Abstract
Numerous studies have shown that Major Depressive Disorder (MDD) in adults is associated with deficits in cognitive control. Particularly, impairment on executive function (EF) tasks has been observed. Research into EF deficits in children and adolescents with MDD has reported mixed results and it is currently unclear whether paediatric MDD is characterised by impairments in EF and attention. PsycInfo, Scopus and Medline were systematically searched to identify all studies that have investigated EF and attention in paediatric depressive disorders between 1994 and 2014. 33 studies meeting inclusion/exclusion criteria were identified. While across different domains of EF some studies identified a deficit in the clinical group, the majority of studies failed to find deficits in response inhibition, attentional set shifting, selective attention, verbal working memory, and verbal fluency. More research is needed to clarify the relationship between depressive disorders in children and adolescents and spatial working memory processing, sustaining attention, planning, negative attentional bias and measures of ‘hot’ EF. There is little support for EF deficits in paediatric depression. However, there are numerous methodological problems that may account for null findings. Alternatively, chronicity and/or severity of symptoms may explain discrepancies between cognitive deficits in adult and paediatric MDD. Recommendations for future studies are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An emerging body of research has shown that executive function (EF) impairments are common in adult patients with depressive disorders [33, 77]. EF relies on prefrontal lobe function, which has been found to differ between patients with major depressive disorder (MDD) and healthy control participants [21, 62]. While much research has been conducted within adult populations, relatively few studies have investigated EF in children and adolescents with depressive disorders. Impairments in cognitive function in the form of trouble making decisions or concentrating are recognised as symptoms of unipolar depression given the diagnostic criteria [2]. Deficits in attention, memory and problem solving may have profound impact on daily activities, particularly in children and adolescents, whose academic achievement may dependent on these skills [4, 9]. Furthermore, disturbances in attention, memory and executive function may limit coping skills, increase the risk of relapse and/or affect treatment compliance [77].
It is currently unclear whether depression in childhood and adolescence is mostly similar or different to adult depression. There may be important differences between early-onset and late-onset depression with early-onset depression being more severe and with higher levels of recurrence [14, 26]. Uncertainties also remain with regard to whether cognitive impairments should be considered vulnerability markers of depression, thus potentially preceding the development of depressive symptoms or, whether cognitive symptoms develop only after the onset of a major depressive episode [14].
Early research has suggested that depression is characterised by general cognitive depletion [56] but others have subsequently proposed that cognitive deficits should be considered as a difficulty in initiating efficient strategies that can be remediated by appropriate cuing or priming [38, 51]. Another potential confounding factor in understanding cognitive impairments in depressive disorders is motivation. It is possible that cognitive deficits are secondary and arise as a result of decreased motivation [51]. A better understanding of the association between cognitive deficits and paediatric depression will aid in the clarification of some of these uncertainties.
EF refers to higher order neurocognitive processes involved in goal-directed behaviour such as, for example, working memory, attentional flexibility, and inhibitory control [59]. While basic EF develops during preschool years, improvements in EF continue throughout childhood and adolescence [8]. This development parallels the prolonged maturation of the prefrontal cortex that continues into early adulthood [28, 53] and is thought to support crucial EF processes. In fact, recent advances in neuroimaging have triggered the distinction between “hot” and “cold” EF [40]. This heuristic framework was developed to reflect a separation of affective processes, for example reward processing, and cognitive processes such as inhibitory control in the orbital/medial and dorsolateral prefrontal regions, respectively. In the context of paediatric depression neuroimaging evidence is slowly accumulating that shows atypical structural and functional changes in prefrontal, limbic and striatal brain regions [42]. While the distinction between affective and cognitive processing regions in the brain is no longer considered helpful as most regions are involved in the processing of both, the separation of hot and cold EF extends the previously sole focus on abstract, decontextualised problem solving to include problems of reward processing and decision-making. Given that neuroimaging evidence suggests abnormalities in regions subserving both hot and cold EF in paediatric depression [42] it is of interest to find out whether behavioural studies also support a deficit across both domains.
The purpose of this review was to systematically identify all studies that have investigated EF and attention deficits in children and adolescents with depressive disorders, to determine whether EF and attention difficulties are common in this clinical group and whether there are specific subdomains of EF and attention that are particularly impaired in paediatric depressive disorders. We did not perform a meta-analysis on the studies, as the number of studies available with comparable data is too limited. The domains of attention, response inhibition, set shifting, working memory, planning and verbal fluency were examined because these have previously been indicated to differ between adult patients with depression and healthy controls. In addition, studies that examined hot EF of reward processing and decision-making were also included.
Method
Search strategy
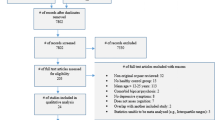
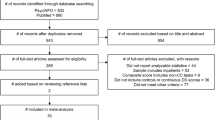
PsycInfo, Scopus and Medline were systematically searched using the following keywords: (child* or kid* or youth or “young people” or boys or girls or adolesc* or teenage*) AND (“unipolar depression” or “major depressive disorder” or “dysthymic disorder” or dysthymia or depression or “depressive episode” or depressive) and (“executive function” or “working memory” or planning or “verbal fluency” or “response inhibition” or “inhibitory control” or shifting or switching or neuropsychologic* or attention or “cognitive control” or “executive dysfunction” or” attention bias” or “decision making” or “selective attention” or reward). The asterisk shortens the word to identify different endings and parentheses are used to group search terms together. We employed a topic search in Medline, an abstract, title and keywords search in Scopus and an abstract search in PsycInfo. In all three databases the search was limited to peer-reviewed English journal articles that were published after 1994 (DSM-IV release) and up until October 2014. To make sure no relevant study was overlooked reference lists of the selected articles were also checked. Figure 1 displays a flow chart of the search and results.
Inclusion/exclusion criteria
The following inclusion criteria were applied:
-
The presence of a nonclinical control group
-
Mean age <19 years
-
Sample size ≥10 of the clinical group
-
DSM-IV or subsequent DSM diagnosis of MDD or dysthymic disorder (DD)
Exclusion criteria:
-
Neuroimaging studies (for a review of neuroimaging studies in paediatric depression see [42, 45]).
