Abstract
Objective
This in vitro study compared the penetration, pH, calcium ion release, solubility, and intradentinal decontamination capacity of calcium hydroxide (CH) pastes with different vehicles and additives.
Materials and methods
Infected standard bovine dentine contaminated with Enterococcus faecalis were treated with propolis extract, chlorhexidine, and camphorated paramonochlorophenol (CPMC) loaded in CH paste for the bacterial viability evaluation made by confocal laser scanning microscopy (CLSM) and microbiological culture. Beside this, 50 acrylic teeth were filled with the previously mentioned pastes to evaluate the pH and calcium ion release (pHmeter and atomic absorption spectrophotometer at time intervals of 7, 15, and 30 days) and solubility (micro-computed tomographic imaging before and after 15 days).
Results
After treatment, all samples decreased intra-dentinal contamination, specially, the CH paste with CPMC. There was no statistically significant difference between the groups when evaluating the intra-canal paste penetration. In the pH measurements, CH with distilled water showed the smallest pH values. Regardless the solubility percentage of the pastes, the paste of CH + PG presented the highest values.
Conclusion
The vehicles and additives tested may increase CH antimicrobial effect, but with small differences. In general, all CH pastes tested here were effective in reducing Enterococcus faecalis and were similar in the penetration, pH, calcium ion release, and solubility of calcium hydroxide when compared to distilled water.
Clinical relevance
The use of calcium hydroxide pastes as intracanal medication with an aqueous or viscous vehicle, as propylene glycol, can be useful, since all formulations of the tested pastes resulted in great bacterial reduction inside root canals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endodontic treatment success depends on biomechanical preparation, microbial control, and complete filling of the root canal system to promote significant reduction in microorganisms and prevent the infection process. However, some microorganisms can survive due to anatomic complexities of root canal system [1,2,3,4] and consequent limited access of instruments and irrigating solutions, and others can also infiltrate through unsatisfactory temporary sealing’s [1, 3, 5, 6]. In this sense, the use of different alternatives to increase endodontic treatment success needs to be investigated. Thus, intracanal medications with antimicrobial action between sessions are used to complement root canal disinfection, as a tentative to prevent a new infection and eliminate remnant bacteria [2, 4].
The use of calcium hydroxide (CH) paste is usually preferred as intracanal medication due to its antimicrobial and biological properties [5], attributed to the alkaline pH and calcium ion release [6]. As a strategy to improve their antimicrobial properties, natural and synthetic substances have been associated with CH to improve its antimicrobial action and intradentinal diffusion [7, 8], especially against resistant bacterial species capable of surviving in an alkaline pH, as Enterococcus faecalis that is observed in primary and secondary infections [9, 10].
A natural substance that has been studied associated to the CH paste is the ethanol extract of propolis (EEP), because of its antimicrobial and anti-inflammatory potential. Thus, it becomes a useful substance to endodontology as an intracanal medication [11, 12], since studies have shown the efficacy of EEP against many bacteria [12]. Furthermore, with the intention of increasing CH paste bacterial spectrum of action, other substances have been added to this intracanal medication [7]. One example is chlorhexidine, an antimicrobial agent with a wide spectrum of action, that has been used as an irrigation solution and intracanal medication in endodontics, because it demonstrated that after a prolonged contact with the root canal, chlorhexidine has shown substantivity and adhesion to anionic substrates [7, 13, 14]. Another substance used in combination with the CH is the camphorated paramonochlorophenol (CPMC), that has been used for a long time and has showing efficacy regardless the antimicrobial action of the medication [7, 15]. Although it is highly toxic when used alone, small amounts of CPMC mixed into the CH paste are significantly less toxic [15, 16].
Thus, the aim of the present study is to investigate the in vitro intradentinal penetrability and antimicrobial ability of CH used with the following vehicles: distilled water (DW group) and propylene glycol (PG group) and the additives EEP (EEP group), chlorhexidine (CLX group), and CPMC (CPMC group). For this, a new contamination protocol for great depths of dentine was used [17], and the antimicrobial effect and penetrability analysis of the pastes was performed by means of microbiological culture and confocal laser scanning microscopy (CLSM). Considering that the calcium hydroxide antimicrobial and biological properties are attributed to the alkaline pH and calcium release, these two properties and the solubility of the previously mentioned calcium hydroxide pastes were also evaluated. The null hypothesis was that the vehicle and additives would not influence the antimicrobial action, intradentinal penetration, pH, calcium ion release, and solubility of calcium hydroxide pastes.
