Abstract
Objectives
To assess the occurrence of coronal and root caries in adults with diabetes mellitus (DM).
Materials and methods
This study was performed accordingly to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. A search strategy was adapted for six databases, as well as gray literature. The risk of bias was assessed using the Joanna Briggs Institute critical appraisal tools for observational studies. Revman 5.3 was used to conduct five meta-analyses. The quality of evidence of meta-analysis was evaluated by GRADE.
Results
From 4047 titles retrieved, 29 studies were included in qualitative synthesis and 20 in quantitative synthesis. Findings showed a higher mean of DMFT in DM individuals compared with healthy controls (mean difference = 1.71; 95% CI 1.08–2.33; p < 0.01; I2 = 55%). Individuals with type 2 DM were three times more likely to have root caries in comparison with non-DM individuals (OR = 3.17; 95% CI 1.19–8.49; p = 0.02; I2 = 70%). Individuals with uncontrolled glycemic levels within the population with DM had higher prevalence of caries than individuals with controlled DM (OR = 3.82; 95% CI 1.12–13.07; p < 0.01; I2 = 89%; DMFT index mean difference = 2.61; 95% CI 1.14–4.08; p < 0.01; I2 = 75%).
Conclusions
Diabetes mellitus may increase the occurrence of coronal and root caries in adults. Poor glycemic control turned diabetic individuals more likely to have caries.
Clinical relevance
Dental caries can be an oral sign to indicate poor glycemic control in individuals with DM. Strategies to prevent root caries should be adopted in individuals with type 2 DM. Besides, dental and medical treatments should synergistically explore whether dietary habits are healthy for controlling both, DM and caries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A global trend of population aging has been associated with an increasing prevalence of chronic diseases such as diabetes mellitus (DM) [1]. It has become a worldwide epidemics: according to the International Diabetes Federation (IDF), 424.9 million individuals are diagnosed with this disease [2]. This metabolic disorder can lead to serious damages and complications in the whole organism that are directly linked to the level of disease control and duration.
It is now well established that periodontal disease is one of the most common complications of DM [3], and it may also impair glycemic control [4]. This fact increases the number of exposed root surfaces and, consequently, the risk of root caries in those individuals. Changes in the salivary flow and composition are also among the oral manifestations of DM [5]. Saliva has a well-known protective role in dental caries [6], and then, DM may predispose to dental caries by causing an imbalance of the oral environment, which favors a cariogenic microbiota establishment [5]. Furthermore, type 2 DM (T2D) and dental caries share the same causal factor: high carbohydrates intake. On the other hand, individuals with good glycemic control must present low sugar intake that could suggest a reduced risk of dysbiosis in the oral biofilm.
There is controversy about scientific evidence for increased risk of caries in individuals with DM. Previous studies have not considered the effect of the glycemic control, which could be linked to the lack of agreement between studies. Two systematic reviews have been conducted reporting oral health of individuals with DM [5, 7]. Mauri-Obradors et al. showed that 40% of studies found an increased caries levels in DM, but this was credited to the low salivary flow [5]. Such approach, however, has failed to address a systematic understanding due to a low number of included studies. Ismail et al. [7] investigated the oral health status of children with type 1 DM (T1D), concluding that it causes a significantly altered salivary flow and buffering capacity, and a consequent increased risk of caries. Nonetheless, no evidence was found regarding the relationship between caries and DM in adults. Although extensive research has been carried out on DM and dental caries, no single meta-analysis exists focusing on whether there are higher or lower chances of DM patients present caries lesions, as well as the correlation between dental caries and periodontal diseases in patients with DM. Therefore, the purpose of this study was to assess the occurrence of both coronal and root caries in adults with DM, by answering the following questions:
-
1.
Are individuals with DM more likely to have dental caries than non-DM?
-
2.
Is there any difference in the occurrence of dental caries among individuals with T1D and T2D?
-
3.
Is there any difference in the occurrence of dental caries among controlled and uncontrolled DM individuals?
Materials and methods
Protocol and registration
This systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) checklist [8]. A study protocol was designed and registered at the International Prospective Register of Systematic Review (PROSPERO) database, under the identification number CRD42018111057.
