Abstract
Objective
The aim of the present study was to assess the extent and severity of periodontal disease among type 1 diabetic patients (T1DM) and to investigate the possible association with systemic markers of glucose control and variability.
Material and methods
Patients were consecutively enrolled in a Diabetic Unit. A full-mouth periodontal evaluation was performed, and data on systemic markers of diabetes were collected. Descriptive statistics and logistic and linear models were performed.
Results
A total of 136 T1DM patients (mean age: 45.5 ± 14.6 years) were examined. Periodontitis was detected in 62% of cases (mean CAL: 3.0 ± 0.9 mm): stage III periodontitis was diagnosed in 32% of patients while stage IV in 8%. Mean level of glycated hemoglobin (HbA1c) was 7.5% ± 1.4. Among the investigated factors, mean CAL (p=0.040) was associated with HbA1c ≥ 7%; 93% of patients with mean CAL > 6 mm showed HbA1c ≥ 7%. Mean CAL (p=0.004), mean PPD (p=0.005), mean FMPS (p=0.030), and stage III/IV periodontitis (p=0.018) predict glucose coefficient of variation (CV).
Conclusions
Periodontitis showed a relevant prevalence in the present, well-controlled T1DM population and predicts poor glycemic control (HbA1c ≥7%) and higher glucose variability. The present findings suggest that periodontal infection may have systemic effects also in T1DM patients.
Clinical relevance
The extent and severity of periodontitis and its possible systemic effects in T1DM patients could be underestimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease (PD) is a chronic inflammation, caused by specific pathogens contained in the dental plaque, leading to host imbalance and destruction of connective tissue, bone resorption, and tooth loss [1]. PD shows a high prevalence (over than 40%) among individuals living in industrialized countries, while severe forms affect more than 10% of the global population [2].
A significant body of evidence demonstrated that PD interacts with several systemic diseases, including cardiovascular diseases and diabetes [3, 4]. Hypothetical mechanisms of interaction imply the possible induction of systemic inflammation from periodontal tissues, increasing the level of circulating inflammatory markers leading to the imbalance of chronic inflammatory processes [5,6,7]. Conversely, less agreement exits on the hypothesis of transient bacteremia caused by periodontal pathogens, reaching the blood stream and interacting with vascular surfaces [8].
A bidirectional association between PD and type 2 diabetes mellitus (T2DM) is supported by current evidence [9, 10]. Poorly controlled T2DM is considered a risk factor for PD [11], leading to the alteration of periodontal tissues via advanced glycation end products (AGEs) deposition and reduction of fibroblastic activity [12, 13], selecting periodontal pathogens [14] and reducing the chemotaxis and diapedesis of polymorphonuclear leukocyte cells (PMN) [15]. Additionally, T2DM could impair oral wound healing process [16].
Conversely, severe PD increases glycated hemoglobin levels (HbA1C) in T2DM patients, and it is associated with a higher prevalence of diabetic complications [17, 18]. A recent consensus concluded that PD is significantly associated with poor glycemic control, measured by HbA1C, in T2DM patients and that the risk is more elevated in patients with poorer HbA1C at baseline [17]. Hypothetical mechanisms of interaction may be related to the increasing levels of pro-inflammatory mediators (tumor necrosis factor-alfa; C-reactive protein and mediators of oxidative stress) and to the associated dyslipidemia in case of PD that may complicate glycemic control [19]. Initial evidence suggested that periodontal treatment may improve glycemic control [20]. Recently, a large, multicenter randomized trial demonstrated that periodontal treatment improves HbA1C levels in T2DM patients compared with controls, after 1 year of follow-up [21].
The association between PD and T1DM is more controversial. A consistent heterogeneity exits among studies in terms of periodontal diagnosis/collected variables, population samplings, and number of enrolled individuals [21]. The overall assessment of studies suggested that there is a higher incidence of PD among T1DM patients compared with healthy individuals, even if the consistency of the association between periodontal variables and diabetic systemic markers was not definitively addressed [21]. A recent consensus report concluded that there is insufficient evidence on the possible association between PD and poor glycemic control among people with T1DM [17].
The aim of the present cohort study was to assess the extent and severity of PD among type 1 diabetic patients and to investigate its possible association with diabetic systemic markers.