-
Studies investigating effects of medication
-
Studies that looked at offspring of parents with a history of affective disorder (note the exception here is [69] as they assessed and compared offspring with a current diagnosis).
Results
33 studies met inclusion criteria for this review and are listed in Table 1. Of those studies the clinical sample size ranged from 11 [18, 36, 75] to 61 [32] with a mean sample size of 22.2 (±9.0) and a median sample size of 20.0 of the clinical group. The included studies covered a total age range from 6 to 19 years of age of participants. In the following we will examine attention and various subdomains of EF: response inhibition, set shifting, working memory, planning, verbal fluency and reward processing in turn and look at evidence for a deficit in children and adolescents with depressive disorders. Table 2 lists and describes common laboratory tasks that were used in the included studies.
Attention
Inattention forms part of the diagnostic criteria of MDD and dysthymic disorder regarding poor concentration or difficulty making decisions [2]. Although not a separate category of EF, attention is a basic building block for EF and we will briefly summarise findings on measures of attention in paediatric MDD. A distinction between measures of sustained attention and selective attention is made.
Of nine studies that have assessed the ability to sustain attention in children and adolescents with depressive disorders five reported difficulties in patients. Six studies included in the review used different versions of the continuous performance test (CPT) to assess the ability to sustain attention in paediatric depression [11, 13, 15, 34, 54, 58]. Two reported medium effect sizes for CPT omissions, commissions and greater inconsistencies in reaction times for the clinical group [13, 15]. Bloch et al. [11] found significant group differences in accuracy and mean response latencies; adolescents with MDD made more errors and responded more slowly than the healthy control participants. On the same task Maalouf et al. [54] noted that the acutely depressed group had more false alarms and responded more impulsively than a remitted and healthy control group. On the other hand, Mayes et al. [58] found no difference on the Gordon Diagnostic System [30] version of the CPT between healthy control participants and anxious/depressed children. Han and colleagues [34] employed a version that manipulated load thus adding a working memory component and found a difference but this disappeared when adjusting for IQ differences between the groups. Günther and colleagues found no evidence for a deficit in sustaining attention in children with depressive disorders in either their 2004 or in their 2011 study. Wilkinson and Goodyer [79] reported a significant group effect showing that the depressed group that was on antidepressant medication was significantly less accurate than the healthy control group on sustained attention. These results remain difficult to interpret, as one would expect antidepressant medication to improve attention.
Five studies have included some measure of selective attention in their neuropsychological assessment of patients and healthy control participants [11, 15, 31, 32, 79]. Most tasks involved a form of target detection in a visual array of similar looking items. No difficulties in selective attention as measured on these tasks were found by any of the studies.
Cold EF
Response inhibition
Sixteen investigations have examined response inhibition in paediatric depression. Out of these only three reported significant group differences [15, 32, 36]. Response inhibition refers to the ability to hold back a prepotent response. Many of the tasks used to measure response inhibition require other cognitive resources such as working memory (keeping rules in mind) and activating an alternative response. Five studies were identified that have used the Stroop to assess inhibitory control in paediatric depressive disorders [13, 15, 19, 23, 64]. While three of these five studies used the standard colour-word Stroop, Neshat-Doost et al. [19] and Dalgleish et al. [64] employed a modified version with positive, neutral, depression-related, threat-related, and trauma-related words. Only one study found that the clinical group, which consisted of 19 children and adolescents with either MDD or DD, performed significantly worse than the healthy control group using the difference in response time between the mismatching word-and-colour condition and the colour-naming condition [15]. Although Brooks et al.[13] did not find a significant group difference they noted a small-to-medium effect size for errors on the Stroop task. On the modified Stroop test, Neshat-Doost et al. [64] noted greater response latencies and more colour-naming errors in the depressed group independent of valence category. In the later study, no group differences were found [19].
Using a go/no-go task with letter stimuli Günther and colleagues [31] found that a group of 31 children with depressive disorders were no different from a group of 33 healthy control children in their task performance. However, the same group found that children with DD or MDD had more false alarms than the healthy control group and were no different to the performance of children with attention-deficit/hyperactivity disorder (ADHD) and those with the comorbid condition in a later study with a larger sample and a narrower age range (10–15 years compared to 6 or 8–17 years in the earlier study) [32].
Cataldo et al. [15] used the walk/don’t walk task, a child friendly version of the go/no-go task to measure response inhibition but found no difference between the depressed and the healthy control group. Because depression has been associated with an attention bias towards negative stimuli, four studies used an affective version of the go/no-go task in which positively and negatively valenced words [34, 47, 55] and faces [34, 48] were presented. Kyte et al. [47] found that adolescents with a recent first depressive episode made more errors when the target stimuli were happy but also found that the clinical participants made fewer errors than the healthy control participants on sad faces. Ladouceur et al. [48] noted faster reaction times to sad faces in children with MDD but no difference in the percentage of correct responses or false alarms compared to typically developing children. No other group differences emerged in any of the other studies.
On an Eriksen Flanker task medication-naïve young people with a first episode of depression and without comorbid anxiety were as accurate as well-matched control children in responding [75]. Although the sample here was very well defined, unfortunately it was very small (n = 11) and covered a wide age range from 7 to 17 years. Han et al. [34] used a similar task but neither found a significant group difference in an older, larger sample of adolescents with MDD.
A few studies have employed other, less commonly used tests to measure response inhibition in paediatric depressive disorders. Klimkeit et al. [46] used a local–global and serial choice reaction time task that both had an inhibition and set-shifting component. No significant group difference emerged for errors made in the serial choice reaction time task while in the local–global task the minor depressed group made significantly less errors than both the major depressed and healthy control group. One group reported no difference in task performance between adolescents with MDD and typically developing children in the neutral condition of a rewarded antisaccade task [44] while a later study using a modified version of the same task found more direction errors in patients than the healthy control group [36].