Eligibility criteria
The acronym PECOs (Population; Exposition; Comparator; Outcomes and Studies) was used to design each synthesis:
Participants/population = adults (> 35 years old).
Exposure(s) =
1. Individuals diagnosed with Diabetes Mellitus type 1 OR type 2;
2. Diabetic individuals with good glycemic index control.
Comparator(s)/control =
1. Individuals without Diabetes Mellitus or different type of diabetes;
2. Diabetic Individuals with poor glycemic index control.
Outcome=
Occurrence (prevalence, incidence, extension) of either coronal or root caries.
Inclusion criteria
Studies eligible for this review were observational studies (cross-sectional, case-control, or cohort studies), with no restriction of publication period. A dental caries index should be provided in either T1D or T2D, independently of the glycemic index state, in comparison with non-DM or different conditions of DM.
Exclusion criteria
Exclusion criteria were the following: (1) reviews, letters, personal opinions, book chapters, conference abstracts, randomized or non-randomized clinical trials, animal and in vitro studies; (2) studies performed in non-DM, individuals with Sjögren syndrome or studies in which samples included individuals with other severe systemic conditions; (3) studies in which DM were not the main systemic condition; (4) studies in which there was no comparison or control group; (5) studies that evaluated periodontal diseases or salivary flow as single outcome, and not dental caries; (6) studies performed in children, adolescents, or young adults/individuals under 35 years old; and (7) studies written in non-Latin alphabet.
Data sources and search strategy
The search process was performed in January 2020. Appendix 1 shows the search strategy. “Dental caries, periodontal disease, type 1 diabetes mellitus, type 2 diabetes mellitus, glycemic control” were used as main search elements that were adapted for each electronic database: MEDLINE via PubMed, LILACS, Web of Science, Scopus, Cochrane, and Livivo. Gray literature search was also performed in Google Scholar, ProQuest, and OpenGrey. Reference lists from included studies were assessed to identify other articles that could be selected. No language or interval time restrictions were applied in the search protocol. Duplicates were identified through EndNoteWeb (Clarivate Analytics, Mumbai) and then manually identified at Rayyan QCRI® (Qatar Computer Research Institute, Qatar).
Study selection and data extraction
The selection process was performed in two phases. First, titles and abstracts were screened by two independent and blinded reviewers (AKAL and JAS). This phase was carried out in a web application tool designed for systematic reviews (Rayyan QCRI®, Qatar Computing Research Institute). Any disagreement was discussed with an expert and the systematic review coordinator (CMS and NDT). In a second phase, reviewers (AKAL and JAS) gathered all the included studies by reading full articles independently. Once a study was selected for the second phase and the full text was not available in any way through online sources, it was performed a protocol in which an email requesting the full text was sent to authors every 3 days for 15 days. A final request via COMUT was tried by the end of this protocol.
Occurrence (prevalence, incidence, extension) of either coronal or root caries was assessed by DMFT (decayed, missing, filled teeth), DFT (decayed, filled teeth), DMFS (decayed, missing, filled surface), and DFS (decayed, filled surface) according to each primary study data. Prevalence of individuals with the outcome was also extracted from the studies. Data were extracted using a specific data extraction form (by AKAL and JAS). Any disagreement was discussed with an expert and the coordinator (CMS and NDT). The study’s authors were consulted to obtain any further information not available in the paper. When study results were published more than once or results were detailed in multiple publications, the most complete data set from all sources was identified, and the data was included only once.
Risk of bias and quality assessment
The risk of bias of the included studies was evaluated by two independent reviewers (AKAL and JAS) using the JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies [9]. The Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) was used to perform the risk of bias figure. Due to the design of included articles, besides all questions of the adopted appraisal tool are considered important, four of them were considered critical domains to this systematic review. These included: “Were the criteria for inclusion in the sample clearly defined?”; “Was the exposure measured in a valid and reliable way?”; “Were objective, standard criteria used for measurement of the condition?”; and “Were the outcomes measured in a valid and reliable way?”.