Material and methods
Source of data and study participants
The PARODIA (PAROdontite and DIAbete) Project is an observational study aimed at investigating the extent and severity of periodontal disease (PD) in patients with type 1 diabetes (T1DM) and the possible association between PD and systemic markers of glucose control and variability. The present manuscript conforms to STROBE guidelines for human observational studies. The study protocol was approved by the Ethical Board (PARODIA Project, approval number: CEAVC 30952/2019). Informed consent was obtained from all subjects included in the study. The present manuscript is a companion paper of a recently published short communication [22].
Patients were recruited at the Diabetes Unit, Azienda Ospedaliera Universitaria Careggi, Firenze (Italy). The following entry criteria were considered:
-
i)
Patients aged ≥ 18 years
-
ii)
Diagnosed with type 1 diabetes and currently treated with multiple daily insulin injections (MDI) or continuous subcutaneous insulin infusion (CSII)
-
iii)
Flash glucose monitoring (FGM) device (FreeStyle Libre FGM, Abbott Diabetes Care, IL) usage for the last 3 months.
Subjects with a history of other systemic diseases such as cancer, HIV, and bone metabolic diseases and history of radiation or immunosuppressive/modulating therapy were excluded, as well as those who had taken antibiotics, corticosteroids, or non-steroidal anti-inflammatory drugs in the last 3 months.
At baseline, all included patients underwent HbA1c, glucose coefficient of variability (CV), height, weight, and blood pressure measurements. Data on comorbidities were retrieved from medical records, and a screening for complications (fundus examination, measurement of serum creatinine, albumin/creatinine ratio, vibratory perception threshold with biothesiometer, and electrocardiogram) was performed. Furthermore, data on total cholesterol, triglycerides, and high-density lipoprotein (HDL) levels were collected.
Periodontal examination implied a full-mouth clinical examination performed with an NCP-15 periodontal probe, collecting data (six sites per tooth of each subject) on:
-
Plaque index (PI)
-
Bleeding on probing (BoP)
-
Probing pocket depth (PPD), measured from the gingival margin to the sulcus/pocket deepest point
-
Gingival recession (REC), measured from the cemento-enamel junction (CEJ) to the gingival margin (GM)
-
Furcation involvements
-
Tooth mobility
The clinical attachment level (CAL) of each site was estimated as the sum of PD + REC.
Information regarding hygiene habits, frequency of dental appointments, smoking habits, and previous dental treatments were also collected during the patient interview. Periodontitis was defined according to the 2017 Classification of Periodontal and Peri-implant Disease and Conditions [23].
All periodontal variables were collected by a single experienced operator (LB) that attended a preliminary calibration session, reporting an intraclass correlation coefficient of 0.87 (95% CI 0.82; 0.91).
Sample size and statistical analysis
The sample size was estimated using the sample size tables for logistic regression [24], with α=0.05, a power of 80%, a percentage of patients with HbA1c ≥ 7% of 70%, and an odds ratio of 1.7, referred to one standard deviation above the mean of the quantitative variables (for example mean CAL or mean PPD). Considering these parameters, a sample size of at least 130 patients was necessary.
Descriptive statistics using mean and standard deviation for quantitative variables, and frequency and percentage for qualitative variables, were used. Outcome variables were HbA1c ≥ 7% and glucose CV. Bivariate analyses were conducted, considering every single variable as a predictor variable. For HbA1c ≥ 7%, logistic regression models were used for quantitative variables and the Fischer exact test for qualitative variables. For glucose CV, linear regression models were used for quantitative variables and ANOVA test for qualitative variables. Stepwise backward analyses were performed using significant variables in the bivariate analyses. The level of significance (p > .05) was considered exclusion criteria for the stepwise backward analysis. A sensitive analysis was performed considering only patients with at least 12 teeth.
Results
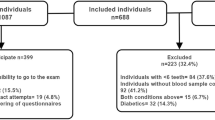
Of the 150 enrolled patients, 136 attended the scheduled periodontal visit. Finally, a total of 133 patients were available for the present analysis (data on FMPS and FMBS were not available for 3 patients).
The mean age was 45.5 ± 14.6 years (19–81). Seventy-three patients (55%) were female, and 22 (17%) were current smokers. The mean value of glycated hemoglobin was 7.5% ± 1.4. Out of the 133 patients, a total of 46 (35%) reported at least one diabetic complication. Data on descriptive statistics regarding systemic markers are reported in Table 1. In the assessed sample, a total of 83 patients (65%) showed periodontitis, while 3% was completely edentulous. The mean CAL was 3.0 ± 0.9 mm. Stage III periodontitis was detected in 32% of patients while stage IV in 8%. Details of periodontal variables are reported in Table 2.