Materials and methods
Calcium hydroxide pastes
The calcium hydroxide pastes used in this study were used in the ratio of 3:1 (powder weight/vehicle weight) or 3:1:1 (powder weight/vehicle weight/additive weight). For each five teeth, 10 g of calcium hydroxide p.a. (Biodinâmica, Londrina, Brazil) and 3.3 g of the vehicle or/and additive were used. The studied groups were group G1–CH in distilled water (DW), G2–CH in propylene glycol (PG), G3–CH in PG and ethanol extract of propolis (EEP), G4–CH in PG and chlorhexidine (CLX), and G5–CH in PG and camphorated paramonochlorophenol (CPMC). The solid green propolis, obtained from the state of Paraná (Brazil), was dissolved in boiling ethanol in an extractor and cooled, and the wax was removed by filtration. This filtrate was concentrated in a rotary evaporator and diluted with pure ethanol (10%) in a volumetric balloon. The EEP was analyzed by using high-performance liquid chromatography (HPLC) to detect its components [18].
Intradentinal contamination
Dentine specimens were obtained from 125 recently extracted bovine incisors and immersed in 1% sodium hypochlorite for 12 h, for surface disinfection. Specimens were standardized to a length of 12 mm, by using a cutting machine (Isomet, Buehler, IL, USA) under cooling to remove dental crown and the five apical millimeters [17, 19], because the apical third has a lower number of tubules, is thinner, and has more anatomical variation. The specimens were instrumented with endodontic files (Dentsply/Maillefer, Ballaigues, Switzerland) to a diameter of 1.20 mm (file K No.120). Afterwards, the specimens were submitted to three ultrasonic baths with a duration of 10 min each, with 1% sodium hypochlorite, 17% EDTA to remove smear layer, and saline to neutralize the previous substances according to the Marinho et al. (2015) protocol [20]. The external surface of specimens was covered with two coats of red nail polish (Colorama, Rio de Janeiro, RJ, Brazil).
The dentine tubules were sterilized in an autoclave (Cristófoli, Campo Mourão, PR, Brazil) at 121 °C for 24 min, inserted in BHI sterile culture media (Brain Heart Infusion, Difco, Detroit, Michigan, USA), and submitted to an ultrasonic bath for 10 min for maximum penetration of the culture broth into the dentinal tubules.
The bacterial reference strain of Enterococcus faecalis (ATCC 29212) was acquired, and the colonial morphology and Gram stain were verified to confirm purity several times throughout the experiment. The microorganisms were cultivated in BHI broth with successive subcultures. Dilutions were made based on the absorbance value, obtained by SF325NM spectrophotometer (Bel Photonics do Brazil Ltda, Osasco, Brazil), to a concentration of 3 × 108 UFC/mL. The specimens were contaminated in a 5-day period, in BHI medium at 37 °C, following the Ma et al. (2011) sequence of centrifugations and the Andrade et al. (2015) protocol [17, 21].
The specimens were placed in a sterilized metal device inside the hood, to insert intracanal medications with the help of a K-file size 90 (Dentsply/Maillefer, Ballaigues, Switzerland). The medication stayed in the specimens that were kept in sterilized microtubes at 37 °C for 15 days. Furthermore, two specimens in each group received only the contamination and no medication (positive controls), and two others in each group were not contaminated and received only the medications (negative controls).
The medications were removed by means of irrigation with 10% citric acid (Specífica Pharmacy, Bauru, SP, Brazil) to inactivate the residual alkaline pH, followed by irrigation with saline and drying with sterilized absorbing paper points.