Set shifting
Three of ten studies provided evidence for a set-shifting deficit in paediatric depression. Set shifting refers to the ability to adjust responses according to changing rules. It is a demanding process that requires both, inhibition and working memory. Two studies used the trail making test (TMT) to assess EF function in children with depression. One reported more perseverative errors in a group of 9- to 11-year-old boys with high self-reported symptoms of depression [22]. This study had a high rate of comorbidity with anxiety, which means that impaired performance on the TMT B may have been due to the presence of anxiety rather than depression. Favre et al. [23] found no significant group differences but noted greater variance in the performance of a larger group of children and adolescents with MDD. They also found no group difference on the Wisconsin Card Sorting Test (WCST). Using a different approach Holler et al. [39] combined WCST perseverative errors and TMT B performance to yield a cognitive flexibility/set shifting subdomain. They reported that the MDD group scored significantly lower than the outpatient control group on this subdomain. This difference was only found for the MDD but not the minor depressed group.
Three studies used the intra-dimensional and extra-dimensional set-shifting task of the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Cambridge Cognition, Cambridge UK) to assess set-shifting skills in adolescents but found no differences in group performance [11, 47, 57]. Other measures of set shifting also failed to reveal significant group differences in error rate although group differences in reaction times were detected [32, 79]. Brooks et al. [13] did find a significant differences and medium effect size for errors on the shifting attention test of the CNS vital signs battery.
Working memory
Working memory refers to a system for temporary storage and manipulation of information. Although limited in its capacity it is crucial for everyday behaviour and supports higher cognitive function such as planning, learning, comprehension and reasoning [5]. The storage components of the working memory model are equal to short-term memory and comprise a system for phonological and visuospatial rehearsal of information. These two storage systems are controlled by a central executive component, which is responsible for attention allocation and necessary for the manipulation of information. It also lies at the interface of short- and long-term memory [6]. Working memory tasks thus differ with regard to whether the information to be remembered is merely maintained over a short delay or whether it needs to be manipulated. For example, the simple digit span requires information to be maintained and repeated. In contrast the complex digit span in which participants are asked to report a number sequence in order or backwards requires manipulation of information.
Visuospatial tasks
The CANTAB test battery offers several different tasks that assess spatial working memory. The delay-match-to sample requires test takers to recognize visual patterns after varying delays. It may be classified as a storage or short-term memory task. Medication-naïve depressed adolescent girls were found to make more mistakes compared to healthy control participants in the delay condition [57] but another study found no group differences on this task between acutely depressed, those remitted and healthy control adolescents [54]. Three studies reported worse performance on the spatial working memory task of the CANTAB in patients [11, 25, 57] but only one of these also found a reduced spatial span in the clinical group [25].
Verbal tasks
In an affective version of the n-back task Ladouceur et al. [49] used letters superimposed onto neutral, positive and negative valenced pictures in a 0- and 2-back condition to assess working memory function. No significant differences across diagnostic groups were detected on correct responses, omissions or commissions. In a similar affectively manipulated n-back task Tavitian et al. [72] found the adolescent MDD group to be less accurate than the healthy control group when letters were flanked by neutral facial expressions. No group differences were detected in conditions with happy or fearful expressions or a blank condition. Because two forms of the verbal digit span form part of the Wechsler Intelligence Scale for Children several studies have reported comparison of performance on the digit span in healthy control participants and children with depression. Mayes and Calhoun [58] did not find the anxious/depressed group to perform worse on the digit span than the healthy control group. In contrast, Klimkeit et al. [46] found a reduced digit span in both adolescents with MDD and those with DD or a depressive disorder not otherwise specified. Three studies included a measure of verbal learning and memory in their investigations some of these tasks require immediate recall and may be considered short-term verbal memory. None reported group differences on immediate recall [13, 18, 31] but two of these noted worse performance on the delayed recall component [13, 31].
Planning
Two of three studies found difficulties in planning in clinical participants with depression. A version of the Tower of London/Hanoi tasks was used in all studies [11, 54, 57]. In Maalouf et al. [54] the acutely depressed group needed more moves than the healthy control group and those that had remitted from depression on the four move problem specifically. Adolescents with MDD needed significantly more time to initiate moves and completed less problems than the healthy control group in the study by Bloch et al. [11]. Matthews and colleagues [57] used the same computerized task but failed to find any difference in performance between depressed girls and controls.
Verbal fluency
Verbal fluency deficits have been noted in two of four studies. Both Cataldo et al. [15] and Klimkeit et al. [46] found a difficulty of the clinical group in the phonemic but not the semantic task. Klimkeit et al. [46] noted this deficit only in the dysthymic/depressive disorder not otherwise specified group but not the major depressed group. Investigating only the phonemic component, two other studies did not observe group differences [23, 39].
Hot EF
Reward processing and decision-making
A popular way to assess decision-making is the Iowa Gambling Task (IGT) [7]. Han and colleagues [34] used the IGT in their sample of 31 children diagnosed with MDD and 30 healthy control children. After controlling for IQ they found a gender effect in that boys with MDD selected more cards from the disadvantageous decks than the healthy control boys. No difference was observed between depressed and healthy control girls. Oldershaw et al. [66] failed to observe significant performance differences on the IGT between adolescents with MDD, adolescents who deliberately self-harm and a healthy control group. Using a similar paradigm where probabilities of winning points and the amount to be won are systematically varied, Forbes et al. [24] observed that 11-year-old boys with DD or MDD chose a large reward less often than children without a diagnosis in a condition where the probability of winning was high. The response pattern during these trials of high probability of winning a large amount predicted internalizing symptoms at age twelve. In one study using a decision-making task with betting options no group differences emerged between adolescents with MDD and healthy control participants [47] but in another study employing a similar task adolescents with depression exhibited less reward seeking by betting lower amounts than healthy control participants [69]. This study also reported that task performance at baseline was associated with severity of depressive symptoms one year later [69].
