Abstract
Objective
To assess any potential association between Helicobacter pylori and oral squamous cell carcinoma/oral potentially malignant disorders.
Materials and methods
Data mining was done using PubMed, Cochrane Library, and SCOPUS databases. The search included articles published up to May 2019. Newcastle-Ottawa scale was used to score the quality of the included articles. Data including the type of study, the sample population, the type of oral lesion, and the resulting statistical data were extracted.
Results
Out of 131 screened articles, only 15 articles fulfilled the eligibility criteria. Among the 15 studies, 9 focused on oral squamous cell carcinoma and 6 focused on oral potentially malignant disorders. Eight out of the 9 oral squamous cell carcinoma studies were included in the meta-analysis. Forest plot was generated using the odds ratio and confidence intervals calculated for each of the included studies. Due to the lack of sufficient studies, the meta-analysis was not performed for oral potentially malignant disorders.
Conclusion
Due to the contradictory results of the included studies, it was not possible to make any conclusive statement on the potential association of H. pylori with oral squamous cell carcinoma. The variations in the methodology, especially the differences in the sensitivity/specificity of the diagnostic modalities could be the cause for differential results.
Clinical relevance
Although the association of H. pylori with oral squamous cell carcinoma could not be confirmed, it is vital to reduce the excess oral microbial load, especially in patients exhibiting oral mucosal changes with no history of associated risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
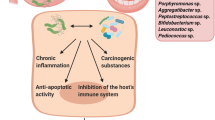
Helicobacter pylori (H. pylori), a Gram-negative microaerophilic bacteria, usually found in the stomach, are frequently associated with gastric and duodenal ulcers [1]. Marshall and Robin Warren have been awarded the Nobel Prize in 2005 by Karolinska Institute, Stockholm, for their discovery of the role of the bacterium in gastritis and peptic ulcers [2]. The bacterium has been established as a co-carcinogen in gastric cancer along with dietary carcinogens and predisposing genetic makeup [3]. Various virulence factors accounted for gastric cancer development include cytotoxin-associated gene A (cagA) and CagA protein (CagA), CagL, vacuolating cytotoxin (VacA), and outer inflammatory protein (OipA) [4]. Various theories have been put forth to establish the role of H. pylori in gastric cancer pathogenesis. According to a meta-analysis conducted in the year 2009 by Lee YC et al., eradication of H. pylori decreases the risk of gastric cancer in previously infected individuals, suggesting the continued presence of H. pylori is required for the carcinogenic effect. H. pylori has been associated with gastric cancer as a potential risk factor, with a relative risk of 65% [5, 6]. Recent studies have shown the presence of H. pylori in the oral cavity of individuals with gastritis/peptic ulcers. In the oral cavity, H. pylori resided primarily in the saliva, supra and subgingival plaques, and periodontal pockets ≥ 5 mm in depth [7].
Conflicting results have been reported in the literature on the isolation of H. pylori from dental plaque. Several studies indicate a low prevalence of H. pylori in the oral cavity of their patients and consider the oral cavity environment to be insignificant for this bacterium [8]. There are studies suggesting that there is only a transient presence of H. pylori in the oral cavity, and the antagonist effects of other oral bacteria against H. pylori, makes it difficult for the bacterium to show any long-term oral colonization [9]. However, authors who found this bacterium in almost all of their study population consider the bacteria to be a part of the normal oral microbiota in the mouth and that the oral cavity may act as a reservoir for re-infection of the stomach [10].
At present, the most common and established risk factors for oral potentially malignant disorders (OPMDs) and oral cancer are tobacco and alcohol. The implementation of restriction in the use of these associated risk factor, especially tobacco products, has been seen in the past few decades in order to curb the growing prevalence of oral cancer. Although tobacco control is on the rise, there is an increasing statistic on the prevalence of oral cancer, which is partly attributed to microbial agents. Unlike oropharyngeal cancer, evidence for an association between microbes such as HPV and oral cancer/OPMDs is not conclusive. Thus, many researchers consider microbes including HPV to be a risk factor only in oropharyngeal cancer. Similar to HPV, there is an increasing number of studies isolating H. pylori in oral cancer and OPMDs, although the nature of the relationship is not elicited. At present, H. pylori is considered as a risk factor for gastric cancer, but its role in oral carcinogenesis is inconclusive [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Thus, the present systematic review and meta-analysis were planned to provide comprehensive data on the current evidence of any potential association between H. pylori and oral cancer/OPMDs.