Microbiological confocal laser scanning microscopy (CLSM) analysis
After removing the medications, six contaminated bovine dentine tubes in each group were longitudinally sectioned with a diamond wheel (Erios, São Paulo, Brazil) in a cutting machine under cooling. Then, they were placed in a 24-well cell culture plate with 17% EDTA for 5 min and washed with 500 μL sterile saline to remove the smear layer resulting from the cutting [19]. The specimens were stained with 30 μL of the LIVE/DEAD® BacLightTM bacterial viability kit dye for 20 min (Invitrogen Molecular Probes, Eugene, OR, USA), to stain the living and the dead bacteria.
The specimens were observed through the Leica TCS-SPE CLSM (Leica Microsystems GmbH, Mannheim, Germany), thus obtaining eight images per tooth of the cervical and middle thirds. A × 40 objective was used to scan every 1 μm of depth. The images were acquired with the Leica Application Suite-Advanced Fluorescence (LAS AF, Leica Mannheim, Germany) program, and the Leica LAS AF Lite software was used for the layer fragmentation of each image. In the bioImagel v2-1 program [22], the living and dead bacteria were quantified with green (SYTO®9) and red (propidium iodide), respectively.
Microbiological culture analysis
After removing the medications from the other ten specimens (10 per group), the samples for microbiological culture analysis were extracted from the dentine specimens by using sterilized Largo burs, numbers 5 and 6.
The dentinal debris was immediately collected in microtubes with 1 mL of BHI broth, agitated, and diluted once (100 μL from the microtube that received the debris to another microtube with 900 μL of BHI broth), and 100 μL of the BHI with the debris from the two microtubes (100 and 10−1) were spread on agar BHI plates. All plates were stored in an incubator at 37 °C for 48 h for a more pronounced colony visualization, for later counting of the colony-forming units per mL (CFU/mL).
Evaluation of paste penetration by CLSM
To test medication penetration, 25 teeth were prepared in the same way as previously described, but without bacterial contamination, and were exposed to the medications of the five groups mixed with 0.1% rhodamine B dye [19] for 15 days. After removing the medication with saline solution and drying with absorbent paper points, the dentine tubes were transversely sectioned with the cutting machine under cooling, at 3 and 5 mm from the apical margin and then taken for CLSM analysis.
Images were also acquired by using the LAS-AF software. The ImageJ 1.48v software (National Institutes of Health, USA) was used to measure the total root canal circumference of the specimens and the perimeter of medication penetration, obtaining the penetration percentages in each tooth section.
Calcium hydroxide pastes pH and calcium ion release
For these tests, 50 artificial acrylic resin maxillary central incisors (n = 10 per group) with an artificial root canal and foramen were instrumented with endodontic files (Dentsply/Maillefer, Ballaigues, Switzerland) to a diameter of 0.40 mm (K-file size 40), filled with the five different calcium hydroxide pastes tested here, and the open access in the crown were sealed with Bioplic (Biodinâmica, Londrina, Brazil).Thus, the only contact of the medications with the external environment was by the apical foramen. Each tooth was individually immersed in a plastic bottle with 10 mL of ultrapure water and moved to new plastic bottles with the same amount of ultrapure water after time intervals of 7, 15, and 30 days. The pH of the solutions was analyzed with a pHmeter (model 371) and the calcium ion release with an atomic absorption spectrophotometer (AA6800; Schimadzu, Tokyo, Japan) equipped with a calcium-ion specific hollow cathode lamp [23]. The measurements were made inside the water of the bottles where the teeth were stored.
Micro-computed tomographic volumetric solubility
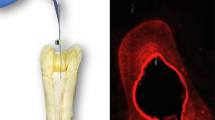
For solubility analysis, 50 artificial acrylic resin maxillary central incisors (n = 10) with an artificial root canal and foramen were instrumented with endodontic files (Dentsply/Maillefer, Ballaigues, Switzerland) to a diameter of 0.40 mm (K-file size 40). After this, they were filled with the five different calcium hydroxide pastes tested here, until the root canal was completely filled, what was achieved when there were no air bubbles, verified by the desktop x-ray micro-focus computed tomographic scanner (SkyScan 1174v2; SkyScan, Kontich, Belgium). Then, the open access in the crown were sealed with Bioplic (Biodinâmica, Londrina, Brazil), and the scanning in the desktop x-ray micro-focus computed tomographic scanner (SkyScan 1174v2; SkyScan, Kontich, Belgium) was started. The image capture parameters were: voxel size of 19.70 μm, 0.5° rotation steps, and a 360° rotation. Each scan consisted of 373 TIFF images with 1024 × 1304 pixels. Subsequently, the samples were individually completely immersed in plastic bottles with 10 mL of ultrapure water and stored at 37 °C. After 15 days, the teeth were removed from their bottles and, without any washing process, new scanning were performed with the same parameters used in the first time. The scanned images were reconstructed, and the volume (mm3) of the pastes were measured with CTan software (CTan v1.11.10.0, Sky-Scan). Then, we had the solubility values by subtracting the final value from the initial value (scanning equal to the total volume lost during immersion). The solubility percentage was calculated by dividing the volume lost by the total value. This methodology was based on Zancan et al.’s methods [24].