Criteria adopted to this systematic review for considering a high risk of bias were the following: two or more “no” answers in those critical domains; or one “no” and two or more “unclear” answers in those critical domains; or one “no” answer in a critical and two or more “no” answers in non-critical domains. Low risk of bias was considered when an article get a maximum one “no” answer or two “unclear” answers in non-critical domains. Articles were considered with a moderate risk of bias when it did not fit the criteria for high or low risk of bias. Decision on critical and non-critical domains and classification system was discussed with research team before the application of the instrument, as described at JBI Reviewer’s Manual [9].
Data analysis
Mean values of the main outcome were directly pooled with weighted mean differences (WMDs) and 95% confidence intervals. Statistical heterogeneity was estimated by the chi-square test (p < 0.05) and I-squared index (I2), which enabled to assess the magnitude of the inconsistency. Values of the I2 over 50% were classified as high, 25% to 50% moderate and less than 25% as low. Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) software was used to conduct five meta-analysis: two meta-analysis comparing coronal caries in DM and non-DM (prevalence or DMFT); two meta-analysis comparing coronal caries (prevalence or DMFT) in different levels of glycemic index control; and one meta-analysis comparing the prevalence of root caries in DM and non-DM.
Risk of bias across studies
The quality of evidence of meta-analysis was evaluated by GRADE (Grading of Recommendations Assessment, Development and Evaluation), performed on GRADEpro GDT (GRADEpro Guideline Development Tool [Software]; McMaster University, 2015, developed by Evidence Prime, Inc., available from gradepro.org).
Results
Studies selection and characteristics
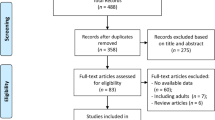
Searches retrieved 3456 titles through databases and 591 titles through gray literature. After removing duplicates, 2764 titles remained for screening. Figure 1 shows PRISMA flowchart depicting the identified, included, and excluded studies with reasons. Appendix 2 shows the excluded articles and reasons for exclusion. After phase 1, n = 125 studies remained for a full-text review, being 29 studies included in qualitative synthesis and 20 included in a quantitative synthesis (meta-analysis). All 29 included studies were cross-sectional and were published from 1988 [10] to 2019 [11, 12]. Studies were conducted in 18 different countries.
Risk of bias within studies
The quality assessment of the selected studies was determined (Appendix 3). Application of defined criteria resulted in eight articles with low risk of bias, nine with moderate, and 12 with high risk of bias. The highest risks of bias observed was for the definition of inclusion criteria and the appropriate description of the study objects, while the lowest risk of bias was observed for the statistical analysis, which shows that overall studies had good quantitative analysis (Fig. 2).
Qualitative and quantitative results for each PECOs question
Table 1 presents the studies characteristics. Results are shown below by the set of questions to be answered.
Are individuals with DM more likely to have dental caries than non-DM?
Nine studies compared the occurrence of caries in DM and non-DM [6, 10, 13, 17, 19,20,21,22,23]. These studies grouped individuals, regardless of the type of DM or glycemia level. Three studies compared the prevalence of caries in DM and non-DM, showing conflicting results [13, 17, 23].
Some studies distinguish the results of non-DM and a specific type of DM. A study compared T1D versus non-DM (DFS T1D = 0.24 ± 0.14; DFS non-DM = 0.28 ± 0.13) [24]. Other 12 studies evaluated T2D compared with a non-DM group [15, 18, 25,26,27,28,29,30,31,32,33,34]. Seven of them showed the prevalence of dental caries in T2D and non-DM individuals, but only one of them had a statistically significant higher prevalence of caries in T2D [15, 26,27,28,29, 31, 34].
Meta-analysis showed no statistically significant difference in the prevalence of dental caries between DM and non-DM (OR = 1.79; 95% CI 0.74–4.34; p = 0.20; I2 = 93%; random effect) (Fig. 3a). However, a higher mean of DMFT in DM individuals was revealed (mean difference = 1.71; 95% CI1.08–2.33; p < 0.01; I2 = 55%; random effect) (Fig. 3b). Furthermore, individuals with T2D were three times more likely to present root caries than non-DM (OR = 3.17; 95% CI 1.19–8.49; p = 0.02; I2 = 70%; random effect) (Fig. 3c). The level of evidence for the prevalence of caries and DMFT between DM and non-DM individuals was considered very low (GRADE system). This result was attributed to the inconsistency, the risk of bias, as well as to the design of included studies (cross-sectional studies). For the prevalence of root caries, the level of evidence was considered low, also essentially due to the design of included studies, which was considered a very severe risk of bias in this conservative analysis. GRADE evidence profile table is presented in Appendix 4.
