preparation of cells for ultrathin sectioning and transmission electron microscopy (TEM) was as described elsewhere (Alain et al. 2002c). Ultrathin sections (50 nm) were prepared on a Reichert-Jung Ultramicrotome. The TEM observations were performed on a LEO 912 electron microscope.
Determination of growth parameters
To determine the optimum temperature, pH, and sea salts concentration for growth, cells were grown in Hungate tubes (27 ml; Bellco) containing 5 ml KA22 medium and H2/CO2 at a pressure of 200 kPa in the headspace. All growth parameters were tested with H2 as the electron donor and NO3 − (S0 was also present in the medium) as the electron acceptor. Experiments were performed in thermostatic aluminum heating blocks (Bioblock) monitored with temperature probes placed in control tubes. Parallel experiments were also performed in a thermostatic oven with shaking. To determine the effect of pH on the growth, KA22 medium was modified with the following 10 mM buffers (Sigma): for pH 3 and 4, no buffer; for pH 5, 5.5, and 6, MES [2-(N-morpholino)-ethane sulfonic acid] buffer; for pH 6.5 and 7, PIPES [piperazine-N,N′-bis-(2-ethane sulfonic acid)] buffer; for pH 7.5 and 8, HEPES [N-(2-hydroxyethyl)-piperazine-N′-(2-ethane sulfonic acid)] buffer; for pH 8.5, AMPSO [3-(1,1-dimethyl-2-hydroxyethyl)-amino-2-hydroxypropane sulfonic acid] buffer. Sodium sulfide was added in the anaerobic chamber and, if necessary, the pH was adjusted with 0.1 M HCl or 0.1 M NaOH. To determine the effect of sea salts concentration on the growth, KA22 medium was prepared with sea salts concentrations ranging from 0 to 70 g l−1. For all salts concentrations, cells were incubated at the optimal temperature and pH for growth. The effects of temperature, pH, and salinity were determined by measuring maximum growth rates calculated by use of linear regression analyses from six to seven points along the logarithmic growth curves in the portion of the curve where the slope was maximal. All growth experiments were carried out in triplicate.
Physiological and biochemical characteristics
The ability of isolate NE1206T to use various electron donors was investigated. For this experiment, the following electron donors were added to a minimal medium (modified KA22 medium without S0 but with 4 g l−1 MgSO4×7H2O to ensure an adequate sulfur source): formate (10 mM), acetate (10 mM), propionate (10 mM), methanol (0.5% v/v), yeast extract (2 g l−1), S0 (12 g l−1), S2O3 2− (10 mM), and H2S (10 mM). These compounds were tested under anaerobic conditions with a N2/CO2 gas phase (80/20, v/v; 200 kPa) with NO3 − as the electron acceptor. H2 was also tested under anaerobic conditions as the sole electron donor (H2/CO2, 80/20, v/v; 200 kPa). The following electron donors were also tested under microaerophilic conditions under an atmosphere of N2/CO2/O2 (80/17/3, v/v/v; 200 kPa) (O2 percentages were measured by gas chromatography before inoculation): Fe2+ (10 mM), S0 (12 g l−1), S2O3 2− (10 mM), H2S (10 mM), and H2 (H2/CO2/O2,80/17/3, v/v/v; 200 kPa).
The ability of the new isolate to grow in the presence of different electron acceptors was tested on a modified KA22 medium [prepared without Mg(NO3)2 and sulfur but supplemented with 1 g l−1 NH4Cl to ensure an adequate nitrogen source]: sulfate was tested at 20 mM on this modified sulfate-free medium. After the demonstration that the strain does not use sulfate as a terminal electron acceptor, the other electron acceptors were tested in the same modified KA22 medium supplemented by 4 g l−1 MgSO4×7H2O to ensure a supplemental sulfur source. Elemental sulfur and l-cystine were tested at 12 g l−1, polysulfides at 10 mM (Blumentals et al. 1990), nitrite at 1 mM, O2 at concentrations ranging from 1% to 20%, and thiosulfate, ferric iron, and nitrate were tested at 20 mM. The headspace gas was H2/CO2 (80/20, v/v; 200 kPa). O2 was also tested as a terminal electron acceptor (at concentrations ranging from 1% to 20%) with S0 (12 g l−1) or S2O3 2− (10 mM) as the electron donor, in experiments where the headspace gas was N2/CO2 (80/20, v/v; 200 kPa).