Two studies showed that incentives were less effective in modulating performance in adolescents with MDD on a rewarded antisaccade task, unlike healthy control participants who showed reductions in latencies and peak velocity [36, 44]. However, anxiety symptoms in particular may have driven these results [36]. Dickstein and colleagues [20] used a reversal learning task in children and adolescents with a range of mood disorders. In this task participants first need to learn stimulus/reward and stimulus/punishment associations by trial and error and then reverse these. There was a trend but large effect size for more reversal errors in the MDD group.
Attentional bias and affective manipulations of cold EF tasks
Rather than a general deficit in attention some have proposed that individuals with depression have an attentional bias towards negative stimuli and potentially away from positive stimuli [1]. Only one of four studies investigating an attentional bias using the dot-probe task found performance differences between clinical and healthy control participants. Neshat-Doost et al. [63] and Dalgleish et al. [19] used emotionally valenced words and did not find a bias in their sample of depressed children but a bias towards threat in children with anxiety disorders. Also using words Taghavi et al.[71] neither observed an attentional bias in an anxious/depressed group of children and adolescents. Hankin et al. [35] used neutral, happy, sad and angry faces in a dot-probe task with 161 children aged 9–17. The depressed group exhibited a bias towards sad faces while an anxiety/depression comorbid group had a bias towards sad and angry faces. The majority of the sample had a past history of MDD, with only a relatively small proportion reporting current symptoms. In the same study there was also a gender divide in that boys but not girls in the comorbid group avoided happy faces.
Information processing bias including attention and other cognitive domains in paediatric depression has previously been reviewed by Jacobs et al. [43]. Although not the main focus of the present review of those studies included here there were eight studies and nine tasks that used some form of affective manipulation within other EF tasks. Four of these found that the affective manipulation of stimuli had no effect on task performance in depressed participants [19, 34, 64]. The other five tasks elicited some effect: [55] observed shorter reaction times to negative than positive stimuli only in the acute MDD group but not in the remitted MDD or healthy control group. However, none of the measures obtained on this task correlated with symptom severity. This is supported by a study that also found faster reaction times to sad faces in depressed adolescents using the same go/no-go task [48]. Also using a response inhibition task Kyte et al. [47] reported that the depressed group committed fewer errors on trials that depicted sad faces but more errors on incongruent sad/happy trials. In contrast, Ladouceur and colleagues [49] noted larger response latencies when neutral stimuli were presented on a negatively valenced background during an n-back task. Tavitian et al. [72] noted that the depressed group’s performance on an n-back task was worse when neutral facial expressions were presented, but not happy or fearful expression.
Discussion
We conducted a systematic review into EF and attention deficits in depressive disorders in children and adolescents. Primarily studies that compared a clinical group to a matched healthy control group were included in the review. Generally, across all domains results have been mixed with a greater tendency for null results. The results have shown little support for impairments in response inhibition, selective attention, set shifting, verbal working memory and verbal fluency. More research, with larger homogenous samples is needed to clarify possible deficits in sustaining attention, planning, spatial working memory and hot EF of reward processing and decision-making. There is some evidence that affectively valenced stimuli, particularly negative stimuli may impact the performance on neuropsychological tasks.
Interpretation of results is difficult given the large number of methodological issues such as sample selection and differing inclusion criteria [e.g. mixed diagnosis (MDD and DD), diagnostic status (current versus past MDD), comorbidities and medication status]. To illustrate this difficulty: on the WCST Holler et al.[39] noted that only the MDD but not the minor depressed group had difficulties. Similarly, planning difficulties have been observed in an acutely depressed group but not in moderately depressed children [54]. Other results suggest that less severe symptoms may also differentially impact certain aspects of EF e.g. phonemic fluency deficits were only observed in a moderately depressed group but not a MDD group [46] as well as a sample of children of which the majority was dysthymic [15].
Medication effects may also differentially impact results. Wilkinson and Goodyer [79] reported that only the medicated MDD group experienced difficulties on a measure of sustained attention. This may explain why Brooks et al. [13] found evidence for a sustaining attention deficit as the majority of clinical participants in their sample were medicated. All other studies investigating sustained attention found no group differences. Other methodological issues are task selection and presentation. On tests of set shifting it was observed that difficulties are less likely to be detected on computerized tasks [32, 47, 57, 79] (except for [13]). On attention tasks face stimuli and affective pictures seem to more reliably evoke a bias than words.
There is some evidence that the affective manipulation of stimuli can impact the performance on EF task of children and adolescents with depression. When a response to a negative/sad stimuli is required individuals with depression appear to react faster [49, 55] while when the negative stimuli is used as a distractor or is presented in incongruent trials with positive stimuli at the same time this may hamper reaction times [47]. It should, however, be noted that other studies have failed to detect such a bias [19, 34, 63, 64]. There may be other variables that drive attentional biases. For example, it has been shown that genetic risk factors, experience of childhood adversity as well as the mother’s history of depression can interact to influence children’s attentional biases [27, 67]. In addition, mother’s negative affect towards their children also resulted in greater attentional bias away from sad faces [16]. This study also found that an emotion regulation strategy differentially influenced this bias in youth with depressive symptoms. While there is some evidence for an attentional bias in paediatric depressive disorders, it is currently unclear whether depressive symptoms alone drive this bias towards negative stimuli. There are a multitude of variables that may mediate such a bias.
The seeming lack of evidence for neuropsychological deficits in depressive disorders in children and adolescents is echoed in community studies that have looked at the relationship of EF and depressive symptoms in nonclinical samples [17, 61]. In the largest community study with more than 1,800 participants that were followed over 5 years a few noteworthy findings emerged [76]: first, this study found significant gender differences in neurocognitive performance in accord with previous findings in developmental studies of typically developing children (e.g. [3]). Affective problems as measured by the Youth Self-report of the CBCL were related to lower baseline reaction times and more variability in reaction times, lower working memory capacity and lower response inhibition in girls only. No association was found between EF measures and self-reported affective problems in boys. Longitudinally the only significant relationship observed showed that response inhibition scores at baseline predicted affective problems in girls at 5 but not at 2.5 years follow-up. Response time variability also predicted subsequent affective problems in girls, but this relationship disappeared when adjusting for baseline affective symptoms. It is unclear why response inhibition would predict affective problems 5 but not 2.5 years later. The authors suggested that response inhibition may be a prodromal factor for affective problems in late adolescence and therefore linked to late-onset rather than early-onset depression.
