Abstract
Objectives
The purpose of this study is to compare bone regeneration properties of recently available collagen-calcium phosphate (C-CP) blend as bone substitute (BS) material in oral surgery with calcium phosphate (CP) as well as collagen material (Collagen). Is C-CP better than the classic loose CP, or is it at least equally effective in the jawbone regeneration with the superiority of a coherent consistency?
Materials and methods
This study included 102 patients during 12-month follow-up. All patients underwent the following surgical procedures: sinus lift (52patients) and tooth extraction (50patients). Patients were divided into 3 groups which are as follows: experimental group with C-CP, CP and pure Collagen as control groups. Texture analysis was performed in intra-oral radiographs. Analyses were performed in the MaZda 4.6 software. Average 2444-pixel ROIs were established in the image of BS materials and normal trabecular bone for texture comparison to evaluate the jawbone regeneration process. Four features were calculated and investigated.
Results
Texture analyses revealed that all 4 features described the healing process well. Reference textural value of feature SumOfSqrs < 102.37 was soft tissue, DifEntr < 1.1 was not the bone, Entr < 2.62 was not a bone and LngREmph > 1.8 was soft tissue. For 12 months, bone regeneration was proved by 3 of 4 investigated features. Only Entr indicated to CP remnants in the ROI (p < 0.05).
Conclusions
This study proves that blended collagen-calcium phosphate as a BS material can bring satisfactory and predictable outcomes in jawbone regeneration.
Clinical relevance
Clinicians can choose a satisfactory and predictable material for bone regeneration treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, various forms of calcium phosphates (CPs) are the most commonly used bone replacement materials due to their ease of application and handling, safety profile, intraoperative cost, time advantages and adaptability to a variety of clinical indications [1,2,3,4]. The biologically active glasses or methyl methacrylate polymer, formerly used, are now marginalised. But alpha, beta and crystalline calcium phosphates have become widespread in oral surgery. Those used in oral surgery are usually available in granular form that does not allow to shape well biomaterials in the surgical field during the surgery procedure.
Developing oral surgery and implantology has led to identification of other needs. It means that surgeons are expecting better and more predictable results of their procedures. Vertical and horizontal bone deficiency and also bone defects are still the biggest challenge. Bone augmentations of the maxilla and mandible are necessary to obtain required bone volume, e.g. before implantological or prosthetic treatment. Still, the gold standard in oral surgery is autologous grafts. Bone tissue has to be harvested from additional surgical site, e.g. iliac crest, ramus and mental of mandible, and maxilla tuberosity. It can lead to further complication, e.g. pain of donor site, harvesting morbidity, more pain and, resulting from it, patient’s fear [1,2,3, 5].
An appropriate solution to this clinical problem is to immerse CP granules in a collagen sponge and create a solid but not hard biomaterial. The blended collagen-calcium phosphate (Collagen-CP) is a combination of collagen and tricalcium phosphates. Collagen does not have adequate mechanical properties as strength and stiffness, but keeps the CP granules in place in bone defect. On the other hand, collagen is the main fibrous protein that is a component of bones [6]. Collagen is non-cytotoxic, compatible with the immune system, and biodegradable, and it also allows for deposition of calcium carbonate and calcium phosphate [6, 7]. It shows that the combination of these two components could reveal interesting properties as a bone substitute material.
It is not known: is Collagen-CP better than the classic loose CP, or is it at least equally effective in the jawbone regeneration with the superiority of a coherent consistency? Thus, the aim of this study is the analysis of the bone regeneration induced by collagen-tricalcium phosphate mixed material in intra-oral radiographs.
Materials and methods
The study was approved by the University Ethical Committee RNN/485/11/KB. A total of 102 patients (46 males and 56 females) aged between 17 and 75 years (49.2 ± 11.75 years) were included. All patients underwent surgical procedures prior to dental implant insertion: sinus lift (52 patients) and tooth extraction (50 patients). Patients were divided into 3 groups depending on the material used: experimental group with blended Collagen-CP (Curasan: Cerasorb Foam), CP (Curasan: Osbone 1000–2000 μm) as positive control group, i.e. typical bone substitute (BS) contemporary often used, and negative control group with pure collagen as BS (B Braun: Osteovit) named here Collagen.