The ability of strain NE1206T to utilize different carbon sources was investigated by adding the following compounds to the KA22 medium prepared without NaHCO3: formate (10 mM), acetate (10 mM), propionate (10 mM), lactate (0.5% v/v), methanol (0.5% v/v), yeast extract (2 g l−1), tryptone (2 g l−1), peptone (2 g l−1), d(+)-glucose (2 g l−1), maltose (2 g l−1), starch (2 g l−1), and CO2 (20%). These experiments were performed under a H2 (100%, 200 kPa) gas phase.
The effect of different growth factors was also tested. In separate experiments the KA22 medium was amended with complex organic substrates (yeast extract, peptone, and tryptone added at 0.1% v/v), organic acids (formate added at 0.5% v/v, and acetate and propionate added at 0.1% v/v), carbohydrates [d(+)-glucose, d(+)-galactose, and maltose tested at 0.1% v/v], and selenite–tungstate solution (1 ml l−1 of a stock solution: 6 mg l−1 Na2SeO3×5H2O; 0.8 mg l−1 Na2WO4×2H2O; 0.4 g l−1 NaOH).
All these tests were performed in triplicate in serum vials. Positive cultures were transferred at least twice on the same substrate combination to confirm growth. Growth was determined by direct cell counts in a Thoma chamber (depth 0.02 mm) with a phase-contrast microscope.
Cytochrome oxidase activity was tested using the "oxidase " kit (Remel), based on the reaction of N,N-dimethyl-1,4-phenylenediamine and alpha-naphthol, where the presence of oxidase activity is confirmed by a dark blue coloration.
Analyses of metabolic products and analytical techniques
H2S formation was detected by the addition of 500 μl of 50 mM HCl–5 mM CuSO4 to 0.2 ml of the culture. A brown precipitate demonstrated the presence of H2S. H2S concentrations were measured spectrometrically at 670 nm 1 h after addition of 0.1 ml of a 100 mM FeCl3 solution and 0.1 ml of a 19 mM N,N-dimethyl-p-phenylenediamine dihydrochloride to 10 ml of culture, by comparison to an H2S calibration curve.
Ammonium/ammonia concentrations were determined by use of an ammonia detection kit (Boehringer Mannheim/R-biopharm, Darmstadt, Germany) according to the manufacturer's instructions. This test was carried out spectrometrically after addition of NADH, 2-oxoglutarate, and glutamate dehydrogenase to the samples yielding glutamate and NAD. Quantitative nitrate determination was performed by use of a nitrate detection kit (Boehringer Mannheim/R-biopharm) following the manufacturer's recommendations. This was carried out spectrometrically after addition of NADPH and nitrate reductase to the sample leading to nitrate reduction. The amount of NADPH oxidized during the reaction is stoichiometric to the amount of nitrate. For nitrate and ammonia determinations, cells were grown at 60 °C on KA22 medium prepared without sulfur and resazurin (pH 6.0, 30 g l−1 sea salts, atmosphere of H2/CO2). For these experiments, samples were deproteinized with 5-sulfosalicylic acid and then degassed to eliminate CO2 before determinations were performed. Nitrite was determined quantitatively using a colorimetric nitrite test kit (chemical method: sulfanilamide) (HI 3873; Hanna Instruments, France).
Gas percentages within gas atmosphere of vials were determined using an MTI microgas chromatograph equipped with a thermal conductivity detector. A molecular sieve with argon as the carrier gas and a temperature of 30 °C were used to detect O2, H2, and N2. CO2 was determined using a Poraplot U column, at 100 °C, with helium as the carrier gas. The analyzed gas phases were pumped from the culture vials directly and without gas contamination, by the suction pump of the MTI system.
DNA extraction and purification
Genomic DNA was extracted as described by Wery et al. (2001). The concentration and purity of the genomic DNA obtained were estimated by use of a GenQuant II spectrophotometer (Pharmacia) at 260, 280, and 320 nm. The quality of the extraction was checked on a 0.8% (w/v) agarose gel containing 0.5 μg ethidium bromide ml−1.
DNA base composition and phylogenetic analysis
The G+C content of the genomic DNA was determined from the melting point according to Marmur and Doty (1962), as described elsewhere (Alain et al. 2002b). A calibration curve was constructed by use of ultrapure DNA from Escherichia coli strain B (50% G+C), Clostridium perfringens (26.5% G+C), and calf thymus (42% G+C) as standards (Sigma).
