Abstract
Polymorphisms in latrophilin 3 (LPHN3) were recently reported to be associated with attention-deficit/hyperactivity disorder (ADHD), and subsequently other researchers tried to replicate the findings in different populations. This study was aimed to confirm the role of the LPHN3 in ADHD and explore the potential interactions with environmental risk factors in Chinese Han population. We examined the association of LPHN3 with ADHD in a population of 473 ADHD children and 585 controls. As a supplement of ADHD diagnosis, Conners Parent Symptom Questionnaire (PSQ) was used to evaluate ADHD symptoms. Blood lead levels (BLLs) were measured by atomic absorption spectrophotometry and other potential environmental risk factors were determined via a questionnaire filled out by the parents. Finally, after validation in an independent sample (284 cases and 390 controls), we observed significant associations between LPHN3 variants rs1868790 and ADHD risk in combined stage within codominant model [TA/AA: OR (95% CI) = 1.636 (1.325–2.021)], dominant model [OR (95% CI) = 1.573 (1.288–1.922)], and additive model [OR (95% CI) = 1.535 (1.266–1.862)]. Furthermore, rs1868790 significantly interacted with BLLs and maternal stress to modify ADHD susceptibility (P < 0.05), and rs1868790 was found to be related with ADHD symptoms (P < 0.05). Expression quantitative trait loci analysis further indicated that rs1868790 took part in the regulation of LPHN3 gene expression. As the first study to comprehensively explore the role of LPHN3 in ADHD in Chinese children, our research suggests that LPHN3 gene has a significant effect on the ADHD in a Chinese population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder in children of school age, occurring in 3–10% of the population in China [1]. ADHD is characterized by elevated levels of inattention and/or hyperactive or impulsive behaviors that cause significant impairments in a child’s academic and social functioning. Twin and adoption studies indicate that genetic factors are critical determinants of ADHD with a heritability estimate of 76% [2]. Although genome-wide association studies of ADHD have not been successful in detecting any significant genome-wide association so far, they provide evidence for associations with some traditional candidate genes such as DRD2, DBH, SLC6A2, ADRA1A, ADRB2, HTR2A, TPH2, CHRNA4, SNAP25, BDNF, and COMT, and also implicate novel candidate genes such as CDH13, GFOD1, and CTNNA2 [3, 4].
Recently, several studies have shown an association between G protein-coupled receptor L3 gene (ADGRL3, also known as latrophilin 3 or LPHN3) and ADHD [5], and replication studies have been conducted in different populations, but not in Chinese [5,6,7,8,9,10,11]. LPHN3 is a member of latrophilins (LPHNs, receptors of α-latrotoxin), and the endogenous functions of LPHNs are linked to cell adhesion and synapse formation or maintenance. LPHN3 is primarily localized in the amygdala, caudate nucleus, cerebral cortex, and cerebellum, which are the key brain regions associated with ADHD [5].
Arcos-Burgos et al. firstly reported the association between LPHN3 and ADHD, and except for susceptibility, the rs6551665 SNP was also associated with MPH treatment response efficacy [5]. Hwang and Choudhry et al. confirmed the association between rs6551665 and ADHD risk in an independent population [6, 12]. LPHN3 rs2305339 was implicated in ADHD and combined subtype in a cohort of Spanish children (P = 0.0153 and 0.0124, respectively), and significant association with ADHD was also found in the male sample (P = 0.0001) [7]. This variant was also related to a refined phenotype of ADHD in the Multimodal Treatment Study [OR (95% CI) = 2.25 (1.28–3.97), P = 0.004] [10]. Single- and multiple-marker analyses showed additional evidence of association between LPHN3 and combined ADHD in adulthood [P = 0.0019, OR = 1.82 (1.25–2.70) and P = 5.1E−05, OR = 2.25 (1.52–3.34), respectively] [8]. Besides, Bruxel et al. reported that CGC haplotype derived from rs6813183, rs1355368 and rs734644 was an ADHD risk haplotype (P = 0.02, OR = 1.46) [9] and family-based genetic analyses identified ADHD-associated SNPs harbored in evolutionarily conserved elements functioning as transcriptional enhancers [11]. Four SNPs (rs1947274, rs2345039, rs6551655, and rs6858066) in LPHN3 were found to have a significant effect in discriminating good responders from non-responders and five tag SNPs (rs1868790, rs6551665, rs1947274, rs6858066, and rs2345039) were associated with behavioral assessment by parents [13]. LPHN3 gene even impacts behavioral and neurophysiological measures of cognitive response control [14].
Furthermore, Jain et al. found a cooperative interaction between LPHN3 and 11q doubled the risk for ADHD [15], and this interaction also explained differences in brain metabolism and pharmacogenetic response to stimulant medication, and predicted ADHD severity and long-term outcome [16]. In later research, highly significant interaction between four LPHN3 tag SNPs (rs1947274, rs2345039, rs6858066, rs6551665) and maternal stress during pregnancy was noted [12]. If confirmed in independent large studies, they may present a step forward in unraveling the complex etiology of ADHD.
Environmental factors, such as maternal smoking, drinking, low birth weight, socioeconomic status, and preterm birth, are thought to contribute to the emergence and severity of the disorder, especially the antenatal factors [17,18,19,20,21]. In particular, environment exposures during pregnancy (maternal smoking, drinking, and stress) always influence the early fetal brain through the blood system and lead to adverse pregnancy outcome [22,23,24]. Besides, childhood blood lead levels (BLLs) were identified as an important risk factor for ADHD in different populations [25, 26] and it has been proposed that gene–environment interaction (G × E) may play a pivotal role in the disorder [12, 17, 27].
So far, no one has systematically investigated the genetic relation between the LPHN3 gene and ADHD in the Chinese Han population. As a supplement to ADHD etiological research, the current study is aimed to confirm the involvement of the LPHN3 gene in the susceptibility to ADHD and to explore the potential G × E model.