The present review has shown that very few studies have found EF impairments in children and adolescents with depressive disorders. The absence of reliable findings, despite methodological issues, suggests that EF deficits are unlikely to play a major role in the aetiology of the majority of paediatric patients with depression. As such attention and EF deficits may be secondary effects that arise out of primary symptoms such as e.g. anhedonia and/or low/irritable mood. Support for this hypothesis comes from adult MDD studies that have shown that cognitive deficits increase with the number of depressive episodes, age and melancholic symptoms [77]. Such an account is in accord with the scarring and/or kindling hypothesis which suggest that a major depressive episode leaves behind biological and/or psychological scars that increase the vulnerability of developing a subsequent episode [26, 52, 68].
An open question in the paediatric depression literature remains as to whether depression in children and adolescents is similar to or different from adult populations. More importantly though developmental differences have been noted not only between child and adult populations but also within paediatric samples. MDD in adolescents and older youth differs in symptom patterns [78]. Additionally, differences have also been noted between depression in preadolescent children and adolescents with the former differing in aspects of epidemiology, aetiology and prognosis [37, 73, 74]. Unfortunately, many of the studies included in the present review covered a wide age range. Rates of depression in prepubertal children are generally low and it may be difficult and costly to identify a sample of children with current MDD. Nevertheless, combining children and adolescents with a diagnosis of MDD into one sample may not be informative given known differences in symptoms patterns.
Recommendations for future studies
Future studies into depressive disorders and EF in paediatric samples should attempt to include larger samples with a narrower age range. Half of the studies included in this review had 21 or fewer participants in the clinical group and many covered wide age ranges. For children and adolescents developmental aspects need to be taken into account. While basic EF develops during preschool years, improvements in EF continue throughout childhood and adolescence [3, 8]. In addition, other skills that are subject to developmental differences may influence task performance e.g. reading skills. Younger children may have more difficulties with tasks that require processing of verbal as compared to visual stimuli.
Another important aspect for future studies is the careful definition of the clinical group. The reviewed studies included a wide range of diagnoses: current depressive episode, past diagnosis of MDD, DD, remitted MDD, depressive disorder not otherwise specified. When these diagnoses are combined to form one ‘depressed’ group it may be more difficult to detect any relationship between each of these diagnosis and neurocognitive function. A better approach may be to combine groups according to symptom severity and/or duration of illness. Participants should be homogenous for either current symptoms or history of depressive episodes. However, this may not suffice. Symptoms may vary across individuals and future studies should collect more information about the specific symptoms that characterise their samples e.g. it may be important to note whether symptoms of inattention, anhedonia, rumination, low self-esteem, sad or primarily irritable mood are present and their severity and duration. Using any of these variables in addition to a MDD diagnosis will help identifying risk factors for attention and EF deficits. Furthermore, family history, neuroimaging markers or genetic risk factors may be used to define groups more precisely. The presence of comorbidities also poses challenges in comparing results across studies. It is well known that MDD is highly comorbid with anxiety disorders [12]. Some of the studies included here explicitly excluded comorbid anxiety while others had high rates of comorbid anxiety. Although including comorbidities may be more representative of the wider population of patients, it makes it more difficult to determine whether depressive symptoms alone are associated with cognitive dysfunction.
Few of the reviewed studies have investigated possible gender effects. Out of the 30 studies that have included mixed gender samples only eight have investigated a possible effect. Five [46, 48, 49, 58, 69] found no difference but three [34, 35, 47] did report significant differences between boys and girls on performance measures. There are known differences in the prevalence of MDD between boys and girls after the age of fifteen [65]. In addition, there are gender differences in brain maturation [50]. Given this evidence of differing developmental trajectories any future studies should consider taking possible gender effects into account.
Other concerns are the selection of neuropsychological tests. We observed a large range of test batteries being used that all assess different constructs of EF. While child friendly tasks may make testing more ‘fun’ for participants these tasks may not be comparable to more traditional measures [41]. It should also be noted that psychometric properties of some EF tasks are relatively weak [10]. This limits the conclusions that can be drawn from any observed group differences. Several studies included in the present review used test batteries with a large number of subtest and report significant results of at least one measure. In the absence of specific hypotheses these results may be false positives. Rather than running a large battery of tests to infer group differences researchers may want to consider selecting a task that specifically addresses an a priori defined hypothesis.
Clinical significance
The discrepancy between results in adults with MDD and children with depressive disorders on measures of EF and attention may suggest that duration and severity of symptoms play a key role in perpetuating those deficits. It is also possible that chronicity alone accounts for EF deficits in depressive disorders. Compared to children and adolescents, adults diagnosed with a depressive disorder may have had a prolonged previous history of subthreshold symptoms or depressive episodes that have remained unnoticed. The kindling hypothesis [60, 68] proposes that the circumstances associated with the occurrence of a first major depressive episode are different to those of recurrent episodes. Cognitive deficits may be less relevant to the development of a first major depressive episode but potentially play a key role in contributing to the recurrence of episodes. Hammar and Ardal [33] reported in their review on cognitive function in adult MDD that there is mounting evidence that improvement of cognitive function does not parallel symptom improvement to the same degree. Monitoring of EF deficits after initial diagnosis and treatment may therefore be important and indicative of risk of relapse but, more importantly may be subject to intervention [70].
Limitations
This was a qualitative and not a quantitative review. To draw firm conclusions as to whether attention and EF deficits are common in paediatric depressive disorders a meta-analysis would be necessary. Currently, there are too few studies for each EF process to warrant conducting a meta-analysis on them individually. However, we hope that the present review will aid researchers in making better decisions on study design and methodology so that a meta-analysis will be facilitated in the future.
Conclusion
Altogether the reviewed studies offer little support for reliable EF and attention deficits in paediatric depression. While the lack of evidence may be due to primarily methodological issues of small, heterogeneous samples the other possibility is that EF deficits in paediatric depression are rare and if they exist may be due to a range of other factors e.g. inattentive symptoms, comorbid anxiety and/or duration of illness. Symptom severity may also play an important role but to date relatively few studies have distinguished between severely depressed individuals and those with mild or moderate symptoms.