The 16S rDNA was selectively amplified from purified genomic DNA by PCR with oligonucleotide primers designed to anneal to conserved positions in the 3′ and 5′ regions of the 16S rRNA genes. The forward primer was SAdir (5′-AGAGTTTGATCATGGCTCAGA-3′) corresponding to positions 8–28 in the E. coli 16S rRNA and the reverse primer was S17rev (5′-GTTACCTTGTTACGACTT-3′), corresponding to positions 1493–1509. The initial denaturation step consisted of heating the reaction mixture to 94 °C for 3 min. This was followed by 30 cycles as follows: denaturation at 94 °C for 1 min, annealing at 49 °C for 1 min 30 s, and extension at 72 °C for 2 min. A final extension step was carried out at 72 °C for 6 min. The PCR products were analyzed on 0.8% (w/v) agarose TAE gels (0.04 M TRIS-acetate, 0.001 M EDTA), containing 0.8 μg ethidium bromide ml−1, and recorded with a Fluor-S multiImager (Bio-Rad).
The 16S rDNA gene PCR product was bidirectionally sequenced by Genome Express S.A. (Grenoble, France) with an automatic DNA analysis system (Applied Biosystems). The following primers, chosen to generate an overlapping set of sequences, were used for sequencing: SAdir, S17rev, 533F (5′-TGBCAGCMGCCGCGGTAA-3′), 517R (5′-ACCGCGGCKGCTGGC-3′), 690R (5′-TCTACGCATTTCACC-3′), and 1100R (5′-AGGGTTGCGCTCGTTG-3′). One thousand five hundred and twenty-one positions of the 16S rDNA were determined. To check for potential polymorphisms in the 16S rDNA genes and to confirm the direct sequencing results, PCR products were cloned into the PCR2.1 vector of the TOPO-TA cloning kit (Invitrogen). Ten independent 16S rDNA clone genes were sequenced.
The 16S rDNA sequence of the strain NE1206T was compared to the sequences of representative members of the phylum Aquificae and clones from hydrothermal vents or hot springs. The CLUSTALW method with weighted residues was used to align the sequences and to calculate similarity levels (Thompson et al. 1994). Alignment was manually refined by use of the multiple sequence alignment editor SEAVIEW and phylogenetic reconstruction was produced by use of PHYLO_WIN (Galtier et al. 1996) with the settings: Jukes-Cantor distance matrix, and successively the neighbor-joining (Saitou and Nei 1987) and maximum likelihood (Felsentein 1981) methods. In the phylogenetic analysis, 1,243 nucleotides were included. Bootstrap values were determined according to Felsentein (1985). Marinitoga piezophila KA3T (AF326121) was used as outgroup. Using the CLUSTALW method with weighted residues, similarity matrices were constructed on 1,399 nucleotides of a subset of Aquificales sequences unequivocally aligned.
The 16S rDNA gene sequence of strain NE1206T has been deposited in the EMBL database under the accession number AJ507320.
Results
Enrichment, isolation, and morphology
In order to enrich for thermophilic chemolithoautotrophic microorganisms, KA22 medium was inoculated with intact, small P. sulfincola tubes (cleaned of their inner content) attached to small fragments of hydrothermal chimney rocks. After 36 h incubation, growth was observed at 60 °C, under an H2/CO2 gas phase. The enriched culture consisted of dense populations of rod-shaped, motile cells. After 10 days incubation of plates under an H2/CO2 atmosphere at 60 °C, pinkish colonies 1–2 mm in diameter were observed. One isolate, referenced as strain NE1206T (DSM 15218T, CIP 107649T), was completely characterized.
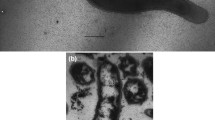
Phase-contrast microscopy indicated that cells of isolate NE1206T were motile, straight to slightly curved rods of 0.9–3.5 μm long and 0.4–0.7 μm wide. When cultivated with shaking under anaerobic conditions, cells were slightly longer. Polar flagella were observed (Fig. 1a). As observed during scanning electron micrography analyses (Fig. 1b), cells divided by constriction. They were Gram-negative (Fig. 1c) and formed balloons during the late stationary phase of growth. Very frequently and systematically when growth occurred into non-reduced media, cells formed aggregates that arranged in macroscopic pinkish to brownish branched streamers. The total length of these pinkish cell networks, in vials, was sometimes up to 8 cm for a diameter that varied from the diameter of a hair to 2 mm diameter. The microbial architecture of these unusual macroscopic structures was highly conserved from one streamer formed in vitro to another (Fig. 1d). This architecture was maintained by a polymeric matrix whose chemical composition and function are under characterization.