Materials and methods
Participants
The discovery sample (stage one) included 473 children and adolescents with ADHD consecutively recruited from Wuhan Medical and Health Center for Women and Children between October 2013 and December 2014, who were diagnosed with ADHD by psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) [28]. The controls were healthy children for physical examination in the same hospital during the same period and were diagnosed with no ADHD by psychiatrists using the DSM-IV. All subjects were required to meet the following criteria: (1) were Chinese Han population between the ages of 6–18 years, (2) had scored 70 and above tested by China-Wechsler Intelligence Scale for Children [29], and (3) individuals with major neurological handicaps, schizophrenia, pervasive development disorder, epilepsy, mental retardation, and other brain disorders were excluded. Finally, 473 cases and 585 controls were enrolled in stage one, and subjects were unrelated ethnic Han Chinese. The validation sample (stage two) included 284 ADHD cases and 390 healthy controls enrolled from the Children’s Hospital of Hunan province (Changsha) from January 2014 to December 2015 according to the criteria mentioned above.
According to DSM-IV, the subtypes of ADHD cases were determined as follows: combined (ADHD-C), predominantly inattentive (ADHD-I), and predominantly hyperactive/impulsive (ADHD-HI). In our study, ADHD-C was the most prevalent at stage one (53.1%) and stage two (54.6%) followed by ADHD-I (36.3% and 31.6%, respectively) and ADHD-HI (10.6% and 13.8%).
At recruitment, peripheral venous blood samples and demographic information were collected from each subject after written informed consent was obtained. This study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology, Wuhan Medical and Health Center for Women and Children, and Children’s Hospital of Hunan province.
Measurement of environmental factors
Information about potential environmental risk factors for ADHD was obtained from the questionnaire, including maternal stress, smoking, and drinking (if the mother ever drank at any time during the pregnancy, the maternal drinking was coded yes, otherwise, no [30, 31]).
Maternal smoking during pregnancy was measured on a three-point scale: no smoking = 0, moderate smoking = 1–9, or heavy smoking = 10 or more cigarettes per day [32,33,34]. In later analysis, maternal smoking was coded as a binary variable, and the last two scales—”moderate smoking” and “heavy smoking”—were both identified as “maternal smoking”, which means maternal smoking was defined as smoking no less than one cigarette per day.
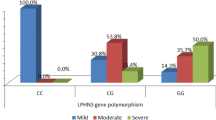
To measure maternal stress during pregnancy, the 30-item Chinese version of Pregnancy Stress Rating Scale (PSRS) was used [35]. The total score is the mean of all items summed, with higher scores indicating higher maternal stress: 0 means that mother experiences no stress; 0.001–1.000, a mild level of stress; 1.001–2.000, a moderate level of stress; and 2.001–3.000, a severe level of stress. During analysis, the variable was dichotomized (no = mild or minimal stress; yes = moderate or severe stress).
BLLs were determined by atomic absorption spectrophotometry (AA-670/GV-5, Shimadzu, Japan) at a commercial laboratory, and a median served as a cutoff point for differentiating BLLs in our study; thus, the median or more was defined as indicative of a high lead level and less than the median denoted a low lead level.
Conners Parent Symptom Questionnaire (PSQ) investigation
As a supplement of ADHD diagnosis, PSQ was applied to measure ADHD symptoms by child psychiatrists blind to genotype [36, 37]. The PSQ contains 48 items and, in addition to a total score, there are six subscale scores: Conduct problem (12 items), Learning problem (4 items), Psychosomatic disorders (5 items), Hyperactivity/Impulsivity (4 items), Anxiety (4 items), and Hyperactivity index (10 items). The parents answered questions concerning their child’s behavior over the past month using a four-point scale, from 0 (not true at all) to 3 (very much true). The three subscales that focus on ADHD symptoms—Hyperactivity/Impulsivity score, Hyperactivity index, and Total score—served as primary outcome measures.
SNP selection
We searched for all the SNPs with minor allele frequencies (MAF) > 0.15 in exon 4 through 19 of the LPHN3 gene in the 1000 Genomes CHB (Han Chinese in Bejing, China) database according to the fine-mapping in another study [5]. Then, MAF > 0.15 and r2 ≥ 0.8 were used as the criteria for tag SNP selection. We placed the selected tag SNPs into an integrated bioinformatics tool “F-SNP” (http://compbio.cs.queensu.ca/F-SNP/) [38] and HaploReg 4.2 (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php) [39] and retrieved a set of functionally predicted SNPs with the possible functions of splicing, transcription, translation, and post-translation processes. Additionally, the variants referred in previous association studies [5,6,7,8,9,10] were also included in our study, and finally 11 SNPs were identified as the candidate SNPs (Fig. 1) and the functional annotation of the 11 SNPs is shown in Table S1 of Online Resource 1.
DNA extraction and genotyping
With the acknowledgement and consent of every subject and their parents, we collected 2 mL of peripheral blood from each participant with vacuum anticoagulant tubes and stored the blood at − 20 °C (immediately). Genomic DNA was extracted from the peripheral blood samples in accordance with the approved guideline of the Relax Gene Blood DNA System DP319-02 (Tiangen, Beijing China) following the manufacturer’s instructions. Genotyping was performed in a 384-well plate format on the Sequenom MassARRAY platform (Sequenom, Inc., San Diego, CA, USA) according to the manufacturer’s iPLEX Application Guide. The primers were designed using the Assay Design 3.0 software of Sequenom.
Bioinformatics analysis
The prediction of the biological functions for significant SNP was achieved through appropriate bioinformatics resources. Specifically, we annotate the functional elements containing significant SNP and its proxies (r2 = 1 in the 1000 Genomes, CHB population) using HaploReg 4.2 (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php) [39], and expression quantitative trait loci (eQTL) analysis was achieved through the BRAINEAC database (http://caprica.genetics.kcl.ac.uk/BRAINEAC/) [40]. Data of BRAINEAC comprise gene expression data from ten brain areas (hippocampus, frontal cortex, temporal cortex, occipital cortex, substantia nigra, frontal white matter, thalamus, putamen, medulla, and cerebellum) from 134 neuropathologically normal donors (16–102 years of age) from the MRC Sudden Death Brain Bank in Edinburgh, UK, and the Sun Health Research Institute.