References
Abela JR, Hankin BL (2008) Cognitive vulnerability to depression in children and adolescents: a developmental perspective. In: Abela JR, Hankin BL (eds) Handbook of depression in children and adolescents. Guilford Press, New York, pp 35–78
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders : DSM-IV. American Psychiatric Association, Washington, DC
Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C (2001) Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol 20:385–406
Aronen ET, Vuontela V, Steenari MR, Salmi J, Carlson S (2005) Working memory, psychiatric symptoms, and academic performance at school. Neurobiol Learn Mem 83:33–42
Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4:829–839
Baddeley A (2012) Working memory: theories, models, and controversies. Annu Rev Psychol 63:1–29
Bechara A, Damasio H, Tranel D, Damasio AR (2005) The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci 9:159–162 (discussion 62–4)
Best JR, Miller PH (2010) A Developmental perspective on executive function. Child Dev 81:1641–1660
Best JR, Miller PH, Naglieri JA (2011) Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learn Individ Differ 21:327–336
Bishop DV, Aamodt-Leeper G, Creswell C, McGurk R, Skuse DH (2001) Individual differences in cognitive planning on the Tower of Hanoi task: neuropsychological maturity or measurement error? J Child Psychol Psychiatry 42:551–556
Bloch Y, Aviram S, Faibel N, Govezensky J, Braw Y, Rabany L, Walter G (2013) The correlation between impaired attention and emotional reactivity in depressed adolescent patients. J Neuropsychiatry Clin Neurosci 25:233–236
Brady EU, Kendall PC (1992) Comorbidity of anxiety and depression in children and adolescents. Psychol Bull 111:244–255
Brooks BL, Iverson GL, Sherman EM, Roberge MC (2010) Identifying cognitive problems in children and adolescents with depression using computerized neuropsychological testing. Appl Neuropsychol 17:37–43
Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J (2008) A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 106:1–27
Cataldo MG, Nobile M, Lorusso ML, Battaglia M, Molteni M (2005) Impulsivity in depressed children and adolescents: a comparison between behavioral and neuropsychological data. Psychiatry Res 136:123–133
Connell AM, Patton E, Klostermann S, Hughes-Scalise A (2013) Attention bias in youth: associations with youth and mother’s depressive symptoms moderated by emotion regulation and affective dynamics during family interactions. Cogn Emot 27:1522–1534
Connolly SL, Wagner CA, Shapero BG, Pendergast LL, Abramson LY, Alloy LB (2014) Rumination prospectively predicts executive functioning impairments in adolescents. J Behav Ther Exp Psychiatry 45:46–56
Constantinidou F, Danos MA, Nelson D, Baker S (2011) Effects of modality presentation on working memory in school-age children: evidence for the pictorial superiority hypothesis. Child Neuropsychol 17:173–196
Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W (2003) Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. J Clin Child Adolesc Psychol 32:10–21
Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E (2010) Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med 40:1089–1100
Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118
Emerson CS, Mollet GA, Harrison DW (2005) Anxious-depression in boys: an evaluation of executive functioning. Arch Clin Neuropsychol 20:539–546
Favre T, Hughes C, Emslie G, Stavinoha P, Kennard B, Carmody T (2009) Executive functioning in children and adolescents with major depressive disorder. Child Neuropsychol 15:85–98
Forbes EE, Shaw DS, Dahl RE (2007) Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry 61:633–639
Franklin T, Lee A, Hall N, Hetrick S, Ong J, Haslam N, Karsz F, Vance A (2010) The association of visuospatial working memory with dysthymic disorder in pre-pubertal children. Psychol Med 40:253–261
Garber J, Rao U (2014) Depression in children and adolescents. In: Lewis M, Rudolph KD (eds) Handbook of developmental psychopathology, 3rd edn. Springer, US, pp 489–520
Gibb BE, Benas JS, Grassia M (2009) Children’s Attentional Biases and 5-HTTLPR genotype: potential mechanisms linking mother and child depression. J ClinChild Adolesc Psychol 38:415–426
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179
Goodman R (1999) The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry 40:791–799
Gordon M, Mettelman B (1987) Technical guide to the Gordon Diagnostic System (GDS). Gordon Systems, DeWitt
Günther T, Holtkamp K, Jolles J, Herpertz-Dahlmann B, Konrad K (2004) Verbal memory and aspects of attentional control in children and adolescents with anxiety disorders or depressive disorders. J Affect Disord 82:265–269
Günther T, Konrad K, De Brito SA, Herpertz-Dahlmann B, Vloet TD (2011) Attentional functions in children and adolescents with ADHD, depressive disorders, and the comorbid condition. J Child Psychol Psychiatry 52:324–331
Hammar A, Ardal G (2009) Cognitive functioning in major depression–a summary. Front Hum Neurosci 3:26
Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A et al (2012) Selective neurocognitive impairments in adolescents with major depressive disorder. J Adolesc 35(1):11–20
Hankin BL, Gibb BE, Abela JR, Flory K (2010) Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. J Abnorm Psychol 119:491–501
Hardin MG, Schroth E, Pine DS, Ernst M (2007) Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J Child Psychol Psychiatry 48:446–454
Harrington R (2001) Adolescent depression: same or different? Arch Gen Psychiatry 58:21–22
Hertel PT (1994) Depression and memory: are timpairments remediable through attention control. Curr Dir Psychol Sci 3:190–193
Holler K, Kavanaugh B, Cook NE (2013) Executive functioning in adolescent depressive disorders. J Child Fam Stud
Hongwanishkul D, Happaney KR, Lee WC, Zelazo PD (2005) Assessment of hot and cool executive function in young children: age-related changes and individual differences. Dev Neuropsychol 28:617–644
Hughes C (2011) Changes and challenges in 20 years of research into the development of executive functions. Infant Child Dev 20:251–271
Hulvershorn LA, Cullen K, Anand A (2011) Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav 5:307–328