a, b Scanning electron micrographs of strain NE1206T in the midexponential phase of growth, showing polar flagella (a) and division by constriction (b). Bar 1 μm. c Transmission electron micrograph of ultrathin section of strain NE1206T showing a Gram-negative cell envelope, with from inside to outside a cytoplasmic membrane (CM), a thin peptidoglycan layer (PG), an outer membrane (OM), and an S-layer (S). Magnification ×60,000. d Scanning electron micrograph of a macroscopic visible filament produced by cells of strain NE1206T in culture vials (KA22 medium, H2/CO2 atmosphere, 60 °C). Bar 500 μm
Growth parameters
The novel isolate NE1206T grew between 50 and 70 °C with an optimum around 60–65 °C. Growth did not occur at 45 or 75 °C (Fig. 2). The strain grew at sea salts concentrations between 20 and 40 g l−1. The optimum sea salts concentration for growth was 30 g l−1. Growth of the isolate was optimal around pH 6.0–6.5, although growth was observed at pH 5.0 and 7.5. Under optimal growth conditions (at 60 °C with shaking, pH 6.5, 30 g l−1 sea salts, with H2 as electron donor, CO2 as carbon source, and NO3 − as terminal electron acceptor) the doubling time was around 1 h 15 min.
Effect of temperature on the maximum growth rate (μmax) of strain NE1206T. The cells were grown under agitation with H2 as electron donor, CO2 as carbon source, and NO3 − as terminal electron acceptor (at pH 6.5 and with 30 g sea salts l−1). The experiment was performed in triplicate. Maximum growth rates were calculated by performing linear regression analysis of the part of the logarithmic growth curves where the slope was maximal
Growth characteristics
Strain NE1206T was an anaerobic, cytochrome oxidase negative, hydrogen-oxidizing, thermophilic bacterium. Tests for growth requirements highlighted the obligate chemolithoautotrophic character of the novel isolate. Growth was possible by reduction of nitrates or elemental sulfur, using molecular hydrogen as electron donor and CO2 as carbon source (Table 1). The shortest generation times were obtained by nitrate reduction. Ammonia was produced from nitrate reduction (Fig. 3). Nitrite could not be detected in the culture medium after growth by nitrate reduction. When nitrate was not present in the culture medium, H2S was produced as a result of sulfur reduction. Final H2S concentrations of 4.0–14.6 mmol l−1 were measured. The novel isolate was unable to grow organotrophically on the complex substrates or small organic molecules tested. On the other hand, the strain was found to tolerate low oxygen concentrations but was demonstrated to not reduce it. It could be grown on non-reduced KA22 medium (up to oxygen concentrations of 3%) but exclusively without shaking or under a relatively gentle one (100–120 rpm). Under these conditions, a pinkish polymer was regularly produced. Moreover, final end products of anaerobic respiration (H2S if S0 was the electron acceptor or ammonia if that was nitrate) were detected in these non-reduced media. When shaken at high velocity (250 rpm), non-reduced media did not allow growth of the novel isolate (while reduced ones allowed it). Under this microoxygenation of the medium homogenized by a strong shaking, cellular lysis was observed only after 2 days incubation at 60 °C. Furthermore, the novel isolate was unable to grow with oxygen (microaerophilic conditions with 1–20% O2 were tested) as the sole terminal electron acceptor when cultivated at 60 °C and at 250 rpm. All these results together demonstrate that the strain is able to tolerate oxygen but does not reduce it.
Nitrate consumption (circles) and ammonium/ammonia formation (squares) during growth (triangles) of strain NE1206T. This experiment was performed on a modified KA22 medium prepared without S0 and resazurin. Cultures were grown at 60 °C, pH 6.0, with 30 g l−1 sea salts under an H2/CO2 atmosphere (80/20, v/v; 200 kPa)
These data suggest that the new isolate is an obligate chemolithoautotrophic bacterium that produces energy by reducing elemental sulfur or nitrate using molecular hydrogen as an electron donor. Routinely, when cultivated under agitation with H2 as an electron donor, NO3 − as an electron acceptor, CO2 as a carbon source (and under optimal temperature, pH, and salinity), cell densities obtained were 5×107–1×108 cells per ml in vials. Occasionally, final cell concentrations of 3×108 cells per ml were obtained. Under our experimental conditions, none of the electron acceptor/donor and carbon source combinations supported growth of strain NE1206T (Table 1). When added individually to the KA22 medium and at the concentrations tested, selenite-tungstate solution, formate, galactose, maltose, and tryptone had no effect on growth (Table 1). On the other hand, the addition of yeast extract, brain–heart infusion, peptone, acetate, and propionate slightly lengthened the generation time of the strain. At the concentration tested, lactate had a complete inhibitory effect on growth.