Is there any difference in the occurrence of dental caries among individuals with T1D and T2D?
Studies that compared dental caries between T1D and T2D showed substantially higher caries levels in T2D individuals [19, 20, 35]. All of them used the DMFT index for the analysis. Individual data regarding the type of DM of the other studies were not recorded due to the range of age including children, adolescents, or young adults [19, 20]. There was not enough data for a meta-analysis.
Is there any difference in the occurrence of dental caries among controlled and uncontrolled DM individuals?
Eleven studies approached for the difference of dental caries occurrence between DM with different levels of glycemic control, considering individuals with controlled or uncontrolled glycemic level [11, 12, 14,15,16, 18, 22, 28, 31, 36, 37]. Eight studies evaluated the glycemic control through glycosylated hemoglobin concentration (HbA1c) [11, 12, 22, 28, 31, 36,37,38]. The value indicated as the limit to consider the DM as uncontrolled ranged from 9% [22, 28] to 6% (7 mmol/L) [38]. Fasting plasma glucose levels were also reported [14, 18]. Goyal et al. applied the fasting plasma glucose higher than 126 mg/dl as a cutoff for considering DM as uncontrolled.
Meta-analysis revealed that uncontrolled DM individuals are 3.8 times more likely to have coronal caries than controlled ones (OR = 3.82; 95% CI 1.12–13.07; p < 0.01; I2 = 89%; random effect) (Fig. 4a), as well as a higher DMFT mean (mean difference = 2.61; 95% CI 1.14–4.08; p < 0.01; I2 = 75%; random effect) (Fig. 4b). The level of evidence was considered very low for the both meta-analysis, mainly due to inconsistency, risk of bias, and the design of included studies (cross-sectional studies).
Forest plot for comparison of dental caries between individuals with controlled glycemia and uncontrolled glycemia: a prevalence of dental caries in controlled versus uncontrolled DM (diabetes mellitus) individuals; b DMFT in controlled versus uncontrolled DM individuals, assessed by Review Manager 5.3
Discussion
Either hyperglycemia caused by T1D or T2D can lead to several complications including the ones on the oral cavity [39]. These complications mostly happen when levels of blood glucose are not controlled. This systematic review and meta-analysis showed that individuals with DM have a higher number of teeth affected by coronal and root caries when compared to non-DM, and individuals with uncontrolled DM have more caries when compared to the ones with controlled DM. A higher prevalence of root caries in T2D in comparison with non-DM individuals was also observed. These findings may contribute to the field of oral health of DM individuals, showing that not only the periodontal diseases but also caries should be carefully controlled in individuals with DM. Dental and medical treatments should synergistically explore whether dietary habits are healthy for DM individuals. Furthermore, oral diseases may be increasingly considered deleterious markers for DM. We aimed to answer three questions in this study, which are discussed below:
Are individuals with DM more likely to have dental caries than non-DM?
The mechanisms that support a higher caries occurrence in DM individuals are not fully explored. Higher sugar intake is considered a common risk factor related to T2D and dental caries. It is also considered that the reduction of the salivary flow, caused by polypharmacy or by the disease per se, increases the risk of dental caries in DM. No statistically significant difference was observed for the prevalence of dental caries between DM and non-DM; however, the DMFT was higher in DM individuals (Fig. 3). A cutoff point considering prevalence can differ depending on the caries index applied, which may affect this result. The DMFT, a measurement of the extension of caries due to the history of the disease, could be related to a life of exposition to carbohydrate intake. An index that evaluates caries activity, such as the Nyvad index [40], would be more likely to demonstrate a real correlation between dental caries caused by DM. Overall, studies have not investigated confounding variables, such as the diet of the individuals, which possibly explain conflicting outcomes. Furthermore, few studies did not present a blood diagnosis for the control group confirming the absence of high levels of glucose, representing an important selection bias that could be reflecting the level of evidence [6, 10, 24, 26, 31, 32].