Inclusion criteria were two-dimensional X-rays and laboratory tests: level of parathormone (PTH in pg/ml), level of thyreotropin (TSH in μIU/ml), level of calcium in serum (Ca in serum in mg/dl), level of low-density lipoprotein (LDL in mg/dl), level of triglycerides (mg/dl), level of glycated haemoglobin (%), level of vitamin D3 (ng/ml) and densitometry (in our work there was T score taken into account (comparison of a person’s bone density with that of a healthy 30-year-old of the same gender) as well as Z score (comparison of a person’s bone density with that of an average person of the same age and gender). Exclusion criteria were as follows: lack of laboratory tests, defected radiographs (in the visual assessment of the researchers) and circumstances required bone grafting. There were no exclusion criteria taking into account general diseases.
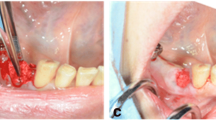
Preliminary investigation was performed to evaluate which values of textural features are connected to cortical bone, trabecular bone and soft tissue as a reference. A total of 29 samples of each of these regions of interests (ROIs) were analysed (Fig. 1).
All two-dimensional radiographs were taken during typical clinical follow-up: starting in the day of surgery–directly after surgery (00 M), 3 months (03 M), 6 months (06 M), 9 months (09 M), and 12 months (12 M) after surgery. Patients should have controls every 3 months from the day of procedure, but it was not always possible. To take an intra-oral radiograph, Digora Optime system of radiography was used. The radiographs were taken in standardised way [8] and with the strictly determined technical parameters: 7 mA, 70 mV and 0.1 s. To make sure that the radiographs will be taken with some repeatability, positioners were used (90° angle of X beam to the surface of phosphor plate).
All radiographs were analysed in the MaZda software version 4.6 invented by University of Technology in Lodz [9, 10]. The main goal of this software is texture analysis. It allows evaluations of texture parameters in digital X-ray images. In this study, 331 X-rays were analysed.
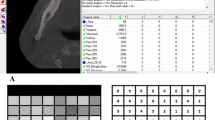
The first step was to load an X-ray image to MaZda in .bmp file format (bit map). Then, the ROIs were marked with average 2444 pixels for the bone area and 2515 pixels for the material area. ROIs were marked on all images: green ROI was for material texture and the reference ROI (the red one) for bone (Fig. 2). The ROIs were normalised to share the same mean and standard deviation of grey level inside the ROI (μ ± 3σ, where μ and σ denote the mean and standard deviation of registered optical density, respectively).
The components of texture analyses are the following (Fig. 3): sum of squares (SumOfSqrs), entropy (Entropy), difference entropy (DifEntr) for 5-pixel distance (all previous features derive from co-occurrence matrix) and long-run length emphasis moment (LngREmph) were utilised. All features were gathered in angles 0°, 45°, 90° and 135° from done pixel, and average value was later calculated. Features were calculated for the reference bone and for the substitute material:
where Σ is the sum; μx are the means and standard deviations; Ng is the number of distinct grey levels in the quantised image, where Ng is equal to 2k, and k is the number of bits per pixel (8 bits, i.e. 256 grey levels); p(i,j) is the number of times there is a run of length and j having grey level i are optical density of pixels 5-image-point distant one from another
where Σ is the sum, p(i,j) is the number of times there is a run of length; j having grey level i are optical density of pixels 5-image-point distant one from another, p is the probability, log is the logarithm [11], Ng is the number of distinct grey levels in the quantised image, where Ng is equal to 2k, and k is the number of bits per pixel (8 bits, i.e. 256 grey levels).