DNA base composition and phylogenetic position of the novel isolate
As determined by melting point analysis, the G+C content of the genomic DNA of strain NE1206T was 36.7±0.8 mol%. Phylogenetic analyses of the almost complete sequence (1,521 bp) of the 16S rDNA gene of strain NE1206T, using the neighbor-joining and maximum likelihood algorithms for tree reconstruction, located the strain in the deeply branching phylum Aquificae, within the genus Desulfurobacterium, in the domain Bacteria (Fig. 4). The phylogenetic position of the organism was determined by comparing the 16S rDNA sequence of strain NE1206T to those of nine representative Aquificae species and to three 16S rDNA sequences of uncultured Aquificae. In all calculations, its closest cultivated relative was D. thermolithotrophum (L'Haridon et al. 1998), sharing 96% 16S rDNA sequence similarity (value obtained by the CLUSTALW method on 1,399 nucleotides of a subset of sequences of unequivocal alignment). It was followed by the recently described genus T. ruber (95%). The novel isolate was very closely related to the 16S rDNA sequence of the uncultured clone VC2.1bac2 (and to other sequences unmarked on the phylogenetic tree) from an Atlantic hydrothermal vent (Reysenbach et al. 2000). They shared 99% 16S rDNA gene sequence similarity.
Phylogenetic position of strain NE1206T within the phylum Aquificae. The alignment was performed with 16S rDNA sequences of representative Aquificae species and uncultured Aquificae from Atlantic hydrothermal vents and terrestrial hot springs. The Thermotogale Marinitoga piezophila was chosen as outgroup. Accession numbers are noted in parentheses. The topology shown corresponds to an unrooted tree obtained by a neighbor-joining algorithm (Jukes and Cantor corrections) established using PHYLO_WIN. In the phylogenetic analysis, 1,243 nucleotides were included. Bootstrap values are displayed on their relative branches. Scale bar indicates 6.7 nt substitutions per 100 nt. The positioning of the new isolate was confirmed by the maximum likelihood method
The secondary structural feature of the 16S rRNA sequence, characterized by a CUC bulge and a single nucleotide bulge in the helix found at positions 198–219 (E. coli numbering), retrieved in the 16S rRNA sequence of D. thermolithotrophum (L'Haridon et al. 1998) and proposed to be a signature of the Aquificales lineage (Reysenbach et al. 1994), was also shared by the novel isolate.
Discussion
Phylogenetic analyses of the 16S rRNA sequence clearly indicated that the novel isolate belonged to a deeply branching lineage of Bacteria. This strain is an anaerobic, thermophilic bacterium growing autotrophically on CO2 by nitrate or sulfur reduction using H2 as an electron donor. On the basis of these characteristics, it resembles the recently described deeply branching genus Thermovibrio (Reysenbach et al. 1994). However, strain NE1206T differs from T. ruber (Reysenbach et al. 1994) by its genotypic characteristics. The G+C contents of the genomic DNA of both strains are almost 10% different (46 mol% for T. ruber strain ED11/3LLKT against 36.7 mol% for strain NE1206T). Moreover, based on the analysis of their 16S rRNA sequences, they are phylogenetically distinct. The novel isolate is phylogenetically more closely related to the genus Desulfurobacterium. Strain NE1206T shares some characteristics with the only described species, D. thermolithotrophum (L'Haridon et al. 1998). Indeed, both strains grow chemolithoautotrophically using CO2 as a carbon source, H2 as an electron donor, and S0 as an electron acceptor; their temperature, salinity, and pH ranges for growth are relatively comparable; both strains are anaerobic; the G+C content of the genomic DNA of D. thermolithotrophum and of the novel isolate are 35 mol% and 36.7±0.8 mol%, respectively; cells stain Gram-negative.
However, some characteristics distinguish both species; D. thermolithotrophum strain BSAT is capable of reducing thiosulfate and sulfite, whereas under our experimental conditions the new strain is not. The novel isolate shows a major metabolic difference from D. thermolithotrophum as a result of its ability to grow by nitrate reduction. The best growth rates of the strain were obtained chemolithoautotrophically, by anaerobic nitrate respiration, using H2 as an electron donor and CO2 as a carbon source.