Statistical analysis
The Hardy–Weinberg equilibrium (HWE) for genotypes was assessed by a goodness-of-fit χ2 test. In the baseline analysis, the distributions of demographic characteristics between the patients and controls were analyzed with Pearson χ2 test, t test, or Mann–Whitney U nonparametric test. Bivariate logistic regression analysis was performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the effects of the SNPs within codominant, dominant, recessive, and additive models, respectively. The associations of PSQ scores with SNPs were explored by ANOVA analysis with post hoc comparisons using the Dunnett t method. All statistical analyses were conducted using IBM SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA).
Two-factor and high-order gene–environment interactions were analyzed in the combined samples (stage one + stage two). The two-factor interactions were measured by logistic regression under multiplicative interaction models [41] in the IBM SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA). To assess the high-order G × E, multifactor dimensionality reduction (MDR) analysis was carried out in the MDR 3.0.2 software (UPenn, Philadelphia, PA, USA) [42]. MDR is a model-free and nonparametric statistical method. At the heart of the MDR approach is a feature or attribute construction algorithm that creates a new variable or attribute by pooling. The best factor models for predicting ADHD risk were found with the maximal balance accuracy cross-validation (CV) testing and CV consistency. The permutation test was carried out to repeat the MDR analysis 1000 times, which was determined using MDR Permutation Testing Software 1.0 beta 2 (UPenn, Philadelphia, PA, USA) and strongly reduced the false positive rate.
Statistical power calculations were carried out in Power version 3.0 [43]. In the Chinese CHB population, the minimal MAF of the ten SNPs is 0.23. Therefore, we found (via calculations) that with our sample size, the power to detect an OR of 1.50 is as follows: stage one power = 0.823; stage two power = 0.630; power of combined stages = 0.957).
Results
Characteristics of the subjects
The baseline characteristics of ADHD patients and controls are shown in Table 1. The cases and controls were matched well on the distributions of age, BMI, IQ score, and gender at two stages (P > 0.05). Significant differences between cases and controls were found in the distribution of maternal stress, and the patients were demonstrated to have higher BLLs compared to the healthy controls at both stages (P < 0.05). For PSQ scores, significant differences have been found at two stages in the Hyperactivity/Impulsivity score and Hyperactivity index (P < 0.05), but the Total score was significant only at stage one (P = 0.041). However, no significant differences between cases and controls were found in the distribution of maternal smoking and drinking.
Association between candidate SNPs and the risk of ADHD
The call rates of the remaining candidate SNPs were all above 95%, except for rs1947274 (call rate < 80%) which was excluded from further analyses. The genotype distribution of the markers did not depart from the HWE (P > 0.05) at both stages, and the estimated genotype frequencies for the ten SNPs investigated herein are shown in Table S2 of Online Resource 1.
Associations of the candidate SNPs with ADHD under different models (codominant, dominant, recessive, and additive models) are partly summarized in Table 2. In the discovery sample, we found that rs1868790, rs4860106, rs2305339, rs6551665, and rs2345039 were involved in ADHD susceptibility. However, after multiple-comparison correction, only rs1868790 and rs4860106 remained statistically significant. Compared with the wild genotype, rs1868790 TA and rs4860106 GA genotypes had increased ADHD risk with OR (95% CI) = 1.612 (1.231–2.110) and 1.584 (1.212–2.070), respectively.
The two SNPs were further genotyped in a validation sample of 284 ADHD children and 390 controls, and we successfully validated the significant association between LPHN3 rs1868790 and ADHD risk [TA/AA: OR (95% CI) = 1.676 (1.194–2.353); dominant model: OR (95% CI) = 1.634 (1.186–2.253); additive model: OR (95% CI) = 1.609 (1.181–2.193), as shown in Table 2]. However, rs4860106 did not pass the multiple-comparison correction. In combined sample (stage one + stage two), the association was further confirmed within the codominant model [TA/AA: OR (95% CI) = 1.636 (1.325–2.021)], dominant model [OR (95% CI) = 1.573 (1.288–1.922)], and additive model [OR (95% CI) = 1.535 (1.266–1.862)].
The association between promising SNPs and ADHD symptom
We then analyzed the relation between LPHN3 rs1868790 and PSQ scores, and found that rs1868790 was associated with ADHD symptoms (Table 3). Compared with the AA group, the rs1868790 TT group showed a higher impulsive–hyperactive score in stage one (P = 0.018), and this trend was verified in stage two and combined stage (P = 0.021 and 0.001, respectively).
Gene–environment interactions
BLLs and maternal stress were found to differ significantly between cases and controls. Therefore, we further analyzed the interactions between rs1868790 and the two environmental factors in the combined sample (stage one + stage two). In two-factor interaction analysis (Table 4), rs1868790 significantly interacted with maternal stress [OR (95% CI) = 1.800 (1.126–2.878), P = 0.014], and the G × E of rs1868790 and BLLs increased OR to 2.012 (95% CI= 1.126–2.878, P = 0.001). In the MDR analysis, the three-factor model including LPHN3 rs1868790, BLLs, and maternal stress was selected as the best predictor for ADHD risk because it had the maximal CVC and balance accuracy of 60.59%, which was significant at the P < 0.001 level after 1000 iterations empirically calculated via permutation testing, with OR= 2.450, 95% CI= 2.002–2.999 (for details, see Table 5).