Methods
Protocol and registration
The present systematic review was performed in accordance with PRISMA (preferred reporting items for systematic reviews and meta-analyses) and MOOSE (meta-analysis of observational studies in epidemiology) guidelines [15]. The systematic review is registered at the International Prospective Register of Systematic Reviews (ID CRD42017059249)
Inclusion criteria
The population (P), intervention (I), comparison (C), outcomes (O), studies (S) framework was used to frame the focussed question. P represents the OPMD and/or oral cancer cases; I represents the diagnostic modality used to identify H. pylori; C represents healthy individuals with no history of OPMD and/or oral cancer; O represents the risk of developing OPMD and/or oral cancer in the presence of H. pylori; and S represents the studies that assessed the association between H. pylori and OPMD/oral cancer. Only the articles in the English language were included.
Exclusion criteria
Exclusion criteria include experimental studies, narrative and systematic reviews, case reports/series, letter to the editor, opinion pieces, conference abstracts, and articles in a language other than English.
Focused question
“Is H. pylori presence associated with increased risk of developing OPMD and/or oral cancer?”
Search strategy
Data mining was done using PubMed, Cochrane Library, and SCOPUS databases. The search included articles published until 25 May 2019. All the screened articles were in turn manually cross-referred to check for any further relevant articles.
The following free terms and medical subject headings (MeSH) were used in various combinations for data mining, i.e., Helicobacter pylori and oral cancer, Helicobacter pylori and oral squamous cell carcinoma, Helicobacter pylori and oral potentially malignant disorders, Helicobacter pylori and oral lichen planus, Helicobacter pylori and oral leukoplakia, and Helicobacter pylori and oral submucous fibrosis.
Study selection and data extraction
Two reviewers (AG and SK) used the selection criteria and independently selected the studies to be included in the systematic review. The selection process consisted of two steps. In the first step, the reviewers screened the titles and the abstracts to identify potential articles. The full texts of the articles selected in the first step were scrutinized using the selection criteria. Each step of the review process was conducted independently by the two reviewers (AG and SK). Only articles wherein both the reviewers had a consensus were included in the systematic review.
The included studies were analyzed by the reviewers, and the data including the type of study, the sample population, the type of oral lesion included (OPMD/oral cancer), and the resulting statistical data were obtained.
Risk of bias assessment
Newcastle-Ottawa scale (NOS) was used to score the quality of the included articles using parameters such as comparability, outcome/exposure, and selection. The maximum score given for the selection was 4, comparability was 2, and outcome/exposure was 4. Thus, a single study could collect a maximum of 10 points in total. A score above or equal to 7 was considered as good.
Statistical analysis
Since there were two reviewers, potential inter-observer bias was assessed by Cohen’s kappa coefficient (κ). All statistical data were reviewed, and NOS was used to score the overall quality.
Results
Study selection
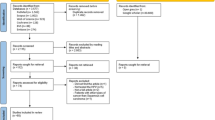
Figures 1 and 2 summarize the workflow of the systematic review for OPMDs and oral squamous cell carcinoma (OSCCs), respectively. Out of the 73 potentially relevant articles identified in the PubMed, Scopus, and Cochrane databases, 14 articles were selected for detailed assessment discussing the role of H. pylori in OSCC; out of which, 9 articles were selected for systematic review [20,21,22,23,24,25,26,27,28]. Similarly, out of 85 potentially relevant articles identified in the three databases, 13 articles were selected for detailed assessment discussing the role of H. pylori in OPMDs; out of which, 6 articles were selected for systematic review [11,12,13,14,15,16]. Studies associating H. pylori with potentially malignant disorders not related to the oral cavity like cutaneous lichen planus and vocal fold leukoplakia were excluded [16, 17]. Studies discussing the mere presence of H. pylori in the oral cavity without discussing its role in OPMDs and OSCCs were excluded [18, 19]. Kappa value for the inter-observer reliability between the two reviewers was 0.98 and 1 for the first and the second step of the review, respectively.