Hydrothermal vent chimney structures result from the mixing of the hot hydrothermal fluid, rich in reduced chemical compounds, with the surrounding cold and oxygenated deep-sea water. Steep thermal and chemical gradients are established in the vicinity of the smokers. Both CO2 and H2,the exclusive carbon source and electron donor used by the strain in our experimental conditions, are abundant in hydrothermal fluids. Elemental sulfur is produced at vents as a result of chemical or biological oxidation of hydrogen sulfide, which is present in high concentration in the hydrothermal fluid. Finally, nitrates used by the strain as preferential electron acceptor are also available in the seawater into which the hydrothermal fluids are discharged. Unlike oxygen which is rapidly reduced by sulfide from the hydrothermal fluid, nitrate is a more stable electron acceptor that can persist in microniches in this ecosystem. Strain NE1206T, capable of growing chemolithoautotrophically either by sulfur reduction or by anaerobic nitrate reduction shows a metabolic plasticity by using several chemical species and gases available in its environment. Consequently, it has the potential to be an important primary producer within its ecological niche.
In addition to the metabolic characteristics that distinguish both strains, the ability of D. thermolithotrophum strain BSAT to form macroscopic colored streamers in liquid culture has not been reported previously, to our knowledge. Polymer production was very frequently observed in cultures of the novel isolate, and occurred systematically when cells were submitted to low oxygen concentrations. Based on the principle that bacterial polymers are generally produced as direct and functional responses to selective pressures in the environment (Whitfield 1988), this observation could suggest that oxygen would be an important environmental constraint for polymer secretion. This hypothesis has still to be corroborated, especially since polymer production was also often observed under anaerobic conditions. A thorough investigation of the architecture of the unusual cellular network built by the novel isolate and an analysis of the chemical composition of the polymer matrix embedding its cells is in progress. This may lead to physiological and ecological insights and to a better understanding of the population dynamic of the strain in vitro and in situ (Alain et al. in preparation).
Furthermore, the novel isolate tolerated oxygen but was unable to use it as a terminal electron acceptor. Growth under non-reductive conditions occurred only under relatively gentle shaking (100–120 rpm) but never at high velocity (250 rpm). As stated in the Results section, products of anaerobic respiration (ammonia or H2S according to the electron acceptor provided in the medium) were detected in the culture media, suggesting the probable occurrence of local anaerobic microniches within the culture media. It is possible that the polymeric matrix, once produced by the cells in an anaerobic microniche, could protect them from oxygen while concurrently the H2S they produced would contribute to reducing the culture medium.
Although D. thermolithotrophum and the novel isolate were both isolated from hydrothermal samples (sulfide chimney sample for D. thermolithotrophum and a tube of the polychaete P. sulfincola mixed with sulfide chimney fragment for the novel isolate), they exhibit major phenotypic differences. They were isolated from geographically well-separated hydrothermal environments. The first strain was isolated from a Mid-Atlantic Ridge sample (L'Haridon et al. 1998) and the novel isolate from Juan de Fuca Pacific Ridge in the Pacific. Up to now, the occurrence of members of the genus Desulfurobacterium, detected either by culture or by molecular method, was restricted to hydrothermal chimneys of the Mid-Atlantic Ridge (Reysenbach et al. 2000). This study expands their geographical localization to deep-sea hydrothermal vents of the northeast Pacific. Considerable further work would be required to determine whether the observed differences between D. thermolithotrophum and the novel isolate are just a biogeographical consequence or are a result of a microflora selection by the different chemical and mineralogical differences between the two environments. Replicate sampling under similar habitat conditions will be required before any biogeographical influences can be considered. Additionally, a thorough examination of the architecture and polymeric composition of the unusual macroscopic network built by cells of the novel isolate (and perhaps by other members of this genus) might give us clues to understand their physiological and ecological behavior in their biotope.
On the basis of the 16S rRNA sequence distance, the morphologic and major metabolic differences between the novel isolate and D. thermolithotrophum, we propose to assign strain NE1206T as the type strain of a novel species for which we propose the name Desulfurobacterium crinifex (=which makes hair).
Amendment of the genus Desulfurobacterium
The description of Desulfurobacterium is based on L'Haridon et al. (1998), plus the following characteristics: Anaerobic. Sulfur, thiosulfate, sulfite, and nitrate can be reduced. Cells, in liquid culture may form macroscopic colored cell masses encased in a polymeric matrix.
Description of Desulfurobacterium crinifex, sp. nov.