Statistical analysis
For all experiments, statistical analysis was performed with the Kruskall-Wallis followed by Dunn tests, with a 5% level of significance.
Results
Microbiological confocal laser scanning microscopy (CLSM) analysis
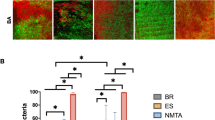
The CLSM analysis showed that according to the bacterial viability detected in the images (Fig. 1), G5 (CH + PG + CPMC) presented the lowest percentage of living bacteria, with statistically significant differences (P < 0.05) in comparison with G1 (CH + DW). The other groups presented intermediated results without statistically significant differences (P > 0.05) (Fig. 2a).
Images of the infected dentine obtained by confocal laser scanning microscopy analysis after the intracanal medications. a, b Calcium hydroxide in distilled water in the superficial and deep areas respectively. c, d Calcium hydroxide in propylene glycol in the superficial and deep areas respectively. e, f Calcium hydroxide in propylene glycol with EEP in the superficial and deep areas respectively. g, h Calcium hydroxide in propylene glycol with chlorhexidine in the superficial and deep areas respectively. i, j Calcium hydroxide in propylene glycol with CPMC in the superficial and deep areas respectively. k, l Positive controls in the superficial and deep areas respectively
a Median percentage of live bacteria per μm3, after intracanal medications for 15 days by confocal laser canning microscopy analysis. b Median percentage of penetrability of the different intracanal medications. DW, calcium hydroxide in distilled water, PG, calcium hydroxide in propylene glycol; EEP, calcium hydroxide in propylene glycol with EEP; CLX, calcium hydroxide in propylene glycol with chlorhexidine; CPMC, calcium hydroxide in propylene glycol with CPMC; C+ positive control
The pastes in the cervical third were shown to be more effective than those in the middle thirds. There was statistically significant difference only between each group and the positive control, when analyzing the thirds. Furthermore, the behavior of the pastes at different depths in the dentine mass was similar, with statistical difference only between each group and the positive control.
Microbiological culture evaluation
Regardless the number of CFU/mL, when the median, minimum, and maximum values were evaluated, there was no statistically significant difference between the studied pastes (P > 0.05). A significant difference (P < 0.05) was observed when the pastes were compared with the positive control. These differences were observed both in the analysis of the most superficial dentine (Largo No. 5), and deeper dentine (Largo No. 6). The negative controls in the microbiological studies showed no bacterial growth; since they received only the pastes, it validated the experiment without contamination.
CLSM evaluation of intratubular paste penetration
There was no statistically significant difference between the groups when evaluating the intra-canal paste penetration, although the paste of G1 (CH with distilled water as a vehicle) demonstrated a lower rate of intra-tubular penetration when compared with the other groups, in which propylene glycol was used (Fig. 2b).
Calcium hydroxide pastes pH and calcium ion release
In the pH measurements, in general, CH with distilled water showed the smallest pH values, presenting significant statistical difference with the pastes of G3, G4, and G5 in 15 and 30 days. In 7 days, there was no significant statistical difference between the pH of the pastes. Regardless the calcium ion release, CH in PG and CLX presented the greatest values in 7 and 15 days, but in 30 days, CH in PG and PP had the biggest values of calcium ion release. Table 1 shows the pH and calcium ion release results.