Study characteristics
Studies discussing the role of H. pylori in OPMDs were all cross-sectional design. Two studies were from India, one of each was from Iran, Egypt, Poland, and Tokyo. Studies based in Iran and Egypt mentioned about age and gender matching between cases and controls; whereas, the study done in Poland did age matching only. However, Indian and Tokyo studies did not mention anything about age and gender matching. Studies conducted by Kazanowska et al. in 2015 in Poland and Attia et al. in 2009 in Egypt used PCR as a method of H. pylori detection. Other three studies including Shimoyama et al., Pourshahidi et al., Hulimavu et al., and Sharma et al. used immunohistochemistry (IHC), ELISA, and microbial cultures, respectively.
Out of the 9 studies discussing the role of H. pylori with OSCC, 7 studies were done on Asian population including China, Japan, Sri Lanka, and India. The remaining two were from Germany and Iran. Six studies did not mention anything about age and gender matching of the individuals between OSCC cases and healthy controls. The method of detection of the association of H. pylori with OSCC included IHC, PCR, ELISA, culture, and special stain Giemsa.
Results of the studies
The results were largely conflicting for OPMDs, with 3 studies (Kazanowska et al., Attia et al., and Sharma et al.) associating H. pylori with OPMDs, and 3 studies (Shimoyama et al., Pourshahidi et al., and Hulimavu et al.) showing no association. Similar to OPMDs, 4 studies associated H. pylori with OSCC, while 4 studies reported no significant increase in H. pylori compared with healthy controls. The conflicting reports in OSCC are augmented by the Meng et al. findings. They found an inverse correlation with healthy subjects having a higher prevalence of H. pylori than oral cancer patients. Tables 1 and 2 summarize the data extracted from the included OPMD and OSCC articles, respectively.
Discussion
Association of H. pylori and OPMDs
Kazanowska-Dygdala et al. [11] compared the H. pylori prevalence with the oral health status between OPMDs and healthy individuals through PCR. The OPMDs included oral lichen planus (OLP) and oral leukoplakia (OL). A total of 23.6% of OLP and 20% of OL were positive for H. pylori, while the control subjects were all negative. The oral health status as assessed by the plaque index, bleeding on probing, and the periodontal pocket depth was found to be significantly poorer in the OLP and OL groups than the control subjects. The study had matched potential confounders such as the age and gender between the comparative groups. The major limiting factor of the study was the lack of histopathological confirmation of OLP and the absence of cell atypia in OL. As there is no evidence to confirm OLP diagnosis and that OL without atypia is most likely hyperkeratosis, the results of the Kazanowska-Dygdala et al. study may not be a true representation of the prevalence of H. pylori in OPMDs [11].
Pourshahidi et al. assessed the H. pylori IgG levels in OLP cases in southwestern Iran through serological examination. The study included 41 clinically diagnosed OLP cases and 82 gender and age-matched controls. They did not find any significant difference between the OLP and the healthy individuals. Similar to the Kazanowska-Dygdala et al. study, even Pourshahidi et al. did not confirm the OLP diagnosis histopathologically. Thus, the results of the Pourshahidi et al. study cannot be used to confirm the association between H. pylori and OPMDs [12].
Attia et al. compared the association of H. pylori between erosive and non-erosive lichen planus with the help of both PCR. Diagnosis of OLP was confirmed histopathologically. The results showed that erosive OLP carried a higher prevalence of H. pylori than non-erosive OLP (p = 0.001). The major limitation of the study was the small sample size (40 samples), which in turn was subdivided into 2 groups (of 20 each) according to the clinical presentation of OLP. Thus, although the study provides a histopathological confirmation of the disease, the limited sample size necessitates further evidence from large-scale samples to confirm the findings [13].
Hulimavu et al. investigated the presence of H. pylori in OLP, normal buccal mucosa. Biopsies of peptic ulcer were taken as positive controls. IHC was used to identify the presence of H. pylori. While the control samples (peptic ulcer tissues) were positive, none of the OLP or normal buccal mucosa samples were positive for H. pylori. Based on the results, the authors questioned the association of H. pylori in OLP. The major limitation of the study was the use of IHC, which is relatively less sensitive than PCR. In addition, the control samples and the OLP cases were not matched for potential confounding factors including age and gender [14].