Desulfurobacterium crinifex sp. nov. (cri′ni.fex; L. masc. n. crinis, hair; L. suffix n. -fex, maker; N. L. nom n. crinifex, hair maker, to indicate the production of streamers by the organism). Cells are rod-shaped (0.9–3.5 μm length×0.4–0.7 μm width), motile with polar flagella, and stain Gram-negative. Cells divide by constriction. Growth occurs between 50 and 70 °C (optimum: 60–65 °C), pH 5.0 and 7.5 (optimum: 6.0–6.5), 20 and 40 g sea salts l−1 (optimum: 30 g sea salts l−1). Optimal doubling time around 1 h 15 min; mean cell yield comprised between 5×107 and 1×108 cells ml−1; maximum cell yield: 3×108 cells ml−1 in vials (with shaking). Anaerobic. Obligate chemolithoautotrophic. Hydrogen-oxidizing. Reduces NO3 − and S0. H2S is formed from S0 and ammonia is formed from NO3 −. G+C content is 36.7±0.8 mol%. Isolated from a tube of the hydrothermal annelid polychaete Paralvinella sulfincola from the Juan de Fuca Ridge (T&S edifice, 130 °01′W, 45 °59′N, 1,581 m depth, CASM vent field). The type strain is Desulfurobacterium crinifex NE1206T (DSM 15218T, CIP 107649T). The EMBL accession number for the 16S rDNA sequence for NE1206T is AJ507320.
References
Alain K, Marteinsson VT, Miroshnichenko ML, Bonch-Osmolovskaya EA, Prieur D, Birrien J-L (2002a) Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 52:1331–1339
Alain K, Quérellou J, Lesongeur F, Pignet P, Crassous P, Raguénès G, Cueff V, Cambon-Bonavita MA (2002b) Caminibacter hydrogeniphilus, gen. nov., sp. nov., a novel thermophilic hydrogen-oxidising bacterium isolated from an East-Pacific rise hydrothermal vent. Int J Syst Evol Microbiol 52:1317–1323
Alain K, Pignet P, Zbinden M, Quillevere M, Duchiron F, Donval J-P, Lesongeur F, Raguenes G, Crassous P, Querellou J, Cambon-Bonavita M-A (2002c) Caminicella sporogenes gen. nov., sp. nov., a novel thermophilic spore-forming bacterium isolated from an East-Pacific Rise hydrothermal vent. Int J Syst Evol Microbiol 52:1621–1628
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Balk M, Weijma J, Stams AJM (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol 52:1361–1368
Baross JA (1995) Isolation, growth and maintenance of hyperthermophiles. In: Robb FT, Place AR (eds) Archaea: a laboratory manual. Thermophiles. Cold Spring Harbor Laboratory, New York, pp 15–23
Blumentals II, Itoh M, Olson GJ, Kelly RM (1990) Role of polysulfides in reduction of elemental sulfur by the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol 56:1255–1262
Buck JD (1982) Nonstaining (KOH) method for determination of Gram reactions of marine bacteria. Appl Environ Microbiol 44:992–993
Davey ME, Wood WA, Key R, Nakamura K, Stahl DA (1993) Isolation of three species of Geotoga and Petrotoga: two new genera, representing a new lineage in the bacterial line of descent distantly related to the "Thermotogales". Syst Appl Microbiol 16:191–200
Eder W, Huber R (2002) New isolates and physiological properties of the Aquificales and description of Thermocrinis albus sp. nov.. Extremophiles 6:309–318
Felsentein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Felsentein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 30:783–791
Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
Götz D, Banta A, Beveridge TJ, Rushdi AI, Simoneit BRT, Reysenbach A-L (2002) Persephonella marina gen. nov., sp. nov. and Persephonella guaymasensis sp. nov., two novel, thermophilic, hydrogen-oxidizing microaerophiles from deep-sea hydrothermal vents. Int J Syst Evol Microbiol 52:1349–1359
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch Microbiol 144:324–333
Huber R, Woese CR, Langworthy TA, Fricke H, Stetter KO (1989) Thermosipho africanus gen. nov., represents a new genus of thermophilic eubacteria within the "Thermotogales". Syst Appl Microbiol 12:32–37
Huber R, Wilharm T, Huber D, Trincone A, Burggraf S, Konig H, Rachel R, Rockinger I, Fricke H, Stetter KO (1992) Aquifex pyrophilus gen. nov. sp. nov., represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst Appl Microbiol 15:340–351
Huber R, Eder W, Heldwein S, Wanner G, Huber H, Rachel R, Stetter KO (1998) Thermocrinis ruber gen. nov. sp. nov., a pink-filament-forming hyperthermophilic bacterium isolated from Yellowstone National Park. Appl Environ Microbiol 64:3576–3583