Micro-computed tomographic volumetric solubility
Regardless the solubility percentage of the pastes analyzed in 15 days, the paste of G2 (CH + PG) presented the highest values. There was statistically significant difference only between the G2 and G5 (CH + PG + CPMC) (P = 0.0018). The results are shown in Table 2.
Discussion
Although small statistical differences were observed between groups, the null hypothesis tested was rejected since there were differences in the antimicrobial action, penetrability, pH, calcium ion release, and solubility of CH pastes according to the different additives used.
In the present study, two different methods were used to evaluate the antimicrobial action of CH pastes inside bovine teeth: microbiological culture and staining evaluation by CLSM, demonstrating that the two analyses could complement each other. Penetration into the dentinal tubules is the most important resistance mechanism of E. faecalis against the antimicrobial agents [25, 26]. The specimen analysis by CLSM with the LIVE/DEAD dye was appropriate for visualizing the bacteria within the dentine, by the fluorescence that indicated lack of integrity of the bacterial membrane.
After autoclaving, specimens were contaminated according to a protocol [17] adapted from Haapasalo; Ørstavik protocol of 1987 [25]. The experimental model was built with the intention of simulating the real situation of endodontic infections occurring in the oral environment, using bovine dentinal tubes with a length of 12 mm [25]. Even though bovine dentine has a similar structure to the human dentine [27], their tubules are bigger which can improve the calcium hydroxide diffusion through the dentine providing a more efficient method of evaluation and standardization. Since the permeability of dentine has been related to the functional diameter of the dentinal tubules; the greater the functional diameter, the higher the flow rate and consequently, the rate of permeation [28]. Therefore, with a bigger proportion between the root canal and the dentine tubules, the intracanal medication action can be better evaluated.
Also related to the specimen preparation, as a manner to ensure that the microorganisms would only penetrate via root canal, the external surfaces of the specimens were varnished with a double coat of nail polish. Furthermore, we used the Ma et al. (2011) protocol of specimen centrifugation [21]. The apical thirds of the teeth were not used due to the reduced number of the dentinal tubules, as previously observed [17].
In the CLSM analysis, the only significant statistical difference was between the paste with DW (G1) and the paste with PG and CPMC (G5), which demonstrates that the other substances added to the calcium hydroxide paste did not have influence on its antibacterial effect. This statistical significant difference between G1 and G5, and the highest antimicrobial effect against E. faecalis of the CH in propylene glycol with CPMC, showed in both methodologies, can be explained by the CPMC ability to eliminate microorganisms in distant regions around the paste and kill bacteria faster than mixtures with inert vehicles, such as distilled water [29]. The results of this study were similar to those of previous studies [2, 30]. The CH paste with propylene glycol as vehicle also presented good results in both thirds, confirming the good diffusion through dentine. Although CPMC toxicity has been proven in vitro and in vivo, the phenols show low toxicity when in contact with living tissues in low concentrations while still maintaining its antimicrobial effect. This can be explained by the production of a weak salt, para-chlorophenolate, which maintains the high pH [15, 31]. Therefore, the CPMC should be used as an additive in low concentrations and not as a vehicle [16, 32]. Other studies showed that the use of this substance as an additive in the CH paste is more effective in eliminating E. faecalis than when it is not used [7, 33]. Thus, since there was a small statistical significant difference between the calcium hydroxide pastes tested here, the hypothesis that the CPMC could overcome the CH effect is rejected. Moreover, Menezes et al. (2004) showed that the combination of CH + CPMC had a greater antimicrobial effect than when CPMC was used alone [33]. Nonetheless, the use of a substance with toxic effect, even if this effect is low, must be considered.
In the present study, the pastes in the cervical third were shown to be more effective than those in the middle thirds. These results can be explained by the decrease in the diameters and amount of dentinal tubules from the cervical to the apical thirds [17, 34]. Also, because the rate of diffusion or the permeability of dentine should vary directly with the surface area. In this way, in the cervical third, the dentinal tubules are bigger and in greater amount than in the middle third, what allows a better penetration, diffusion, and efficacy of the medications on this area [28, 32]. Beside this, when the calcium hydroxide paste is applied in the root canal, during an endodontic treatment, it produces a gradient of hydroxyl ions, with a decreased amount in the apical dentine tubules. The bacteria that are sheltered in the deep layers of the dentine mass therefore respond to the stresses of the endodontic treatment procedures and as the intracanal medication, upregulating virulence genes promoting biofilm growth [35, 36].