A bivariate analysis considering all possible predictive periodontal and systemic variables and HbA1c ≥ 7% as outcome variable was performed (Table 3). Among all investigated factors, mean CAL (p=0.040) was associated with HbA1c ≥ 7%. Interestingly, the increase in CAL for each mm showed an OR = 1.79 [1.03; 3.12] for HbA1c ≥7%; 93% of patients with mean CAL > 6 mm showed HbA1c ≥7%. Mean CAL was significantly associated to HbA1c ≥ 7%, also in patients with at least 12 teeth (p=0.045).
Similarly, a bivariate analysis considering all possible predictive variables and glucose CV as outcome was performed (Table 4). Among all investigated factors, mean CAL (p=0.004), mean PPD (p=0.005), FMPS (p=0.030), and stage III/IV periodontitis (p=0.018) were associated with glucose CV.
Stepwise backward analyses with glucose CV as outcome variable were also performed, showing that mean PPD (2.87 ± 0.99 [0.90–4.84]; p=0.005) predicts glucose CV (Table 5). Finally, stage III and IV periodontitis were significantly associated with glucose CV (p=0.018) compared to stage I and II (Table 6).
Discussion
Over 30% of adults in the USA and Northern Europe showed periodontal disease; in 13% of these individuals, the condition was severe [2, 25]. A meta-regression assessing the global burden of PD in the last two decades showed that the prevalence of more severe forms was stable around 11% worldwide [2]. The progression of untreated PD leads to tooth loss, impaired quality of life, and significant systemic inflammation [26]. A significant body of evidence has shown that PD interplays with several important chronic diseases, including diabetes mellitus. Data on T2DM showed a significant, bidirectional association between the two diseases, but current data on the systemic impact of PD in T1DM are inconclusive [17]. The aim of the present study was to explore the possible association between PD and type 1 diabetes in a sample of Italian patients.
In the present analysis, 62% of T1DM patients showed signs of PD. In details, 32% was diagnosed with stage III and 8% with stage IV PD. Interestingly, the reported prevalence of PD in type 1 diabetic patients is significantly higher than that described in a recent systematic review [21]. Possible reasons may be related with great heterogeneity in periodontal diagnosis, mainly using screening methods or partial-mouth probing evaluation. On the other hand, a standard full-mouth periodontal charting was performed in the present cohort of patients, according to the recent classification presented at the World Workshop in Periodontology 2017 [23]. Our findings suggested a high prevalence of PD among type 1 diabetic patients, thus supporting the role of T1DM as a significant risk factor for PD. A large body of evidence showed that type 2 diabetes mellitus is a significant risk factor for PD [27] and this seems to be related to a number of factors including impairment of immune response, selection of periodontal pathogens, and alteration of periodontal connective tissues due to AGEs deposition [9]. Data regarding the possible association between T1DM and PD are lacking and controversial, even if a large study on diabetic children showed a greater incidence of periodontal destruction in these individuals compared with healthy controls [27]. Findings from our study suggest that early periodontal diagnosis and treatment are critical in T1DM patients. It should be kept in mind that 3% of the patients were completely edentulous and 40% showed stage III and IV periodontitis, thus supporting the hypothesis of a rapid progression of PD in T1DM patients.
The present results showed a significant association between mean CAL and the threshold value of HbA1c ≥ 7%. Interestingly, the increase in CAL for each mm showed an OR= 1.79 [1.03; 3.12] for HbA1c > 7%. Additionally, 93% of patients with mean CAL > 6 mm showed HbA1c ≥ 7%, thus corroborating the association between PD and poorly controlled diabetes. From this point of view, an association between PD and HbA1c levels has been clearly demonstrated in type 2 diabetes. The recent update of the EFP/AAP systematic review showed that type 2 diabetic patients with PD showed higher HbA1c levels and higher incidence of diabetic complications when compared with periodontally healthy type 2 diabetic controls [19]. Conversely, the level of existing evidence linking PD and type 1 diabetes is more controversial [21]. In the present sample of patients, a clear association between PD severity and HbA1c ≥ 7% was shown. The significance of the reported association seems to be very interesting since it was observed in a sample of well-controlled T1DM patients. None of the possible systemic markers investigated in the analysis showed association with HbA1c ≥ 7%, thus supporting the correct lifestyle and compliance of this sample of patients. Although further studies are necessary, this observational study seems to suggest that the “two-way” relationship [28] may also exist for T1DM and periodontitis. The possible link between PD and T1DM was also supported in the present study by the association between mean PPD and glucose CV, a novel parameter capturing daily fluctuations in blood glucose levels, thus predicting the risk of hypoglycemia. Interestingly, more severe PD stages were associated with higher values of glucose CV, suggesting that higher loss of bone and clinical attachment could probably stimulate more systemic inflammation also in T1DM patients. This finding seems to corroborate the possible systemic effect of periodontal infection, supporting the hypothesis that PD may generate a low-grade systemic inflammation via acute-phase and neutrophil oxidative stress response, impacting T1DM general conditions.