Is there any difference in the occurrence of dental caries between individuals with T1D and T2D?
The lifestyle is considered a critical risk factor for T2D [35]. The results of the qualitative analysis showed that individuals with T2D had higher DMFT than T1D ones. T1D individuals have multifactorial causes and it usually affects young people. The condition obligates them to have a more controlled diet during life, avoiding fermentable carbohydrates in most cases. Contrarily, T2D is directly related to high carbohydrate intake, which is also known as the crucial factor in the initiation of dental caries [41]. It is important to point out that both, T1D and T2D, could have reduced saliva flow. Although there were industry efforts to minimize the need for sugar consumption control to untie dental caries [42, 43], the results of the present study can further support that sugar intake restriction is important to caries control in individuals with T2D.
Is there any difference in the occurrence of dental caries between controlled and uncontrolled DM individuals?
Even though fasting plasma glucose (FPG) is often used for the diagnostic of DM [44], it can also be applied to evaluate the level of glycemic control of DM. However, HbA1c can acquire a more representative long-term mean of the glycemic control than FPG, due to the later glycemic levels changes in HbA1c than in FPG [45]. In this review, studies using HbA1c as a parameter were most frequently observed, but a high variation of the value used as a cutoff point (6% to 9%) could be a confounding factor to this analysis. It is important to observe that this variation is inherent to changes in the cutoff point over time in the medical protocols: the older the study, the higher the cutoff.
The hypothesis that there is a higher occurrence of caries when individuals have uncontrolled glycemic control was confirmed, suggesting that the sugar intake could be considered a common risk factor for those individuals for both, caries and DM. The knowledge of dental caries as a dysbiosis of resident microbiota of individuals with high sugar consumption [46] could explain this outcome. Individuals with DM that maintain medical and nutritional monitoring may intake fewer carbohydrates in general and, therefore, have a lower prevalence and extension of dental caries. Likewise, the higher the blood glucose level, the lower the saliva secretion [47] and the higher salivary levels of glucose. Although the interference of salivary glucose in caries is still unclear, it could lead a growth of acidogenic microorganisms [48] that could also prompt the environment to caries.
Quality of evidence, strength, and limitations
Despite our endeavors to include studies with high-quality evidence (extensive search in six databases and gray literature), only cross-sectional studies were found. Therefore, odds ratios of the meta-analysis could be overestimated. To overcome this issue, the GRADE criteria “risk of bias” was considered “very severe” for all meta-analysis (Appendix 4). Also, the risk of bias analysis was strict and conservative.
Even though most studies were well designed, no reference to the STROBE checklist was observed. Additionally, some studies did not present statistical analysis [10, 32, 37] or did not isolate the numerical values by age for all outcomes, including a range of age that does not meet the interests of this systematic review [19, 20]. These studies usually have convenience samples, getting demand from hospitals and, therefore, may present low external validity. The age limit imposed in inclusion criteria may have hampered the comparison of dental caries between T1D and T2D since studies of T1D were basically developed in children. However, this limitation was necessary, considering that grouping individuals with a large age variation could result in bias due to the duration of exposure to both DM and risk factors. In this study, the “P” from the acronyms PECOS was “adults > 35 years,” since including youth individuals could add a bias due to the difference in the occurrence of T2D [49]. This criteria definition was also based on the cumulative characteristics of dental caries. Younger individuals are unlikely to present root caries, and lifetime may favor a greater prevalence of overall lesions. Also, information is missing in most studies over the time of diagnosis of DM and it could add some bias in the analysis. More accurate studies considering this issue, as well as considering caries activity, are needed. We believe that new studies following the STROBE checklist should be developed to improve the level of evidence in this field. This study will be updated periodically as new evidence emerges.
Conclusions
It can be concluded for each review question that:
-
1.
Similar caries prevalence was observed in DM and non-DM individuals. DM individuals have higher DMFT index than non-DM ones. T2D individuals have higher prevalence of root caries in comparison with non-DM;
-
2.
Qualitative analysis showed that individuals with T2D had higher caries prevalence and DMFT than T1D in the same age group (no data available for quantitative synthesis);
-
3.