Jacobs RH, Reinecke MA, Gollan JK, Kane P (2008) Empirical evidence of cognitive vulnerability for depression among children and adolescents: a cognitive science and developmental perspective. Clin Psychol Rev 28:759–782
Jazbec S, McClure E, Hardin M, Pine DS, Ernst M (2005) Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry 58:632–639
Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ (2014) Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin 4:209–231
Klimkeit EI, Tonge B, Bradshaw JL, Melvin GA, Gould K (2011) Neuropsychological deficits in adolescent unipolar depression. Arch Clinl Neuropsychol 26:662–676
Kyte ZA, Goodyer IM, Sahakian BJ (2005) Selected executive skills in adolescents with recent first episode major depression. J Child Psychol Psychiatry 46:995–1005
Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ (2006) Processing emotional facial expressions influences performance on a go/nogo task in pediatric anxiety and depression. J Child Psychol Psychiatry 47:1107–1115
Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey BJ (2005) Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. J Abnorm Child Psychol 33:165–177
Lenroot RK, Giedd JN (2006) Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30:718–729
Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA (2007) cognitive deficits in depression and functional specificity of regional brain activity. Cogn Ther Res 31:211–233
Lewinsohn PM, Steinmetz JL, Larson DW, Franklin J (1981) Depression-related cognitions: antecedent or consequence? J Abnorm Psychol 90:213–219
Luna B, Padmanabhan A, O’Hearn K (2010) What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn 72:101–113
Maalouf FT, Brent D, Clark L, Tavitian L, McHugh RM, Sahakian BJ, Phillips ML (2011) Neurocognitive impairment in adolescent major depressive disorder: state vs. trait illness markers. J Affect Disord 133:625–632
Maalouf FT, Clark L, Tavitian L, Sahakian BJ, Brent D, Phillips ML (2012) Bias to negative emotions: a depression state-dependent marker in adolescent major depressive disorder. Psychiatry Res 198:28–33
Mathews A, MacLeod C (1994) Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol 45:25–50
Matthews K, Coghill D, Rhodes S (2008) Neuropsychological functioning in depressed adolescent girls. J Affect Disord 111:113–118
Mayes SD, Calhoun SL (2007) Learning, attention, writing, and processing speed in typical children and children with ADHD, autism, anxiety, depression, and oppositional-defiant disorder. Child Neuropsychol 13:469–493
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41:49–100
Monroe SM, Harkness KL (2005) Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev 112:417–445
Muris P, van der Pennen E, Sigmond R, Mayer B (2008) Symptoms of anxiety, depression, and aggression in non-clinical children: relationships with self-report and performance-based measures of attention and effortful control. Child Psychiatry Hum Dev 39:455–467
Murray EA, Wise SP, Drevets WC (2011) Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry 69:e43–e54
Neshat-Doost HT, Moradi AR, Taghavi MR, Yule W, Dalgleish T (2000) Lack of attentional bias for emotional information in clinically depressed children and adolescents on the dot probe task. J Child Psychol Psychiatry 41:363–368
Neshat-Doost HT, Taghavi MR, Moradi AR, Yule W, Dalgleish T (1997) The performance of clinically depressed children and adolescents on the modified Stroop paradigm. Pers Individ Dif 23:753–759
Nolen-Hoeksema S, Girgus JS (1994) The emergence of gender differences in depression during adolescence. Psychol Bull 115:424–443
Oldershaw A, Grima E, Jollant F, Richards C, Simic M, Taylor L, Schmidt U (2009) Decision making and problem solving in adolescents who deliberately self-harm. Psychol Med 39:95–104
Owens M, Goodyer IM, Wilkinson P, Bhardwaj A, Abbott R, Croudace T, Dunn V, Jones PB, Walsh ND, Ban M, Sahakian BJ (2012) 5-HTTLPR and early childhood adversities moderate cognitive and emotional processing in adolescence. PLoS One 7:e48482
Post RM (1992) Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 149:999–1010
Rawal A, Collishaw S, Thapar A, Rice F (2013) ‘The risks of playing it safe’: a prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychol Med 43:27–38
Segal ZV, Williams JM, Teasdale JD, Gemar M (1996) A cognitive science perspective on kindling and episode sensitization in recurrent affective disorder. Psychol Med 26:371–380
Taghavi MR, Neshat-Doost HT, Moradi AR, Yule W, Dalgleish T (1999) Biases in visual attention in children and adolescents with clinical anxiety and mixed anxiety-depression. J Abnorm Child Psychol 27:215–223
Tavitian LR, Ladouceur CD, Nahas Z, Khater B, Brent DA, Maalouf FT (2014) Neutral face distractors differentiate performance between depressed and healthy adolescents during an emotional working memory task. Eur Child Adolesc Psychiatry 23:659–667
Thapar A, Collishaw S, Pine DS, Thapar AK (2012) Depression in adolescence. The Lancet 379:1056–1067
Thapar A, McGuffin P (1994) A twin study of depressive symptoms in childhood. Br J Psychiatry 165:259–265
van der Meere J, Borger NA, Pirila S, Sallee F (2011) Interference control in children with first episode major depression: a brief report. Child Neuropsychol 17:96–104
van Deurzen PA, Buitelaar JK, Agnes Brunnekreef J, Ormel J, Minderaa RB, Hartman CA, Huizink AC, Speckens AE, Oldehinkel AJ, Slaats-Willemse DI (2012) Response time variability and response inhibition predict affective problems in adolescent girls, not in boys: the TRAILS study. Eur Child Adolesc Psychiatry 21:277–287
Wagner S, Doering B, Helmreich I, Lieb K, Tadic A (2011) A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatr Scand 125:281–292
Weiss B, Garber J (2003) Developmental differences in the phenomenology of depression. Dev Psychopathol 15:403–430
Wilkinson PO, Goodyer IM (2006) Attention difficulties and mood-related ruminative response style in adolescents with unipolar depression. J Child Psychol Psychiatry 47:1284–1291
Achenbach TM, Edelbrock CS (1983) Manual for the child behavior checklist and behavior profile. University of Vermont, Burlington
Ambrosini PJ, Metz C, Bianchi MD, Rabinovich H, Undie A (1991) Concurrent validity and psychometric properties of the beck depression inventory in outpatient adolescents. J Am Acad Child Adolesc Psychiatry 30:51–57