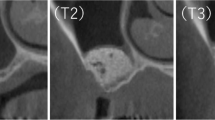
Textural features calculated in this study. Radiograph samples taken from location presented in Fig. 2 (raw RTG). Below these radiograph samples, there are a series of maps of investigated texture features (SumOfSqrs, Entropy, DifEntr and LngREmph) which were analysed for 12 months of observation. The brighter areas in these maps indicate where a given texture feature is more intense
where Σ is the sum, log is the logarithm, Ng is the number of distinct grey levels in the quantised image, where Ng is equal to 2k, and k is the number of bits per pixel (8 bits, i.e. 256 grey levels).
where Σ is the sum, p(i,j) is the number of times there is a run of length j having grey level i. Ng is the number of distinct grey levels in the quantised image, where Ng is equal to 2k, and k is the number of bits per pixel (8 bits; i.e. 256 grey levels in this study was reduced to 6 bit/pixel due to noise removal) Nr is the number of pixel in series (5 pixels); p is the probability.
Then, the medians of SumOfSqrs, Entropy, DifEntr and LngREmph for reference bone, Collagen-CP, CP (as positive reference material—bone substitute, i.e. crystalline tricalcium phosphate) and for collagen ( negative control group) individually after each control period, i.e. 00 M, 03 M, 06 M, 09 M and 12 M were evaluated for statistics to describe texture structure variability.
Statistical analysis
The Kruskal-Wallis test (to compare time-dependent alternations in medians) was applied for statistical analysis. Next, a multiple comparison procedure was used to determine which means were significantly different from the other (which). The method discriminates among the variables Fisher’s least significant difference (LSD) procedure. The difference was considered as significant if p < 0.05. Stargraphics Centurion XVI, StarPoint Technologies. INC., Virginia, USA was used for statistical analyses.
Results
The preliminary characteristics evaluating radiotextural features were investigated in the cortical bone, the trabecular bone and the gingiva as soft tissue reference. SumOfSqrs indicates trabecular bone pretty well (p < 0.05). ROI presenting a lower value of this textural parameter (in this study lower than 102.37) is the cortical bone or the soft tissue, i.e. gingiva, but not trabecular bone. The other 3 features indicate where the bone tissue image is (without distinction whether cortical or trabecular one) contrary to soft tissue where significantly higher values are noted (p < 0.001), e.g. for LngREmph: 1.8 (higher values are soft tissue, p < 0.001). Entr values < 2.62 are not bone but soft tissues (p < 0.001). DifEntr < approx. 1.1 are not the bone but soft tissue (p < 0.001) (Table 1). Based on that analysis, the reference data were acquired and will be used to verify transformation of the BS and evaluate regenerative effectiveness of the recent Collagen-CP blended material.
In LngREmph analyses, ROI for bone versus ROI for material (Collagen-CP, CP and Collagen) were calculated for each separately. At 00 M, there was a statistical difference between collagen and CP groups (p < 0.05). After 3 months (03 M), there was statistical difference between Collagen-CP and CP texture (value for Collagen-CP 1.65 and for CP was 1.49), p < 0.05, but there was also difference between Collagen-CP and pure collagen (for Collagen 1.77). After 6 months (06 M), the value for LngREmph was lower (Collagen-CP - 1.54; CP - 1.53, Collagen - 1.71), still p < 0.05. From month 6 to month 9, LngREmph for collagen decreased, for Collagen-CP increased. After 1 year (12 M) of observation after surgery, the LngREmph values were on the level: Collagen-CP - 1.50; CP - 1.64; Collagen - 1.77, p < 0.05 (Table 2) (Fig. 4a, b, c).