Uncontrolled DM individuals are more likely to have dental caries than controlled ones. DMFT was also higher in uncontrolled DM individuals when compared to the controlled ones;
Implications for clinical practice include the suggestion of dental caries as an oral sign of uncontrolled DM, and that strategies to prevent root caries should be adopted in individuals with DM. We suggest that individual and population-based strategies to control sugar consumption should be adopted for controlling both conditions. Future longitudinal researches are necessary using more appropriate parameters to evaluate caries activity.
References
World Health Organization, Global Health and Aging, Hum. Serv. (2011).
International Diabetes Federation, Eighth edition 2017, 2017. https://doi.org/10.1016/S0140-6736(16)31679-8.
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D (2018) Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 137:231–241. https://doi.org/10.1016/j.diabres.2017.12.001
Graziani F, Gennai S, Solini A, Petrini M (2017) A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J Clin Periodontol 45:1–21. https://doi.org/10.1111/jcpe.12837
Mauri-Obradors E, Estrugo-Devesa A, Jane-Salas E, Vinas M, Lopez-Lopez J (2017) Oral manifestations of diabetes mellitus. A systematic review. Med. Oral Patol. Oral y Cir. Bucal 22. https://doi.org/10.4317/medoral.21655
Seethalakshmi C, Jagat Reddy RC, Asifa N, Prabhu S (2016) Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: a cross-sectional study. J. Clin. Diagnostic Res. 10:ZC12–ZC14. https://doi.org/10.7860/JCDR/2016/16310.7351
Ismail AF, McGrath CP, Yiu CKY (2015) Oral health of children with type 1 diabetes mellitus: a systematic review. Diabetes Res. Clin. Pract. 108:369–381. https://doi.org/10.1016/j.diabres.2015.03.003
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, Clark J, Clarke M, Cook D, D’Amico R, Deeks JJ, Devereaux PJ, Dickersin K, Egger M, Ernst E, Gøtzsche PC, Grimshaw J, Guyatt G, Higgins J, Ioannidis JPA, Kleijnen J, Lang T, Magrini N, McNamee D, Moja L, Mulrow C, Napoli M, Oxman A, Pham B, Rennie D, Sampson M, Schulz KF, Shekelle PG, Tovey D, Tugwell P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6. https://doi.org/10.1371/journal.pmed.1000097
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P (2017) Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Inst. Rev. Man. 340:1–3. https://doi.org/10.1136/BMJ.C846
Albrecht M, Banoczy J, Tamas G (1988) Dental and oral symptoms of diabetes mellitus. Community Dent. Oral Epidemiol. 16:378–380. https://doi.org/10.1111/j.1600-0528.1988.tb00586.x
Schmolinsky J, Kocher T, Rathmann W, Völzke H, Pink C, Holtfreter B (2019) Diabetes status affects long-term changes in coronal caries-the SHIP Study. Sci. Rep. 9:1–11. https://doi.org/10.1038/s41598-019-51086-z
Suzuki S, Yoshino K, Takayanagi A, Ishizuka Y, Satou R, Nara N, Kamijo H, Sugihara N (2019) Relationship between blood HbA1c level and decayed teeth in patients with type 2 diabetes: a cross-sectional study. Bull. Tokyo Dent. Coll. 60:89–96. https://doi.org/10.2209/tdcpublication.2018-0039
Bharateesh J, Ahmed M, Kokila G (2012) Diabetes and oral health: a case-control study. Int. J. Prev. Med. 3:806–809
Goyal D, Kaur H, Jawanda MK, Verma S, Parhar S (2012) Salivary pH and dental caries in diabetes mellitus. Int. J. Oral Maxillofac. Pathol. 3:7–10
Malvania E, Sharma A, Sheth S, Mansuri S, Shaikh F, Sahani S (2017) Dental caries prevalence among type II diabetic and nondiabetic adults attending a hospital. J. Int. Soc. Prev. Community Dent. 6, 232. https://doi.org/10.4103/2231-0762.197202
Nimbal AV, Desai VC, Rathod SBAI A comparative study in the diabetes mellitus patients for oral manifestation at tertiary care hospital in North Karnataka. Asian Journal of Pharmaceutical and Clinical Reseach 9(2016):9–11
Ramana DPV, Rao VUM (2014) Status of periodontal diseases and dental caries among type-2 diabeteic and non-diabetic patients. Res. J. Pharm. Biol. Chem. Sci. 5:1251–1260
Sharma R, Raj SS, Vinod K, Reddy YG, Desai V, Bailoor D (2011) Comparison of oral health indicators in type 2 diabetes mellitus patients and controls. J. Indian Acad. Oral Med. Radiol. 23:168–172. https://doi.org/10.5005/jp-journals-10011-1121
Arrieta Blanco FJ, Arrieta Blanco JJ, Bartolomé Villar B, Jiménez Martínez E, Saavedra Vallejo P (2003) Problemas bucodentales en pacientes con diabetes mellitus. I: Índice de placa y caries dental. Med. Oral. 8:97–109 http://www.medicinaoral.com/medoralfree/v8i2/medoralv8i2p97.pdf.