Huber H, Diller S, Horn C, Rachel R (2002) Thermovibrio ruber gen. nov., sp. nov., a novel extremely thermophilic, chemolithoautotrophic deeply branching bacterial nitrate-reducer. Int J Syst Evol Microbiol 52:1859–1865
Kawasumi T, Igarashi Y, Kodama T, Minoda Y (1984) Hydrogenobacter thermophilus gen. nov., sp. nov., an extremely thermophilic, aerobic, hydrogen-oxidizing bacterium. Int J Syst Bact 34:5–10
L'Haridon S, Jeanthon C (2001) Genus incertae sedis. I. Desulfurobacterium. In: Garrity GM (ed) Bergey's manual of systematic bacteriology, 2nd edn, vol 1. Springer, Berlin Heidelberg New York, pp 359–367
L'Haridon S, Cilia V, Messner P, Raguénès G, Gambacorta A, Sleytr UB, Prieur D, Jeanthon C (1998) Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Bact 48:701–711
Marmur J, Doty P (1962) Determination of the base composition of desoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch Microbiol 141:63–69
Powers EM (1995) Efficacy of the Ryu nonstaining KOH technique for rapidly determining Gram reactions of food-borne and waterborne bacteria and yeasts. Appl Environ Microbiol 61:3756–3758
Ravot G, Ollivier B, Magot M, Patel BKC, Crolet J-L, Fardeau M-L, Garcia J-L (1995) Thiosulfate reduction, an important physiological feature shared by members of the order Thermotogales. Appl Environ Microbiol 61:2053–2055
Reysenbach A-L (2001) Phylum BI. Aquificae phy. nov. In: Garrity GM (ed) Bergey's manual of systematic bacteriology, 2nd edn, vol 1. Springer, Berlin Heidelberg New York, pp 359–367
Reysenbach A-L, Wickham GS, Pace NR (1994) Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus Spring, Yellowstone National Park. Appl Environ Microbiol 60:2113–2119
Reysenbach AL, Longnecker K, Kirshtein J (2000) Novel bacterial and archaeal lineages from an in situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl Environ Microbiol 66:3798–3806
Ryu E (1940) A simple method of differentiation between Gram-positive and Gram-negative organisms without staining. Kitasato Arch Exp Med 17:58–63
Saitou M, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Stöhr R, Waberski A, Völker H, Tindall BJ, Thomm M (2001) Hydrogenothermus marinus gen. nov., sp. nov., a novel thermophilic hydrogen-oxidizing bacterium, recognition of Calderobacterium hydrogenophilum as a member of the genus Hydrogenobacter and proposal of the reclassification of Hydrogenobacter acidophilus as Hydrogenobaculum acidophilum gen. nov., comb. nov., in the phylum 'Hydrogenobacter/aquifex'. Int J Syst Evol Microbiol 51:1853–1862
Takai K, Komatsu T, Horikoshi K (2001) Hydrogenobacter subterraneus sp. nov., an extremely thermophilic, heterotrophic bacterium unable to grow on hydrogen gas, from deep subsurface geothermal water. Int J Syst Evol Microbiol 51:1425–1435
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wery N, Lesongeur F, Pignet P, Derennes V, Cambon-Bonavita MA, Godfroy A, Barbier G (2001) Marinitoga camini gen. nov., sp. nov., a rod-shaped bacterium belonging to the order Thermotogales, isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 51:495–504
Whitfield C (1988) Bacterial extracellular polysaccharides. Can J Microbiol 34:415–420
Acknowledgments
We thank Prof. H. Trüper (University of Bonn, Germany) for correction of the etymology of the new species. We are grateful to Christian Jeanthon and Stéphane L'Haridon for stimulating and critical discussions. We acknowledge the officers and crew of the R/V Brown and the ROV Ropos operations team. We thank the 'Service de microscopie électronique', IFR de biologie intégrative, CNRS/Paris VI, for transmission electron microscopy. This work was financially supported by Ifremer, Programme Dorsales, Région Bretagne, and the Natural Sciences and Engineering Research Council of Canada. The GenBank/EMBL accession number for the 16S rDNA is AJ507320.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian
Rights and permissions
About this article
Cite this article
Alain, K., Rolland, S., Crassous, P. et al. Desulfurobacterium crinifex sp. nov., a novel thermophilic, pinkish-streamer forming, chemolithoautotrophic bacterium isolated from a Juan de Fuca Ridge hydrothermal vent and amendment of the genus Desulfurobacterium . Extremophiles 7, 361–370 (2003). https://doi.org/10.1007/s00792-003-0329-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0329-4