Sharma et al. assessed the prevalence of H. pylori in OPMDs and OSCC. Unlike previous studies, Sharma et al. used salivary samples for estimating the presence of H. pylori. The OPMDs included oral submucous fibrosis (OSMF) and OL. The study used a specialized Campylobacter Supplement medium (Skirrow’s) to detect H. pylori. Overall, OSCC showed a higher prevalence than OPMDs and healthy subjects. Within OPMDs, OSMF showed higher H. pylori prevalence than OL. The higher prevalence of H. pylori in OSMF was attributed to the salivary pH alterations caused by lime. Lime forms a major etiologic factor along with areca nut and tobacco for OSMF. Lime-induced disturbance in the buffering capability of saliva was presumed to have promoted H. pylori growth [26].
Shimoyama et al. analyzed 22 oral ulcerative lesions including recurrent aphthous stomatitis, herpes simplex virus, and lichen planus. They used microbial culture to detect the association of the bacterium with the disease which was then confirmed with the help of ELISA. Though none of the three lichen planus samples shows positivity for H. pylori with culture, one of the samples was positive with ELISA but was not significant enough to infer association [15].
Association of H. pylori and oral cancer
Similar to HPV studies with cancer, most studies on H. pylori have included cancer cases classified under general terms such as oropharyngeal cancers and head and neck cancers. As the results of these studies could misled (over-represent) the prevalence of H. pylori in oral cancer, the present systematic review selected only those studies which either have assessed only oral cancer or have specified separate statistics for oral cancer.
Dayama et al. compared the prevalence of H. pylori between 20 oral cancer cases and 20 age and gender-matched healthy subjects using culture and PCR. However, the odds ratio by both culture and PCR (3.0, 95% CI 0.342-6.4 and 1.5, 95% CI 0.28–8.03, respectively) were statistically non-significant. The small sample size could have been the cause of the insignificant result [20].
Fernando et al. assessed the prevalence of H. pylori in oral cancer and healthy individuals. The samples were further subdivided based on the history of betel chewing. The oral mucosal samples were subjected to culture to identify H. pylori. The serological samples of the subjects were studied for H. pylori IgG antibodies. The results showed that the H. pylori prevalence varied significantly between betel chewers and non-chewers (chi square p < 0.05), irrespective of the presence of cancer. Thus, the presence of betel chewing was proposed as a potential contributing factor to H. pylori rather than the presence of oral cancer [21]. Areca nut extracts were hypothesized to have modulated the periodontal microenvironment promoting bacterial colonization as proposed by the previous studies [29,30,31,32].
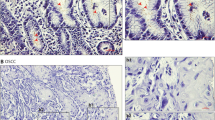
Grimm et al. showed an increased immunohistochemical expression of H. pylori and TLR5 in OSCC. Though TLR5 ligands have been associated with detection of bacterial flagella and promoting migration and proliferation of cancer cells including non-small-cell lung cancer cells and cervical cancer cells [33], the present study could not prove the association of TLR5 to the increased progression of OSCC. However, the authors reported the study to be the first of its kind providing immunohistochemical evidence of H. pylori expression in OSCC [22].
Ravali did a cross-sectional study on H. pylori prevalence in 60 OSCC subjects and 60 age and gender-matched healthy controls with ELISA. The author reported a significant association (p < 0.05) of H. pylori with OSCC [25].
Sharma et al. did a microbial culture study on a sample size of 50, each for healthy individuals and patients with OPMDs and OSCC. The incidence of H. pylori in OPMDs was more than that in the healthy individuals and was highest in OSCC. A significant (p < 0.05) association was noted between H. pylori and OPMDs/OSCC. They also reported an increased incidence of H. pylori count in OSMF compared with leucoplakia [26].
Irani et al. through immunohistochemistry reported an increased incidence of H. pylori in ulcerative and inflammatory lesions as compared with OSCC. They also reported the incidence pattern of H. pylori in normal oral tissues, with H. pylori positivity most commonly being found in tonsils and tongue followed by buccal mucosa and oropharynx [27].
Gupta et al. presented a non-significant association of H. pylori with OSCC through PCR, but suggested the need for more studies in the field with large sample size and proper matching of cases and controls [28].