a Distribution of test results of texture features in the three-dimensional space described by the patient’s age (in years), test period (in months) and the value of the radiotexture feature for Collagen implant sites. b Distribution of test results of texture features in the three-dimensional space described by the patient’s age (in years), test period (in months) and the value of the radiotexture feature for blended Collagen-CP implant sites. c Distribution of test results of texture features in the three-dimensional space described by the patient’s age (in years), test period (in months) and the value of the radiotexture feature for calcium phosphate derived bone substitute material (CP) implant sites
Analysing entropy, it has been observed that the value for bone was on the same level throughout the whole period of observation (00 M–12 M) 2.76. Entropy value for Collagen-CP, CP and collagen in 00 M (Collagen-CP - 2.84; CP - 2.89; Collagen - 2.73) contrary to bone entropy was similar, so there was no statistical difference (p > 0.05). There were differences between collagen group to Collagen-CP and CP. Then throughout months of observations, the structure of materials was rebuilt. The entropy value for CP decreased in 03–12 M, and in 03 M–09 M, collagen-CP decreased and the same value started increasing up to 12 M of observation, and for collagen entropy, the value from 06 to 12 M of observation was increasing, p < 0.05 (Table 2) (Fig. 4a, b, c). Entropy feature can successfully become the second prognostic indicator.
Taking into account SumOfSqrs value, analyses show that the value of p < 0.05 (statistically significant) was after the surgery for 00 M and 03 M. During the healing (06 M, 09 M, 12 M), the value of p > 0.05. SumOfSqrs should not be a prognostic feature in the observation of BS healing process (statistically not significant).
The last feature that was analysed was DifEntr, through the whole period 00 M–12 M (p < 0.05) (value of DifEntr for Collagen-CP, CP decreased for Collagen-CP from 1.23 to 1.16 and for CP from 1.28 to 1.20), but for collagen, this feature grew from 1.15 to 1.21. During the observation from 00 to 03 M, the difference between collagen and CP was observed. The next feature that can be used for comparison (Table 2) (Fig. 4a, b, c).
Evaluation of the structure that bone substitution material becomes can be verified by comparing SumOfSqrs, Entr., DifEntr. and LngREmph of the cortical and trabecular bone and gingiva as soft tissue to same features for the material through a 12-month period (Table 2) (Fig. 4a, b, c).
Additionally the age as a factor has been analysed. In all used materials, there is no statistical significance between texture analyses and age of the patients in the day of surgery (p > 0.05).
Discussion
The current problem in regenerative oral surgery is to choose and use the right and the most predictable BS. To observe and visually assess the bone structure, dentists/surgeons take 2D radiographs usually. This is one of the most popular diagnostics in dental clinics, and it also allows to visually analyse the structure of the bone. 2D X-rays have got minimal exposure dose and are safest for patients [12]. Presented in the literature and commonly used radiological method of bone assessment is DXA. DXA is a dual-energy X-ray absorptiometry. Unfortunately, this method does not allow for distinction of the structure of the bone; DXA reveals the level of bone mineral density [13, 14]. CBCT is the next option for the assessment bone deficiency; it ensures high accuracy of radiological assessment, but it carries with it a higher dose of radiation [15]. 2D radiographs are a sufficient method to evaluate the texture of bone/material healing process.
The development of radiology and the need for more accurate radiographs led to producing the digital intra-oral sensor: charge-coupled device (CCD) and complementary metal oxide semi-conductor (CMOS) [16]. Nevertheless, photostimulable phosphor (PSP) plates in the DIGORA Optime system indicate agreement with the gold standard [17]. To evaluate the texture features, ROIs were marked for the bone (average 2444 pixels ) and for the material (average 2515 pixels). It has been proved that an ROI of more than 800-pixel area is enough to perform repeatable texture analyses [18].
Four texture features were analysed: SumOfSqrs analyses differentiation of the bone microarchitectonic. This feature analysed five pixels in four directions for occurrence of pixels in the same shade of grey. Contrary to our reference bone texture, if the value of SumOfSqrs was higher than 102.37, we could conclude that the arising tissue became a bone. LngREmph—long-run emphasis inverse moment map described longer occurrence of pixels, e.g. long series of dark pixels in bone defects or in gingiva. If the value of this feature was decreasing < 1.8, the healing material became similar to reference bone. Probably ShrtREmph (short-run emphasis inverse moment) could be better for the detection of human bone. DifEntrp—the difference entropy map indicated the area (white) where the original image presented a very chaotic/fine texture. Entropy is a measure of structure disarrangement. We assumed that it attains minimal values in a bone loss region which is structurally homogeneous. In the course of bone healing process, when heterogeneity in optical density (complicated new bone elements) appears, the entropy value should increase, achieving a maximum level in a healthy or a totally regenerated trabecular bone tissue.