Functional annotation of LPHN3 rs1868790 and eQTL analysis
To explore the potential function of promising SNPs, we first used the HaploReg v.4.1 to annotate the functional elements containing rs1868790 or its proxies. As shown in Table 6, rs1868790 is located in the region containing the enhancer histone marks of embryonic stem cell (ESC) and induced pluripotent stem cell (IPSC), and possible motifs to alter transcription factor binding (AP-1, Foxj1, Gfi1, Ik-2, Nanog, and STAT), which is still a conserved site identified by SiPhy and the accessible region of DNase in gastrointestinal cell (GI). These findings suggested that rs1868790 may affect transcription regulation via these regulatory elements.
SNPs associated with complex diseases are likely to function as eQTL, and the tissues of unaffected individuals can be used for gene expression association analysis. Subsequently, using the eQTL data from the BRAINEAC database, we have found that rs1868790 affected LPHN3 expression in intralobular white matter (P = 0.0012), with the T allele indicating lower mRNA levels compared to A allele (Fig. 2).
Association of rs1868790 with LPHN3 expression in human brain tissues in the BRAINEAC database CRBL cerebella cortex, FCTX frontal cortex, HIPP hippocampus, MEDU medulla (specifically, inferior olivary nucleus), OCTX occipital cortex (specifically, primary visual cortex), PUTM putamen, SNIG substantia nigra, TCTX temporal cortex, THAL thalamus, WHMT intralobular white matter
Discussion
Our study is the first trial to comprehensively investigate the relation between the LPHN3 gene variants and ADHD susceptibility in the Chinese Han population, and we also identified their possible interactions with BLLs and maternal stress during pregnancy. The main results suggest that variants of rs1868790 are associated with ADHD susceptibility, and G × E analysis consistently revealed the potential interactions of LPHN3 rs1868790 collaborating with BLLs and maternal stress during pregnancy to modify ADHD risk. Furthermore, rs1868790 was found to be related with ADHD symptoms measured by PSQ and still took part in the regulation of LPHN3 gene expression.
The association between LPHN3 and ADHD was first observed from fine-mapping of Paisa population in Colombia [5], and replication studies had been performed in other populations, but not in Chinese [5,6,7,8,9,10,11]. SNPs within the LPHN3 gene interact with SNPs spanning the 11q region that contains DRD2 and NCAM1 not only to double the risk of developing ADHD, but also to increase ADHD severity [15, 16], which in turn may predict long-term ADHD outcome. Moreover, common variants of the LPHN3 gene predict the effectiveness of stimulant medication [5, 9, 13] and affect behavioral and neurophysiological measures of cognitive response control [14].
In our two-stage association study, we found that rs1868790, rs6551665, rs2345039, rs2305339, and rs4860106 were involved in ADHD susceptibility, but after multiple-comparison correction and validation in another independent sample, only rs1868790 was still statistically significant. In a Spanish sample, rs1868790 were nominally associated with combined ADHD under different models and haplotype-based analysis showed over-representation of the T-C-A haplotype (rs1868790/rs6813183/rs12503398) in ADHD and combined subtype [P = 1.9e−04, OR = 1.80 (1.31–2.48); P = 7.5e−05, OR = 2.06 (1.46–2.90), respectively] [8]. The role of rs1868790 in ADHD risk was further validated by Martinez et al. (P = 0.00988) [11], but not Gomez-Sanchez or Sánchez-Mora et al. [7, 44]. As for the treatment, rs1868790 did not have a significant effect in discriminating good responders from non-responders [13]. Although the remaining four SNPs (rs6551665, rs2345039, rs2305339, and rs4860106) were associated with ADHD risk in previous studies, we did not replicate the association. The contradiction between the previous studies and our research may result from ethnic differences and limited sample size in our study.
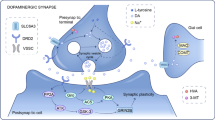
LPHN3 is a brain-specific receptor, and is located in the cerebral cortex, cerebellum, caudate nucleus, and amygdala, which are the areas of important lesions in ADHD (see Fig S1 of Online Resource 1) [5, 45]. The endogenous function of LPHN3 is linked to cell adhesion and synapse formation or maintenance. Animal experiments validated the function of the LPHN3 gene in the brain and linked LPHN3 and dopamine (DA) system together. Loss of lphn3.1 (ortholog of LPHN3) function caused a reduction and misplacement of DA-positive neurons in the ventral diencephalon and a hyperactive/impulsive motor phenotype, and the behavioral phenotype could be rescued by the ADHD drugs methylphenidate and atomoxetine [46]. The hyperactivity of lphn3.1 morphants was recently confirmed in an independent study [47]. Pharmacological analysis suggests that saturated dopaminergic signaling could underlie the ADHD-like locomotor hyperactivity in zebrafish lphn3.1 morphant larvae, and compared with the controls, lphn3.1 morphants have an overall hyposensitivity to dopamine agonists and antagonists [48]. Moreover, Lphn3 null mice display increased reward motivation and activity levels [49, 50], and gene expression changes in those mice, including DA and serotonin receptors and transporters, and neurotransmitter metabolism genes, as well as neural developmental genes [50]. Seeing that dopaminergic neurotransmission system is one of the most important components in the etiology of ADHD [51, 52] and studies on physiological function of LPHN3 are very limited, further research is still needed to further explore the physiological function of LPHN3 and the relation between the LPHN3 gene and dopaminergic system in the etiology of ADHD.
Considering the importance of G × E in the pathogenesis of ADHD, we investigated the roles of the potential environmental risk factors in ADHD, including maternal stress, maternal smoking, maternal drinking, and BLLs. However, only maternal stress and BLLs were found to differ significantly between cases and controls. Therefore, only the two environmental factors were included in the later G × E analysis, and significant interactions were found to modify the ADHD risk in two- and three-factor models (P < 0.05). In another study, highly significant interaction between four LPHN3 tag SNPs (rs6551665, rs1947274, rs6858066, and rs2345039) and maternal stress during pregnancy was noted [12]. It has been proposed that in the G × E, the genotype of the individual modulates the sensitivity or response to the environmental risk factor [53].