In contrary to the above-mentioned studies, an inverse association between H. pylori and oral cancer was found by Meng et al. They found that the H. pylori prevalence was statistically lower (Spearman’s correlation coefficient = − 0.191, p = 0.012) in OSCC cases than healthy controls. Also, there was no significant correlation between the presence of H. pylori and lymph node metastasis and tumor size. The finding of inverse correlation led to the authors discussing the potential protective role of H. pylori infection in the cancers of the esophagus and oral cancers [30,31,32]. However, among several limitations of the Meng et al. study, including small sample size, was the lack of information about habits of OSCC patients which could have affected the results of the study [23].
Okuda et al. studied the presence of H. pylori in gastric and oral samples through RT-PCR. The study results showed that the subjects who are positive for H. pylori in oral swab samples were also positive for gastric samples. H. pylori was reported to be present in the oral cavity only as a transient organism and were presumed to be derived from the stomach [24].
There were notable differences in the methodology between OSCC studies with and without significant H. pylori association. Although PCR, ELISA, and culture were employed in at least one of the studies with [20, 25, 26] and without [21, 24] H. pylori association, there were two studies with positive H. pylori correlation [22, 27] which exclusively used only IHC. It is possible that the relatively lower sensitivity and specificity of IHC compared with PCR could have resulted in a positive correlation. There were only minor differences between the sample size of the positive correlation studies (32 controls and 83 cases by Irani et al.; 50 controls and 50 cases by Sharma et al.; 60 cases and 60 controls by Ravali; 20 cases and 20 controls by Dayama et al.; 191 cases and 10 controls by Grimm et al.) and the studies with no significant associations (58 cases and 58 controls by Okuda et al.; 53 cases and 120 control by Fernando et al.; 40 cases and 10 controls by Gupta et al.) [20, 21, 24,25,26]. In addition, despite employing strong diagnostic modalities (ELISA, PCR, and Giemsa) and having sample size (68 cases and 104 control) relatively similar to other included studies, a negative H. pylori correlation (greater H. pylori detection in the control than OSCC) was obtained by Meng et al. [23].
Two of the 3 studies exhibiting no significant H. pylori association had employed PCR as the diagnostic modality. In addition, as mentioned above, the Meng et al. study which revealed a negative correlation with H. pylori had also employed PCR. Thus, given the higher sensitivity of PCR compared with the other diagnostic modalities used in the included studies, the negative correlation and lack of significant association cannot be ignored. A possible explanation for the contradictory results could be due to the population-specific high H. pylori prevalence. All the studies with no association and negative correlation were from the Asian population. In Asia, the prevalence of H. pylori is in the range of 54 to 76% [34]. Thus, the control samples of the included studies from Asia could have exhibited H. pylori levels similar to OSCC cases. In such cases, it is not clear if the H. pylori in the OSCC are chance findings in a highly H. pylori prevalent population or has a potential causal association.
In addition to the qualitative analysis, the potential association between H. pylori and OSCC was evaluated quantitatively through a meta-analysis. Unlike OSCC, there was not a sufficient number of OPMD studies available for a meta-analysis, thus, only qualitative analysis was carried out for assessing the association between OPMD and H. pylori.
Meta-analysis
H. pylori and OPMDs
Out of the 6 studies included to associate H. pylori with OPMDs, 3 studies (Attia et al., Shimoyama et al., and Hulimavu et al.) did not take healthy controls in their studies. Hence, it was not possible to calculate the odds ratio in those studies. The fourth study by Kazanowska et al. could not find any association in healthy controls making the odds ratio value infinity. Thus, these four studies were excluded from the meta-analysis. A quantitative analysis using only the 2 studies would not provide sufficient evidence for a conclusive inference. Thus, OPMDs association to H. pylori was not assessed using meta-analysis.