00 M observation—analyses of texture just after surgery revealed that taking into account entropy value, collagen-CP and CP materials was similar to reference bone tissue. The DifEntr feature also shows that CP was closer to the bone structure. 03 M observation—after 3 months from surgery, the CP material becomes more similar to bone structure taking into account Entr., DifEntr. and LngREmph. Entropy shows that all materials were under a rebuilding process. 06 M observation—after 6 months from surgery, the materials were still rebuilding. However, the CP microstructure was more similar to the reference bone tissue taking into account texture analyses. 09 M observation—after 9 months of observation period, the study shows that all materials were still rebuilding, but the CP material was similar to the bone one taking into account the two features (Entr and LngREmph). 12 M observation—the last period of observation revealed progressive healing process. All materials were similar to the bone. Nevertheless, the CP bone substitute had higher entropy contrary to collagen-CP and pure collagen, while its microarchitecture was most similar to the reference bone tissue without distinction to trabecular or cortical after 1 year of observation (Fig. 4a, b, c). Based on the texture analyses, it can be presumed that after a 12-month observation of the healing process, the microstructure of used CP material becomes compound cortical and trabecular tissue.
Texture analyses can be confirmed by radiographs from 3 groups of presented materials. It has been observed that materials after the 12-month period can be similar to the bone, but the healing process looks different every 3 months and for each material (Fig. 3). Our study proves that using collagen-CP material ensures bone tissue formation better than pure collagen. Nevertheless, the CP bone substitute material revealed a slightly better healing process contrary to Collagen-CP, and it can be caused due to its resorbability level. The mineral part of human bones consists of crystalline calcium phosphate, i.e. hydroxyapatite. Now, synthetic hydroxyapatite (CP) reveals osteoconductive and biocompatible properties. It is widely used in oral and orthopaedic surgery to fill bone defects thanks to its support proliferation properties. Nevertheless, its low bioresorbability effects remain after surgery for a long time [12, 19, 20]. Texture analyses have shown that Collagen-CP becomes similar to the reference bone just after few months of healing. The CP material is also considered, but we have to take into account its low level of bioresorbability and the need of longer period of observation of this material.
As it is known, collagen is one of the most important fibrous tissues which builds our bones and more. It has great properties as a BS, e.g. increase a platelet aggregation, very low host response [6, 7]. But also, it has a big disadvantage in its properties; i.e., it has not enough endurance as a osteoconductive material. Collagen does not hold its dimension through the healing process period and allows ingrowth of the soft tissue. Blending collagen with CP created something like modified collagen without his disadvantages and better properties. It becomes osteogenic, odontogenic and angiogenic, and as it was said, it keeps his dimension through the healing process period [6]. Collagen-CP revealed better clinical outcomes than pure collagen in this research taking into account the texture analyses of two-dimensional X-ray images.
The suspension of CP particles in the collagen scaffold (Collagen-CP material) improves the control of the arrangement of the biomaterial in the bone defect. The material granules do not disperse in the operating field. The use of this type of material is much easier than powders, pastes or loose granules in a difficult manual oral cavity environment. The limitation is that CP compositions are not available from the manufacturers.
Conclusion
This study revealed that Collagen-CP has a similar influence on bone healing process as CP as pure collagen. Mineralised tissue from CP appeared a little faster and had a microstructure similar to the reference bone as the texture analysed in the radiographs. Collagen-CP and pure collagen need more time for the healing process to complete.