Out of the present sample of T1DM patients, 35% showed at least one systemic complication, and diabetic retinopathy (29%) and neuropathy (11%) were the most frequent. Considering the relatively low mean age of the present sample of patients (45.5 ± 14.6 years), this finding seems to strengthen the importance of the HbA1c control optimization, in order to prevent and/or delay the related progression of diabetic complications. Interestingly, considering that classical factors failed to predict HbA1c in the current sample of patients and that only PD was associated with T1DM systemic glucose profile, the findings of the present study underline the importance of proper periodontal diagnosis and treatment also in order to prevent possible severe clinical sequelae of T1DM.
Limitations of the present study may be related to the sample of enrolled patients that could be considered not representative of the whole population, since it was recruited among patients in a university-based diabetes center. Furthermore, larger samples of patients are necessary for further observational studies. Finally, the cross-sectional study design precludes definitive, causal associations between the two investigated diseases.
Conclusions
The present study suggests that:
-
Periodontitis is highly prevalent in a well-controlled T1DM population, selected in a university-based Diabetic Unit.
-
Periodontal disease predicts HbA1c ≥ 7% levels and higher glucose CV, thus supporting the hypothesis of a systemic effect of PD also in T1DM patients.
References
Sanz M, Quirynen M, European Workshop in Periodontology Group A (2005) Advances in the aetiology of periodontitis. Group A: consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 32(Suppl. 6):54–56. https://doi.org/10.1111/j.1600-051X.2005.00827.x
Kassebaum NJ, Bernab E, Dahiya M, Bhandari B, Murray CJ, Marcenes W (2014) Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dental Re 93(11):1045–1053. https://doi.org/10.1177/0022034514552491
Chapple IL, Genco R, Working group 2 of the joint EFP/AAP workshop (2013) Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 84(4 Suppl):S106–S112. https://doi.org/10.1902/jop.2013.1340011
Kapila YL (2021) Oral health’s inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 87(1):11–16. https://doi.org/10.1111/prd.12398
Cairo F, Castellani S, Gori AM, Nieri M, Baldelli G, Abbate R, Pini-Prato GP (2008) Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J Clin Periodontol 35(6):465–472. https://doi.org/10.1111/j.1600-051X.2008.01228.x
Cairo F, Castellani S, Gori AM, Nieri M, Abbate R, Pini-Prato GP (2009) Periodontol variables predict sub-clinical atherosclerosis and systemic inflammation in young adults. Eur J Oral Implant 2:125–133
D’Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, Masi S, Tsakos G, Hurel S, Hingorani AD, Donos N, Deanfield JE, TASTE Group (2018) Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol 6(12):954–965. https://doi.org/10.1016/S2213-8587(18)30038-X
Cairo F, Gaeta C, Dorigo W, Oggioni MR, Pratesi C, Pini Prato GP, Pozzi G (2004) Periodontal pathogens in atheromatous plaques. A controlled clinical and laboratory plaques. J Periodontal Res 39:442–446. https://doi.org/10.1111/j.1600-0765.2004.00761.x
Genco RJ, Borgnakke WS (2013) Diabetes as a potential risk for periodontitis: association studies. Periodontol 83(1):40–45. https://doi.org/10.1111/prd.12270
Genco RJ, Borgnakke WS (2020) Diabetes as a potential risk for periodontitis: association studies. Periodontol 83(1):40–45. https://doi.org/10.1111/prd.12270
Polak D, Sanui T, Nishimura F, Shapira L (2020) Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontol 83(1):46–58. https://doi.org/10.1111/prd.12298
Golub LM, Schneir M, Ramamurthy NS (1978) Enhanced collagenase activity in diabetic rat gingiva: in vitro and in vivo evidence. J Dent Res 57(3):520–525. https://doi.org/10.1177/00220345780570032101
Federoff HJ, Lawrence D, Brownlee M (1993) Nonenzymatic glycosylation of laminin and the laminin peptide CIKVAVS inhibits neurite outgrowth. Diabetes 42(4):509–513. https://doi.org/10.2337/diab.42.4.509