H. pylori and OSCC
Out of the 9 studies included in the systematic review, only 8 were included for the meta-analysis. One study (Grimm et al.) was excluded as the odds ratio calculated was infinity. A random effect model was used for the meta-analysis where overall odds ratio calculated was 2.29 with 0.61–8.68 as 95% confidence interval. The odds ratio of all the included studies has been provided in Table 3. Out of the 8 studies subjected to meta-analysis, 3 studies (Ravali, Sharma, et al., and Irani et al.) showed a positive association of H. pylori with OSCC. However, the confidence interval in the study by Ravali was very broad. Two studies (Xiu et al. and Okuda et al.) reported an inverse correlation of H. pylori with OSCC. The remaining 3 studies (Dayama et al., Fernando et al., and Gupta et al.) did not show any significant association. Overall, the meta-analysis revealed a non-significant association between the bacterium and OSCC (Fig. 3).
Conclusion
All the included studies were designed as case-control studies. The most significant variation between the studies was the different diagnostic modalities (IHC, ELISA, PCR, culture) used for the detection of H. pylori. Due to the heterogeneity of the included studies, individual odds ratio and confidence intervals were calculated for each study. The data from the included studies showed contradictory results, which in turn could be attributed to the variations in the methodology. Thus, based on the results of the included studies, it was not possible to make any conclusive statement on the potential association of H. pylori with oral cancer. Conclusive evidence of the true carcinogenic potential of H. pylori in the oral mucosa would require further large-scale multi-center prospective in vitro and in vivo studies.
References
Brown LM (2000) Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 22(2):283–297
Blaser MJ (2006) Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep 7(10):956–960
Liu H, Merrell DS, Semino-Mora C, Goldman M, Rahman A, Mog S, Dubois A (2009) Diet synergistically affects helicobacter pylori-induced gastric carcinogenesis in non-human primates. Gastroenterology 137(4):1367–1379
Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F (2009) Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 151(2):121–128
Sokic-Milutinovic A, Popovic D, Alempijevic T, Dragasevic S, Lukic S, Pavlovic-Markovic A (2014) Helicobacter pylori infection and gastric cancer — is eradication enough to prevent gastric cancer. In: Roesler B (ed) Trends in Helicobacter pylori infection. In Tech, Rijeka, pp 155–173
Dye BA, Kruszon-Moran D, McQuillan G (2002) The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am J Public Health 92:1809–1815
Adler I, Muiño A, Aguas S, Harada L, Diaz M, Lence A, Labbrozzi M, Muiño JM, Elsner B, Avagnina A, Denninghoff V (2014) Helicobacter pylori and oral pathology: relationship with the gastric infection. World J Gastroenterol 20(29):9922–9935
Oliver BJ, Bond RP, van Zyl WB, Delport M, Slavik T, Ziady C, Terhaar Sive Droste JS, Lastovica A, van der Merwe SW (2006) Absence of Helicobacter pylori within the oral cavities of members of a healthy South African community. J Clin Microbiol 44:635–636
Ishihara K, Miura T, Kimizuka R, Ebihara Y, Mizuno Y, Okuda K (1997) Oral bacteria inhibit Helicobacter pylori growth. FEMS Microbiol Lett 152:355–361
Umeda M, Kobayashi H, Takeuchi Y, Hayashi J, Morotome-Hayashi Y, Yano K, Aoki A, Ohkusa T, Ishikawa I (2003) High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol 74:129–134
Kazanowska-Dygdala M, Dus I, Radwan-Oczko M (2016) The presence of Helicobacter pylori in oral cavities of patients with leukoplakia and oral lichen planus. J Appl Oral Sci 24(1):18–23
Pourshahidi S, Fakhri F, Ebrahimi H, Fakhraei B, Alipour A, Ghapanchi J, Farjadian S (2012) Lack of association between Helicobacter pylori infection and oral lichen planus. Asian Pac J Cancer Prev 13:1745–1747
Attia EAS, Abdel Fattah NSA, Abdella HM (2009) Upper gastrointestinal findings and detection of Helicobacter pylori in patients with oral lichen planus. Clin Exp Dermatol 35:355–360