References
Schaaf H, Lendeckel S, Howaldt HP, Streckbein P. Donor site morbidity after bone harvesting from the anterior iliac crest. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. 2010;109(1):52–8. https://doi.org/10.1016/j.tripleo.2009.08.023
Kalk WWI, Raghoebar GM, Jansma JBG (1996) Morbidity from iliac crest bone harvesting. J Oral Maxillofac Surg. 54:1424–1429
Goulet JA, Senunas LE, DeSilva GL, Greenfield MLVH (1997) Autogenous iliac crest bone graft: Complications and functional assessment. Clin Orthop Relat Res. 339:76–81
Pryor LS, Gage E, Langevin C-J, Herrera F, Breithaupt AD, Gordon CR et al (2009) Review of bone substitutes. Craniomaxillofac Trauma Reconstr 2(3–4):151–160
Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017;3(1).
Sarker B, Hum J, Nazhat SN, Boccaccini AR (2015) Combining collagen and bioactive glasses for bone tissue engineering: a review. Adv Healthc Mater. 4(2):176–194
Stenzel KH, Miyata T, Rubin AL (1974) Collagen as a biomaterial. Annu Rev Biophys Bioeng. 3(0):231–253
Kozakiewicz M, Maroniak-Hoffman A, Olszycki M (2010) Comparative analysis of three bone substitute materials based on co-occurrence matrix. Dent Med Probl. 47(1):23–29
Szczypiński P, Strzelecki M MA. MaZda – a software for texture analysis. In: Proceedings of ISITC. Jeonju, Republic of Korea, p. 245–249.
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda-A software package for image texture analysis. Comput Methods Programs Biomed. 94(1):66–76
Kołaciński M, Kozakiewicz M, Materka A (2015) Textural entropy as a potential feature for quantitative assessment of jaw bone healing process. Arch Med Sci. 11(1):78–84
Lurie AG (2019) Doses, benefits, safety, and risks in oral and maxillofacial diagnostic imaging. Health Phys. 116(2):163–169
Ranjanomennahary P, Ghalila SS, Malouche D, Marchadier A, Rachidi M, Benhamou C, Chappard C (2011) Comparison of radiograph-based texture analysis and bone mineral density with three-dimensional microarchitecture of trabecular bone. Med Phys. 38(1):420–428
Guo Z, Du X, Wang L, Li K, Jiao J, Guglielmi G, et al. Measurements of volumetric bone mineral density in the mandible do not predict spinal osteoporosis. Br Inst Radiol. 2019;49(3).
Suphanantachat S, Tantikul K, Tamsailom S, Kosalagood P, Nisapakultorn K, Tavedhikul K (2017) Comparison of clinical values between cone beam computed tomography and conventional intraoral radiography in periodontal and infrabony defect assessment. Dentomaxillofacial Radiol. 46(6):1–8
Aziman C, Hellén-Halme K, Shi XQ. A comparative study on image quality of two digital intraoral sensors. Dentomaxillofacial Radiol. 2019;48(7).
Buchanan A, Morales C, Looney S, Kalathingal S. Fish scale artefact on an intraoral imaging receptor. Dentomaxillofacial Radiol. 2017;46(8).
Zieliński KW, Strzelecki M (2002) Komputerowa analiza obrazu biomedycznego. Wstęp do morfometrii i patologii ilościowej. PWN, Warszawa
Yamaguchi K, Hirano T, Yoshida G, Iwasaki K (1995) Degradation-resistant character of synthetic hydroxyapatite blocks filled in bone defects. Biomaterials. 16(13):983–985
Kamitakahara M, Ohtsuki C, Miyazaki T (2008) Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J Biomater Appl. 23(3):197–212
Acknowledgements
The authors thank Medif the for Collagen-CP sample delivery (www.medif.com)
Funding
This research was funded by the Medical University, grant number 503/5-061-02/503-51-001-18, 503/5-061-02/503-51-001-17, 503/5-061-02/503-51-002-18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the University Ethical Committee RNN/485/11/KB. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wach, T., Kozakiewicz, M. Are recent available blended collagen-calcium phosphate better than collagen alone or crystalline calcium phosphate? Radiotextural analysis of a 1-year clinical trial. Clin Oral Invest 25, 3711–3718 (2021). https://doi.org/10.1007/s00784-020-03697-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03697-4