Maternal cortisol is the most widely proposed mechanism by which maternal stress during pregnancy is associated with negative outcomes in the offspring. Elevated maternal cortisol in response to stress can exceed the placental capacity to degrade it, cross the placental barrier, and influence the developing brain and/or ‘program’ the fetal hypothalamic–pituitary–adrenal axis [54]. Though researches involving LPHN3 function were very limited, current evidence suggested that LPHN3 belonged to the G protein-coupled receptors (GPCRs) family. Dependent on G protein, GPCRs regulate multiple intracellular signal transduction, for example, activation of adenylate cyclase, phospholipase, and Ca2+ channel activity [55]. Also, G(α)q protein mediated PLC-β activation, regulated IP3 and DAG, and subsequent intracellular Ca2+ release. Intracellular calcium was implicated in an array of physiological processes, including the formation and maintenance of neuronal connections, neurotransmitter release, and hormone secretion [56]. Therefore, we can hypothesize that decreased LPHN3 expression led to elevated intracellular calcium level and subsequent cortisol secretion.
Another explanation for these findings is that mood problems (such as anxiety and stress) and ADHD may share common genetic factors, which are passed from mothers to their children. Besides, epigenetic modifications induced by stress in the uterus may lead to perinatal reprogramming, resulting in ADHD in the offspring [57]. These may partly explain how LPHN3 work with maternal stress to modify ADHD risk.
Lead is known to play an important role in the etiology of ADHD [58, 59] and has been proved to be associated with ADHD symptoms (inattention, hyperactivity, and impulsivity) [60, 61]. Even low-level lead exposure (at concentrations much lower than the action limit of 100 μg/L) has been associated with a clinical diagnosis of ADHD in several recent studies [59, 62]. The presence of lead affects mostly the prefrontal cortex, hippocampus, basal ganglia, and cerebellum [63] and disrupts the dopaminergic, cholinergic, and glutamatergic neurotransmission circuitry [64]. The mechanism of the G × E may be that lead displaces multivalent cations, such as calcium and zinc [65], and the olfactomedin-like domain of LPHN3 is a five-bladed β propeller with a Ca2+ ion bound in the central pore. These changes will directly negatively affect the normal physiological function of LPHN3. Nevertheless, LPHN3 is an adhesion class G protein-coupled receptor and ligand binding will activate the cAMP signal pathway; lead accumulation in the brain causes the dysfunction of intracellular cAMP [66]. Moreover, Luo et al. discovered the epigenetic mechanism bridging lead and ADHD at the histone modification level [67], which might partly explain the G × E we had identified in our study.
The results of the eQTL analysis indicated that intronic variant rs1868790 was related with LPHN3 mRNA expression with the T allele indicating lower mRNA levels compared to A allele, which could partly explain how rs1868790 played a role in the etiology of ADHD. The promising SNP identified in other studies were largely located in the noncoding region, and researchers found extensive functionally relevant noncoding variants through the bioinformatics approach [51]. Variations in the noncoding regions participate in a disease through a range of regulatory mechanisms. Martinez et al. were the first to explore the functional mechanism of LPHN3 intron sequences in ADHD, and they found that an ultraconserved element, formed by rs17226398, rs56038622, and rs2271338, functions as a transcriptional enhancer, and the risk haplotype reduced enhancer activity by 40% (P < 0.0001) [11]. The rs2271338 risk allele disrupts binding of YY1 transcription factor, and eQTL analysis revealed an association between rs2271338 and reduced LPHN3 expression in the thalamus [11].
To our knowledge, this is the first two-stage case–control study to comprehensively explore the role of the LPHN3 gene in ADHD and its interactions with environmental risk factors in the Chinese Han population. Our results provide clues to LPHN3’s involvement in ADHD. Nonetheless, our study has several limitations. First, the association we found in our study still needs to be verified in a larger sample. Second, subsequent functional research should be conducted on positive SNP to determine the potential mechanisms of how LPHN3 plays roles in ADHD. Third, the biological mechanism of interactions between the LPHN3 gene and risk environmental factors (BLLs and maternal stress) was ambiguous and required further investigation. Besides, future research is still needed to explore the role of other environmental factors (for example, socioeconomic status, preterm birth, and low birth weight), and the mechanism of G × E model in the pathogenesis of ADHD.