Angold A, Costello EJ (1995) A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the child and adolescent psychiatric assessment (CAPA-C). Psychol Med 25:755–762
Angold A, Costello EJ, Pickles A, Winder F (1987) The development of a questionnaire for use in epidemiological studies of depression in children and adolescents. Unpublished Manuscript. University of London
Beck AT, Steer RA, Brown GK (1996) Manual for the Beck depression Inventory-II. Psychological Corporation, San Antonio
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Birleson P (1981) The validity of depressive disorder in childhood and the development of a self-rating scale: a research report. J Child Psychol Psychiatry 22:73–88
Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM (1997) The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 36:545–553
Conners CK (1997) Conners’ rating scales-revised: CRS-R: MHS, multi-health systems
Costello EJ, Angold A (1988) Scales to assess child and adolescent depression: checklists, screens, and nets. J Am Acad Child Adolesc Psychiatry 27:726–737
Elternfragebogen über das Verhalten von Kindern und Jugendlichen—2. Auflage mit deutschen Normen. Köln: Arbeitsgruppe Deutsche Child Behavior Checklist
Elliott D, Huizinga D, Ageton S (1985) Explaining delinquency and drug USE. Sage, Thousand Oaks
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–296
Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for Affective disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988
Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U (2000) K-Sads-Pl. J Am Acad Child Adolesc Psychiatry 39:1208
Kaufmann J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1996) The schedule for affective disorders and Schizophrenia for school-age children. University of Pittsburgh Medical Center, Pittsburgh
Kovacs M (1982) The children’s depression inventory. Unpublished manuscript. University of Pittsburgh
Kovacs M (1985) The childhood depression inventory (CDI) manual. Multi-Health Systems, New York
Kovacs M (1992) Children’s depression inventory. Multi-Health Systems, New York
Kovacs M, Beck AT (1977) An empirical clinical approach towards a definition of child depression. In: Schulterbrandt JG, Raskin A (eds) Depression in children: Diagnosis, treatment, and conceptual models. Raven Press, New York, pp 1–25
Lindgren SD, Koeppl GK (1987) Assessing child behavior problems in a medical setting: development of the pediatric behavior scale. In: Prinz RJ (ed) Advances in behavioral assessment of children and families. JAI, Greenwich
March JS, Parker JD, Sullivan K, Stallings P, Conners CK (1997) The multidimensional anxiety scale for children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36:554–565
Mazza JJ, Reynolds WM (1998) A longitudinal investigation of depression, hopelessness, social support, and major and minor life events and their relation to suicidal ideation in adolescents. Suicide Life Threat Behav 28:358–374
Nolen-Hoeksema S (1991) Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol 100:569–582
Petersen AC, Crockett L, Richards M, Boxer A (1988) A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 17:117–133
Poznanski EO, Freeman LN, Mokros HB (1985) Children’s depression rating scale-revised. Psychopharmacol Bull 21:979–984
Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R (1984) Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry 23:191–197
Poznanski EO, Mokros HB (1996) Children’s depression rating scale, Revised (CDRS-R) manual. Western Psychological Services Publishers and Distributors, Los Angeles
Reich W (2000) Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 39:59–66
Reynolds CR, Richmond BO (1978) What I think and feel: a revised measure of children’s manifest anxiety. J Abnorm Child Psychol 6:271–280
Reynolds CR, Richmond BO (1985) Revised children’s manifest anxiety scale. RCMAS manual. Western Psychological Services, Los Angeles
Reynolds CR, Richmond BO (2008) Revised children’s manifest anxiety scale, second edition (RCMAS-2): manual. Western Psychological Services, Los Angeles
Reynolds WM (2002) The Reynolds adolescent depression scale (2nd edition) professional manual. Psychological Assessment Resources, Florida
RUPP (2002) The Research Units On Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): development and Psychometric Properties. J Am Acad Child Adolesc Psychiatry 41:1061–1069
Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S (1983) A children’s global assessment scale (CGAS). Arch Gen Psychiatry 40:1228–1231
Silverman WK, Albano AM (1996) Anxiety disorders interview schedule for DSM-IV: clinician’s manual: Psychological Corporation
Silverman WK, Nelles WB (1988) The anxiety disorders interview schedule for children. J Am Acad Child Adolesc Psychiatry 27:772–778
Spielberger C (1983) Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto
Spielberger C, Edwards D, Lushene R, Montuori J, Platzek D (1973) State-trait anxiety inventory for children. Consulting Psychologists Press Inc, Palo Alto
Stiensmeier-Pelster J, Schurmann M, Duda K (2000) Depressions-Inventar für Kinder und Jugendliche (2). Hogrefe Verlag für Psychologie, Göttingen
Unnewehr S, Schneider S, Margraf J (1995) Kinder DIPS—Diagnostisches Interview bei psychischen Sto ̈rungen im Kindes und Jugendalter. Springer, Heidelberg
Walkup J, Davies M (1999) The Pediatric Anxiety Rating Scale (PARS): a reliability study. Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 19–24
Acknowledgments
This research was conducted within the Developmental Imaging research group, Murdoch Childrens Research Institute and the Academic Child Psychiatry Unit, Department of Paediatrics, The University of Melbourne. Royal Children’s Hospital, Melbourne, Victoria. It was supported by the Murdoch Childrens Research Institute, the Royal Children’s Hospital, The Royal Children’s Hospital Foundation, Department of Paediatrics The University of Melbourne and the Victorian Government’s Operational Infrastructure Support Program. TS was supported by a NHMRC Career Development Award.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilgis, V., Silk, T.J. & Vance, A. Executive function and attention in children and adolescents with depressive disorders: a systematic review. Eur Child Adolesc Psychiatry 24, 365–384 (2015). https://doi.org/10.1007/s00787-015-0675-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-015-0675-7