Bacic M, Ciglar I, Granic M, Plancak D, Sutalo J (1989) Dental status in a group of adult diabetic patients. Community Dent Oral Epidemiol. 17:313–316
Iqbal S, Kazmi F, Asad S, Mumtaz M, Khan AA (2011) Dental caries and diabetes mellitus. Pakistan Oral Dent. J. 31:1–5
Lin BPJ (1999) Dental caries in older adults with diabetes mellitus. Spec. Care Dent. 19:8–14. https://doi.org/10.1111/j.1754-4505.1999.tb01361.x
Tanriverdi Ö, Ayman D, Türker N, Kaya B (2006) Investigation of prosthesis complications in diabetics and determination of prognosis of gingival diseases and dental caries. BTDMJB 2:50–54
Tavares M, Depaola P, Soparkar P, Joshipura K (1991) The prevalence of root caries in a diabetic population. J. Dent. Res. 70:979–983
Vaziri PB, Vahedi M, Abdollahzadeh SH, Abdolsamadi HR, Hajilooi M, Kasraee SH (2009) Evaluation of salivary albumin in diabetic patients. Iran. J. Public Health. 38:54–59
Zielinski MB, DMD DF, Forman LJ, Pomerantz SC (2002) Oral health in the elderly with non-insulin-dependent diabetes mellitus. Spec. Care Dentist. 22:94–98. https://doi.org/10.1111/j.1754-4505.1996.tb00844.x
Cherry-Peppers G, Ship JA (1993) Oral health in patients with type II diabetes and impaired glucose tolerance. Diabetes Care. 16:638–641. https://doi.org/10.2337/diacare.16.4.638
Collin H-L, Uusitupa M, Niskanen L, Koivisto A-M, Markkanen H, Meurman JH (1998) Caries in patients with non-insulin-dependent diabetes mellitus. Oral Surg. Oral Med. Oral Pathol. 85
Hintao J, Teanpaisan R, Chongsuvivatwong V, Ratarasan C, Dahlen G (2007) The microbiological profiles of saliva, supragingival and subgingival plaque and dental caries in adults with and without type 2 diabetes mellitus. Oral Microbiol. Immunol. 22:175–181. https://doi.org/10.1111/j.1399-302X.2007.00341.x
Leung WK, Siu S-C, Chu FCS, Wong KW, Jin L, Sham ASK, Tsang CSP, Samaranayake LP (2008) Oral health status of low-income, middle-aged to elderly Hong Kong Chinese with type 2 diabetes mellitus. Oral Health Prev. Dent. 6:105–118 http://www.ncbi.nlm.nih.gov/pubmed/18637388
Mohamed HG, Idris SB, Ahmed MF, Bøe OE, Mustafa K, Ibrahim SO, Anne NA (2013) Association between oral health status and type 2 diabetes mellitus among sudanese adults: a matched case-control study. PLoS One 8. https://doi.org/10.1371/journal.pone.0082158
Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF (2000) Type 2 diabetes and oral health: a comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract 50:27–34
Soto OPL, Rodriguez LDJ (2009) Conductas preventivas orales, actitudes, percepciones y estado de salud bucal en pacientes diabéticos. Hacia La Promoción La Salud. 14:13–23
Sukminingrum N, Ishak I, Masudi M, Alam MK (2013) Comparison of Decayed , Missing or filled teeth (DMFT) indexes between diabetic and non-diabetic patients. Int. Med. J.:1–4
Weinspach K, Staufenbiel I, Memenga-Nicksch S, Ernst S, Geurtsen W, Günay H (2013) Level of information about the relationship between diabetes mellitus and periodontitis-results from a nationwide diabetes information program. Eur. J. Med. Res. 18. https://doi.org/10.1186/2047-783X-18-6
Malicka B, Kaczmarek U (2011) Periodontal condition in adult patients with diabetes mellitus type 1 and 2. Dent. Med. Probl. 48:9–24