References
Faraone SV, Biederman J, Mennin D, Gershon J, Tsuang MT (1996) A prospective four-year follow-up study of children at risk for ADHD: psychiatric, neuropsychological, and psychosocial outcome. J Am Acad Child Adolesc Psychiatry 35(11):1449–1459. https://doi.org/10.1097/00004583-199611000-00013
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005) Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57(11):1313–1323. https://doi.org/10.1016/j.biopsych.2004.11.024
Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch K-P, Faraone SV, Nguyen TT, Schäfer H, Holmans P, Daly M, Steinhausen H-C, Freitag C, Reif A, Renner TJ, Romanos M, Romanos J, Walitza S, Warnke A, Meyer J, Palmason H, Buitelaar J, Vasquez AA, Lambregts-Rommelse N, Gill M, Anney RJL, Langely K, O’Donovan M, Williams N, Owen M, Thapar A, Kent L, Sergeant J, Roeyers H, Mick E, Biederman J, Doyle A, Smalley S, Loo S, Hakonarson H, Elia J, Todorov A, Miranda A, Mulas F, Ebstein RP, Rothenberger A, Banaschewski T, Oades RD, Sonuga-Barke E, McGough J, Nisenbaum L, Middleton F, Hu X, Nelson S (2010) Meta-analysis of genome-wide association studies of attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 49(9):884–897. https://doi.org/10.1016/j.jaac.2010.06.008
Li Z, S-h Chang, L-y Zhang, Gao L, Wang J (2014) Molecular genetic studies of ADHD and its candidate genes: a review. Psychiatry Res 219(1):10–24. https://doi.org/10.1016/j.psychres.2014.05.005
Arcos-Burgos M, Jain M, Acosta M, Shively S, Stanescu H, Wallis D, Domene S, Karkera J (2010) A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry 15(11):1053–1066. https://doi.org/10.1038/mp.2010.6
Hwang IW, Lim MH, Kwon HJ, Jin HJ (2015) Association of LPHN3 rs6551665 A/G polymorphism with attention deficit and hyperactivity disorder in Korean children. Gene 566(1):68–73. https://doi.org/10.1016/j.gene.2015.04.033
Gomez-Sanchez CI, Riveiro-Alvarez R, Soto-Insuga V, Rodrigo M, Tirado-Requero P, Mahillo-Fernandez I, Abad-Santos F, Carballo JJ, Dal-Ré R, Ayuso C (2015) Attention deficit hyperactivity disorder: genetic association study in a cohort of Spanish children. Behav Brain Funct BBF 12(1):2. https://doi.org/10.1186/s12993-015-0084-6
Ribases M, Ramos-Quiroga JA, Sanchez-Mora C, Bosch R, Richarte V, Palomar G, Gastaminza X, Bielsa A, Arcos-Burgos M, Muenke M, Castellanos FX, Cormand B, Bayes M, Casas M (2011) Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: a replication study. Genes Brain Behav 10(2):149–157. https://doi.org/10.1111/j.1601-183X.2010.00649.x
Bruxel EM, Salatino-Oliveira A, Akutagava-Martins GC, Tovo-Rodrigues L, Genro JP, Zeni CP, Polanczyk GV, Chazan R, Schmitz M, Arcos-Burgos M, Rohde LA, Hutz MH (2015) LPHN3 and attention-deficit/hyperactivity disorder: a susceptibility and pharmacogenetic study. Genes Brain Behav 14(5):419–427. https://doi.org/10.1111/gbb.12224
Acosta MT, Swanson J, Stehli A, Molina BSG, The MTAT, Martinez AF, Arcos-Burgos M, Muenke M (2016) ADGRL3 (LPHN3) variants are associated with a refined phenotype of ADHD in the MTA study. Mol Genet Genom Med 4(5):540–547. https://doi.org/10.1002/mgg3.230
Martinez AF, Abe Y, Hong S, Molyneux K, Yarnell D, Löhr H, Driever W, Acosta MT, Arcos-Burgos M, Muenke M (2016) An ultraconserved brain-specific enhancer within ADGRL3 (LPHN3) underpins attention-deficit/hyperactivity disorder susceptibility. Biol Psychiatry 80(12):943–954. https://doi.org/10.1016/j.biopsych.2016.06.026
Choudhry Z, Sengupta SM, Grizenko N, Fortier ME, Thakur GA, Bellingham J, Joober R (2012) LPHN3 and attention-deficit/hyperactivity disorder: interaction with maternal stress during pregnancy. J Child Psychol Psychiatry 53(8):892–902. https://doi.org/10.1111/j.1469-7610.2012.02551.x
Labbe A, Liu A, Atherton J, Gizenko N, Fortier ME, Sengupta SM, Ridha J (2012) Refining psychiatric phenotypes for response to treatment: contribution of LPHN3 in ADHD. Am J Med Genet Part B Neuropsychiatr Genet 159B(7):776–785. https://doi.org/10.1002/ajmg.b.32083
Fallgatter AJ, Ehlis A-C, Dresler T, Reif A, Jacob CP, Arcos-Burgos M, Muenke M, Lesch K-P (2013) Influence of a Latrophilin 3 (LPHN3) risk haplotype on event-related potential measures of cognitive response control in attention-deficit hyperactivity disorder (ADHD). Eur Neuropsychopharmacol 23(6):458–468. https://doi.org/10.1016/j.euroneuro.2012.11.001
Jain M, Velez J, Acosta M, Palacio L, Balog J, Roessler E, Pineda D, Londono A, Palacio J, Arbelaez A (2012) A cooperative interaction between LPHN3 and 11q doubles the risk for ADHD. Mol Psychiatry 17(7):741–747. https://doi.org/10.1038/mp.2011.59
Acosta M, Velez JI, Bustamante M, Balog J, Arcos-Burgos M, Muenke M (2011) A two-locus genetic interaction between LPHN3 and 11q predicts ADHD severity and long-term outcome. Transl Psychiatry. https://doi.org/10.1038/tp.2011.14
Riva V, Marino C, Giorda R, Molteni M, Nobile M (2015) The role of DCDC2 genetic variants and low socioeconomic status in vulnerability to attention problems. Eur Child Adolesc Psychiatry 24(3):309–318. https://doi.org/10.1007/s00787-014-0580-5
Riva V, Battaglia M, Nobile M, Cattaneo F, Lazazzera C, Mascheretti S, Giorda R, Mérette C, Émond C, Maziade M, Marino C (2015) GRIN2B predicts attention problems among disadvantaged children. Eur Child Adolesc Psychiatry 24(7):827–836. https://doi.org/10.1007/s00787-014-0627-7
van Baar AL, Vermaas J, Knots E, de Kleine MJK, Soons P (2009) Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics 124(1):251–257. https://doi.org/10.1542/peds.2008-2315
Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC (2011) Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 13(5):333. https://doi.org/10.1007/s11920-011-0221-3
Silva D, Colvin L, Hagemann E, Bower C (2014) Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 133(1):e14–e22. https://doi.org/10.1542/peds.2013-1434
Eichler A, Hudler L, Grunitz J, Grimm J, Raabe E, Goecke TW, Fasching PA, Beckmann MW, Kratz O, Moll GH, Kornhuber J, Heinrich H (2018) Effects of prenatal alcohol consumption on cognitive development and ADHD-related behaviour in primary-school age: a multilevel study based on meconium ethyl glucuronide. J Child Psychol Psychiatry 59(2):110–118. https://doi.org/10.1111/jcpp.12794
Huang L, Wang Y, Zhang L, Zheng Z, Zhu T, Qu Y, Mu D (2018) Maternal smoking and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Pediatrics. https://doi.org/10.1542/peds.2017-2465
Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, Schwab M (2017) Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2017.07.003
Byun Y-H, Ha M, Kwon H-J, Hong Y-C, Leem J-H, Sakong J, Kim SY, Lee CG, Kang D, Choi H-D, Kim N (2013) Mobile phone use, blood lead levels, and attention deficit hyperactivity symptoms in children: a longitudinal study. PLoS One 8(3):e59742. https://doi.org/10.1371/journal.pone.0059742
Huang S, Hu H, Sánchez BN, Peterson KE, Ettinger AS, Lamadrid-Figueroa H, Schnaas L, Mercado-García A, Wright RO, Basu N, Cantonwine DE, Hernández-Avila M, Téllez-Rojo MM (2016) Childhood blood lead levels and symptoms of attention deficit hyperactivity disorder (ADHD): a cross-sectional study of mexican children. Environ Health Perspect 124(6):868–874. https://doi.org/10.1289/ehp.1510067
Gu X, Yuan F-F, Huang X, Hou Y, Wang M, Lin J, Wu J (2018) Association of PIK3CG gene polymorphisms with attention-deficit/hyperactivity disorder: a case–control study. Prog Neuro Psychopharmacol Biol Psychiatry 81(Supplement C):169–177. https://doi.org/10.1016/j.pnpbp.2017.10.020
Dadds MR, Schollar-Root O, Lenroot R, Moul C, Hawes DJ (2016) Epigenetic regulation of the DRD4 gene and dimensions of attention-deficit/hyperactivity disorder in children. Eur Child Adolesc Psychiatry 25(10):1081–1089. https://doi.org/10.1007/s00787-016-0828-3
Gong Y, Cai T (1993) Chinese-Wechsler intelligence scale for children Hunan. Map Press, Beijing
Furtado EF, Roriz STdS (2016) Inattention and impulsivity associated with prenatal alcohol exposure in a prospective cohort study with 11-years-old Brazilian children. Eur Child Adolesc Psychiatry 25(12):1327–1335. https://doi.org/10.1007/s00787-016-0857-y
Yuan F-F, Gu X, Huang X, Hou Y-W, Zhong Y, Lin J, Wu J (2017) Attention-deficit/hyperactivity disorder associated with KChIP1 rs1541665 in Kv channels accessory proteins. PLoS One 12(11):e0188678. https://doi.org/10.1371/journal.pone.0188678
Skoglund C, Chen Q, D´Onofrio BM, Lichtenstein P, Larsson H (2014) Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry 55(1):61–68. https://doi.org/10.1111/jcpp.12124
Browne HA, Modabbernia A, Buxbaum JD, Hansen SN, Schendel DE, Parner ET, Reichenberg A, Grice DE (2016) Prenatal maternal smoking and increased risk for Tourette syndrome and chronic tic disorders. J Am Acad Child Adolesc Psychiatry 55(9):784–791. https://doi.org/10.1016/j.jaac.2016.06.010
Langley K, Heron J, Smith GD, Thapar A (2012) Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol 176(3):261–268. https://doi.org/10.1093/aje/kwr510
Li Y, Zeng Y, Zhu W, Cui Y, Li J (2016) Path model of antenatal stress and depressive symptoms among Chinese primipara in late pregnancy. BMC Pregnancy Childbirth 16(1):180–186. https://doi.org/10.1186/s12884-016-0972-2
Fan J, Du YS, Wang LW (2005) The norm and reliability of the Conners Parent Symptom Questionnaire in Chinese urban children. Shanghai Arch Psychiatry 17(6):321–323
Young J, Rugino T, Dammerman R, Lyne A, Newcorn JH (2014) Efficacy of guanfacine extended release assessed during the morning, afternoon, and evening using a modified Conners’ Parent Rating Scale–revised: short form. J Child Adolesc Psychopharmacol 24(8):435–441. https://doi.org/10.1089/cap.2013.0134
Lee PH, Shatkay H (2008) F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res 36(Database issue):D820–D824. https://doi.org/10.1093/nar/gkm904
Ward LD, Kellis M (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 40(D1):D930–D934. https://doi.org/10.1093/nar/gkr917
Ramasamy A, Trabzuni D, Guelfi S, Varghese V, Smith C, Walker R, De T, Consortium UKBE, North American Brain Expression C, Coin L, de Silva R, Cookson MR, Singleton AB, Hardy J, Ryten M, Weale ME (2014) Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci 17(10):1418–1428. https://doi.org/10.1038/nn.3801
Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A (2005) Calculating measures of biological interaction. Eur J Epidemiol 20(7):575–579. https://doi.org/10.1007/s10654-005-7835-x
Hahn LW, Ritchie MD, Moore JH (2003) Multifactor dimensionality reduction software for detecting gene–gene and gene–environment interactions. Bioinformatics 19(3):376–382. https://doi.org/10.1093/bioinformatics/btf869
Lubin JH, Gail MH (1990) On power and sample size for studying features of the relative odds of disease. Am J Epidemiol 131(3):552–566. https://doi.org/10.1093/oxfordjournals.aje.a115530
Sánchez-Mora C, Richarte V, Garcia-Martínez I, Pagerols M, Corrales M, Bosch R, Vidal R, Viladevall L, Casas M, Cormand B, Ramos-Quiroga JA, Ribasés M (2015) Dopamine receptor DRD4 gene and stressful life events in persistent attention deficit hyperactivity disorder. Am J Med Genet Part B Neuropsychiatr Genet 168(6):480–491. https://doi.org/10.1002/ajmg.b.32340