Hulimavu SR, Mohanty L, Tondikulam NV, Shenoy S, Jamadar S, Bhadranna A (2014) No evidence for Helicobacter pylori in oral lichen planus. J Oral Pathol Med 43:576–578
Shimoyama T, Horie N, Kato T, Kaneko T, Komiyamat K (2000) Helicobacter pylori in oral ulcerations. J Oral Sci 42:225–229
Zenouz AT, Mehdipour M, Heydarlou MJ, Gholizadeh N (2010) Relationship between Lichen planus and Helicobacter pylori infection. J Dent Res Dent Clin Dent Prospect 4(1):17–20
Chen M, Chen J, Yang Y, Cheng L, Wu HT (2018) Possible association between Helicobacter pylori infection and vocal fold leukoplakia. Head Neck:1–10
Veiga N, Pereira C, Resende C, Amaral O, Ferreira M, Nelas P, Chaves C, Duarte J, Cirnes L, Machado JC, Ferreira P, Correia IJ (2015) Oral and gastric Helicobacter pylori: effects and associations. PLoS One 10(5):e0126923. https://doi.org/10.1371/journal.pone.0126923
Viganò L, Cinzia C, Oliveira A, Guerrieri P (2018) Helicobacter pylori: is there an association with oral pathologies? A Traditional Review. Acta Sci Microbiol:32–39
Dayama A, Srivastava V, Shukla M, Singh R, Pandey M (2011) Helicobacter pylori and oral cancer: Possible association in a preliminary case control study. Asian Pac J Cancer Prev 12:1333–1336
Fernando N, Jayakumar G, Perera N, Amarasingha I, Meedin F, Holton J (2009) Presence of Helicobacter pylori in betel chewers and non-betel chewers with and without oral cancers. BMC Oral Health 9:23–28
Grimm M, Munz A, Exarchou A, Polligkeit J, Reinert S (2014) Immunohistochemical detection of Helicobacter pylori without association of TLR5 expression in oral squamous cell carcinoma. J Oral Pathol Med 43:35–44
Meng X, Wang Q, He C, Chen M, Liu J, Liu W, Yuan Y (2016) An inverse association of Helicobacter pylori infection with oral squamous cell carcinoma. J Oral Pathol Med 45:17–22
Okuda K, Ishihara K, Miura T, Katakura A, Noma H, Ebihara Y (2000) Helicobacter pylori may have only a transient presence in the oral cavity and on the surface of oral cancer. Microbiol Immunol 44(5):385–388
Ravali CT (2017) Association of Helicobacter pylori in oral cancer patients. Int J Appl Dent Sci 3(3):185–192
Sharma P, Gawande M, Chaudhary M (2015) Evaluation of prevalence of bacteria Helicobacter pylori in potentially malignant disorders and oral squamous cell carcinoma. World J Dent 6(2):82–86
Irani S, Esfahani AM, Zerehpoush FB (2013) Detection of Helicobacter pylori in oral lesions. J Dent Res Dent Clin Dent Prospect 7(4):230–237
Gupta AA, Kheur S, Mamatha GS, Shetty L, Kheur M (2016) Helicobacter pylori as a risk indicator of oral squamous cell carcinoma – a PCR based study. Int Jr Current Research 8(7):34109–34119
Chang MC, Kuo MY, Hahn LJ, Hsieh CC, Lin SK, Jeng JH (1998) Areca nut extract inhibits the growth, attachment, and matrix protein synthesis of cultured human gingival fibroblasts. J Periodontol 69(10):1092–1097
Hung SL, Chen YL, Wan HC, Liu TY, Chen YT, Ling LJ (2000) Effects of areca nut extracts on the functions of human neutrophils in vitro. J Periodontal Res 35(4):186–193
de Miranda CM, van Wyk CW, van der Biji P, Basson NJ (1996) The effect of areca nut on salivary and selected oral microorganisms. Int Dent J 46(4):350–356
Gebara EC, Pannuti C, Faria CM, Chehter L, Mayer MP, Lima LA (2004) Prevalence of Helicobacter pylori detected by polymerase chain reaction in the oral cavity of periodontitis patients. Oral Microbiol Immunol 19(4):277–280
Shaykhiev R, Behr J, Bals R (2008) Microbial patterns signaling via Toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS One 3:e1393
Eusebi LH, Zagari RM, Bazzoli F (2014) Epidemiology of Helicobacter pylori infection. Helicobacter. 19(Suppl 1):1–5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, A.A., Kheur, S., Raj, A.T. et al. Association of Helicobacter pylori with oral potentially malignant disorders and oral squamous cell carcinoma—a systematic review and meta-analysis. Clin Oral Invest 24, 13–23 (2020). https://doi.org/10.1007/s00784-019-03125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03125-2