Zambon JJ, Reynolds H, Fisher JG, Shlossman M, Dunford R, Genco RJ (1988) Microbiological and immunological studies of adult periodontitis in patients with noninsulin-dependent diabetes mellitus. J Periodontol 59(1):23–31. https://doi.org/10.1902/jop.1988.59.1.23
Salvi GE, Lawrence HP, Offenbacher S, Beck JD (1997) Influence of risk factors on the pathogenesis of periodontitis. Periodontol 14:173–201. https://doi.org/10.1111/j.1600-0757.1997.tb00197.x
Ko KI, Sculean A, Graves DT (2021) Diabetic wound healing in soft and hard oral tissues. Transl Res 236:72–86. https://doi.org/10.1016/j.trsl.2021.05.001
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D (2018) Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol 45(2):138–149. https://doi.org/10.1111/jcpe.12808
Genco RJ, Graziani F, Hasturk H (2020) Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol 83(1):59–65. https://doi.org/10.1111/prd.12271
Graziani F, Gennai S, Solini A, Petrini M (2018) A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J Clin Periodontol 45(2):167–187. https://doi.org/10.1111/jcpe.12837
Engebretson S, Kocher T (2013) Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol 84(Suppl 4):S153–S169. https://doi.org/10.1902/jop.2013.1340017
Dicembrini I, Serni L, Monami M, Caliri M, Barbato L, Cairo F, Mannucci E (2020) Type 1 diabetes and periodontitis: prevalence and periodontal destruction-a systematic review. Acta Diabetol 57(12):1405–1412. https://doi.org/10.1007/s00592-020-01531-7
Dicembrini I, Barbato L, Serni L, Caliri M, Pala L, Cairo F, Mannucci E (2021) Glucose variability and periodontal disease in type 1 diabetes: a cross-sectional study-the “PAROdontopatia e DIAbete” (PARODIA) project. Acta Diabetol 58(10):1367–1371. https://doi.org/10.1007/s00592-021-01720-y
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol 45(Suppl 20):S149–S161. https://doi.org/10.1111/jcpe.12945
Hsieh FY (1989) Sample size tables for logistic regression. Stat Med 8(7):795–802. https://doi.org/10.1002/sim.4780080704
Hugoson A, Norderyd O, Slotte C, Thorstensson H (1998) Distribution of periodontal disease in a Swedish adult population 1973, 1983 and 1993. J Clin Periodontol 25:542–548. https://doi.org/10.1111/j.1600-051x.1998.tb02485.x
Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J (2017) Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol 44:456–462. https://doi.org/10.1111/jcpe.12732
Preshaw PM, Alba AL, Herrera D, Jepsen S, Kostantinidis A, Makrilakis K, Taylor R (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55(1):21–31. https://doi.org/10.1007/s00125-011-2342-y
Taylor GW (2001) Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 6:99–112. https://doi.org/10.1902/annals.2001.6.1.99
Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, Goland RS, Lamster I (2007) Diabetes mellitus promotes periodontal destruction in children. J Clin Periodontol 34:294–298. https://doi.org/10.1111/j.1600-051X.2007.01054.x
Funding
This study was self-funded.
Author information
Authors and Affiliations
Ethics declarations
Ethical approval
The study protocol was approved by the Ethical Board (PARODIA Project, approval number: CEAVC 30952/2019).
Conflict of interest
Francesco Cairo has received consultancy fees and grants from Straumann and Geistlich Biomaterials. Ilaria Dicembrini has received speaking fees from Merck, Novartis, AstraZeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli-Lilly, Novo Nordisk, Sanofi, and Novartis. Edoardo Mannucci has received consultancy fees from Merck and Novartis; speaking fees from AstraZeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli-Lilly, Merck, Novo Nordisk, Sanofi; and Novartis, and research grants from Merck, Novartis, and Takeda. Other authors have nothing to disclose.
Informed consent
Informed consent was obtained from all subjects included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cairo, F., Dicembrini, I., Serni, L. et al. Periodontitis predicts HbA1c levels and glucose variability in type 1 diabetic patients: the PARODIA Florence Project study. Clin Oral Invest 26, 3585–3591 (2022). https://doi.org/10.1007/s00784-021-04326-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04326-4