Konrad K, Eickhoff SB (2010) Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 31(6):904–916. https://doi.org/10.1002/hbm.21058
Lange M, Norton W, Coolen M, Chaminade M, Merker S, Proft F, Schmitt A, Vernier P, Lesch KP, Bally-cuif L (2012) The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol Psychiatry 17(9):946–954. https://doi.org/10.1038/mp.2012.29
Reuter I, Knaup S, Romanos M, Lesch K-P, Drepper C, Lillesaar C (2016) Developmental exposure to acetaminophen does not induce hyperactivity in zebrafish larvae. J Neural Transm 123(8):841–848. https://doi.org/10.1007/s00702-016-1556-z
Lange M, Froc C, Grunwald H, Norton WHJ, Bally-Cuif L (2018) Pharmacological analysis of zebrafish lphn3.1 morphant larvae suggests that saturated dopaminergic signaling could underlie the ADHD-like locomotor hyperactivity. Prog Neuro Psychopharmacol Biol Psychiatry 84(Pt A):181–189. https://doi.org/10.1016/j.pnpbp.2018.02.010
Orsini CA, Setlow B, DeJesus M, Galaviz S, Loesch K, Ioerger T, Wallis D (2016) Behavioral and transcriptomic profiling of mice null for Lphn3, a gene implicated in ADHD and addiction. Mol Genet Genom Med 4(3):322–343. https://doi.org/10.1002/mgg3.207
Wallis D, Hill DS, Mendez IA, Abbott LC, Finnell RH, Wellman PJ, Setlow B (2012) Initial characterization of mice null for Lphn3, a gene implicated in ADHD and addiction. Brain Res 1463:85–92. https://doi.org/10.1016/j.brainres.2012.04.053
Hawi Z, Cummins TDR, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA (2015) The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry 20(3):289–297. https://doi.org/10.1038/mp.2014.183
Wu J, Xiao H, Sun H, Zou L, Zhu L-Q (2012) Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol 45(3):605–620. https://doi.org/10.1007/s12035-012-8278-5
Wermter A-K, Laucht M, Schimmelmann BG, Banaschweski T, Sonuga-Barke EJS, Rietschel M, Becker K (2010) From nature versus nurture, via nature and nurture, to gene × environment interaction in mental disorders. Eur Child Adolesc Psychiatry 19(3):199–210. https://doi.org/10.1007/s00787-009-0082-z
Seckl JR, Holmes MC (2007) Mechanisms of disease: glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat Clin Pract Endocrinol Metab 3(6):479–488. https://doi.org/10.1038/ncpendmet0515
Eichel K, von Zastrow M (2018) Subcellular organization of GPCR signaling. Trends Pharmacol Sci 39(2):200–208. https://doi.org/10.1016/j.tips.2017.11.009
Gustavsson N, Wu B, Han W (2012) Calcium sensing in exocytosis. In: Islam MS (ed) Calcium signaling. Springer, Dordrecht, pp 731-757. https://doi.org/10.1007/978-94-007-2888-2_32
Van Den Bergh BRH (2011) Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Dev Med Child Neurol 53:19–23. https://doi.org/10.1111/j.1469-8749.2011.04057.x
Joo H, Lim M-H, Ha M, Kwon H-J, Yoo SJ, Choi K-H, Paik K-C (2017) Secondhand smoke exposure and low blood lead levels in association with attention-deficit hyperactivity disorder and its symptom domain in children: a community-based case–control study. Nicotine Tob Res 19(1):94–101. https://doi.org/10.1093/ntr/ntw152
Eubig PA, Aguiar A, Schantz SL (2010) Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect 118(12):1654–1667. https://doi.org/10.1289/ehp.0901852
Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J (2016) Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6–11 years of age: CHMS 2007–2009. NeuroToxicology 54(Supplement C):89–98. https://doi.org/10.1016/j.neuro.2016.03.014
Goodlad JK, Marcus DK, Fulton JJ (2013) Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: a meta-analysis. Clin Psychol Rev 33(3):417–425. https://doi.org/10.1016/j.cpr.2013.01.009
Aguiar A, Eubig PA, Schantz SL (2010) Attention deficit/hyperactivity disorder: a focused overview for children’s environmental health researchers. Environ Health Perspect 118(12):1646–1653. https://doi.org/10.1289/ehp.1002326
Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP (2004) Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol 44:87–110. https://doi.org/10.1146/annurev.pharmtox.44.101802.121424
Cory-Slechta DA (1995) Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol 35(1):391–415. https://doi.org/10.1146/annurev.pa.35.040195.002135
Godwin HA (2001) The biological chemistry of lead. Curr Opin Chem Biol 5(2):223–227. https://doi.org/10.1016/S1367-5931(00)00194-0
Flora GJ, Seth PK (2000) Alterations in some membrane properties in rat brain following exposure to lead. Cytobios 103(403):103–109
Luo M, Xu Y, Cai R, Tang Y, Ge M-M, Liu Z-H, Xu L, Hu F, Ruan D-Y, Wang H-L (2014) Epigenetic histone modification regulates developmental lead exposure induced hyperactivity in rats. Toxicol Lett 225(1):78–85. https://doi.org/10.1016/j.toxlet.2013.11.025
Acknowledgements
We are sincerely grateful to the participating families for their cooperation. We thank Dr. Jun Lin from Wuhan Medical and Health Center for Women and Children, and Dr. Yan Zhong from Children’s Hospital of Hunan province for their widespread support for our study and sharing their knowledge. This work was supported partially by the National Natural Science Foundation of China (81773456), the Fundamental Research Funds for the Central Universities, HUST (2016 YXMS218) supported Dr. Jing Wu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, X., Zhang, Q., Gu, X. et al. LPHN3 gene variations and susceptibility to ADHD in Chinese Han population: a two-stage case–control association study and gene–environment interactions. Eur Child Adolesc Psychiatry 28, 861–873 (2019). https://doi.org/10.1007/s00787-018-1251-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-018-1251-8