Commisso L, Monami M, Mannucci E (2011) Periodontal disease and oral hygiene habits in a type 2 diabetic population. Int. J. Dent. Hyg. 9:68–73. https://doi.org/10.1111/j.1601-5037.2009.00439.x
Malvania E, Sheth S, Sharma A, Mansuri S, Shaikh F, Sahani S (2016) Dental caries prevalence among type II diabetic and nondiabetic adults attending a hospital. J. Int. Soc. Prev. Community Dent 6:232. https://doi.org/10.4103/2231-0762.197202
World Health Organization, Global report on diabetes, Isbn. 978 (2016) 88. ISBN 978 92 4 156525 7.
Nyvad B, Baelum V (2018) Nyvad criteria for caries lesion activity and severity assessment: a validated approach for clinical management and research. Caries Res. 52:397–405. https://doi.org/10.1159/000480522
Sheiham A, James WPT (2015) Diet and dental caries: the pivotal role of free sugars reemphasized. J. Dent. Res. 94:1341–1347. https://doi.org/10.1177/0022034515590377
Kearns CE, Bero LA (2019) Conflicts of interest between the sugary food and beverage industry and dental research organisations: time for reform. Lancet. 394:194–196. https://doi.org/10.1016/S0140-6736(19)31277-2
Beaglehole RH, Beaglehole R (2019) Promoting radical action for global oral health: integration or independence? Lancet. 394:196–198. https://doi.org/10.1016/S0140-6736(19)31610-1
Hong S, Kang JG, Kim CS, Lee SJ, Lee CB, Ihm SH (2016) Fasting plasma glucose concentrations for specified HbA1c goals in Korean populations: data from the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-2, 2011). Diabetol. Metab. Syndr. 8:4–9. https://doi.org/10.1186/s13098-016-0179-8
Dekker JH, van der Does FE, Bouma M, de Vries H, Kostense PJ, Heine RJ, Kriegsman DM, van Eijk JT, de Sonnaville JJ (2007) How valid is fasting plasma glucose as a parameter of glycemic control in non-insulin-using patients with type 2 diabetes? Diabetes Care. 22:904–907. https://doi.org/10.2337/diacare.22.6.904
Marsh PD (1994) Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 8:263–271. https://doi.org/10.1177/08959374940080022001
Chávez EM, Borrell LN, Taylor GW, Ship JA (2001) A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 91:166–173. https://doi.org/10.1067/moe.2001.112054
Miko S, Ambrus SJ, Sahafi S, Dinya E, Tamas G, Albrecht MG (2010) Dental caries and adolescents with type 1 diabetes. Br. Dent. J. 208:E12–E12. https://doi.org/10.1038/sj.bdj.2010.290
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology 14:88–98
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ana Karolina Lima declares any conflict of interest. Juliana Amorim dos Santos declares any conflict of interest. Cristine Miron Stefani declares any conflict of interest. Adriano de Almeida de Lima declares any conflict of interest. Nailê Damé-Teixeira declares any conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 81 kb)
Rights and permissions
About this article
Cite this article
de Lima, A.K.A., Amorim dos Santos, J., Stefani, C.M. et al. Diabetes mellitus and poor glycemic control increase the occurrence of coronal and root caries: a systematic review and meta-analysis. Clin Oral Invest 24, 3801–3812 (2020). https://doi.org/10.1007/s00784-020-03531-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03531-x