The association of chlorhexidine and CH did not show satisfactory results, corroborating with the results of another study [37]. These results can be attributed to the fact that chlorhexidine degrades in alkaline environments [38, 39]. Furthermore, chlorhexidine can release by-products, such as para-chloroaniline and reagent oxygen species, alone or associated with CH paste [39, 40]. Waris and Ahsan reported that some carcinogens can exert part of their carcinogenic effect by the reagent oxygen species release [41]. Even though, some researchers have found good antimicrobial results combining CH and chlorhexidine [27, 42]; the differences between results may be attributed to the different methodologies used [43]; therefore, more studies with this association are necessary.
The CH paste with 10% EEP, presented intermediate results, although without statistical difference. The different formulations and sources of propolis can also produce different results [44]; hence, the use of typified propolis samples in the studies is indispensable [18]. Propolis purification and wax elimination are necessary, as well as identification of its compounds. The sample used in this study was analyzed by high-performance liquid chromatography (HPLC) to determine and standardize the compounds. Ferreira et al. (2007) and Bhandari et al. (2014) investigated propolis individually, and this substance demonstrated greater antimicrobial effect in comparison with the CH substance. The mechanisms of propolis activity against microorganisms have been attributed to flavonoids and phenolic compounds [12, 44]. Propolis prevents bacterial cellular division and acts on the microbial membrane, causing structural damage, similar to the action of some antibiotics [45], without toxic effects to the host cells.
Propylene glycol was chosen as a vehicle in most groups because of its lower surface tension than water, allowing greater diffusion of medication through the bacterial cellular membrane. This vehicle presents little antimicrobial activity and does not irritate the periapical tissues [46]. Ganesh et al. (2014) compared the antimicrobial effectiveness of the CH in distilled water, propylene glycol, and CPMC by the agar diffusion test and found results similar to those of the present study [47].
The advantage of propylene glycol over distilled water as a vehicle was also shown when the pH and calcium ion release was evaluated. In the pH measurements, the use of different additives did not influence the results. In calcium ion release evaluation, the paste with chlorhexidine as additive, showed greater results in great part of the comparisons. Considering this, it can be assumed that, all pastes reacted similarly, and that the pastes with propylene glycol as a vehicle had better results, in concordance with Duarte et al. 2009 study [23].
CH manipulated with a vehicle that has good solubility will favor the ion dissociation ratio and diffusion through the dentinal tubules [48]. Nerwich et al. 1993, reported that calcium hydroxide used as an intracanal medication significantly increased the pH in the apical region after only 2–3 weeks [49]. Thus, the solubility test was made by micro-computed tomographic imaging to find the volumetric loss of the pastes after 15 days. The calcium hydroxide paste with propylene glycol as vehicle presented the highest percentage values of solubility, and the other pastes with the additives did not present statistical significant differences between each other. These results can also suggest that a viscous vehicle can improve the solubility of the calcium hydroxide paste. Figure 3 illustrates the micro-computed tomographic 3-dimensional reconstructions of the initial and final (superimposed) intracanal dressings in root canals, showing that the pastes were more soluble in the apical thirds for all groups. The solubility test results are in concordance with the study of Zancan et al. 2016 [24].
Conclusion
The vehicles and additives tested may increase CH antimicrobial effect, but with a small statistical difference. In general, all CH pastes tested here were effective in reducing Enterococcus faecalis and were similar in the penetration, pH, calcium ion release, and solubility of calcium hydroxide when compared to distilled water.
References
El Karim I, Kennedy J, Hussey D (2007) The antimicrobial effects of root canal irrigation and medication. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103(4):560–569. https://doi.org/10.1016/j.tripleo.2006.10.004
Bystrom A, Claesson R, Sundqvist G (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol 1(5):170–175
Li GH, Niu LN, Zhang W, Olsen M, De-Deus G, Eid AA, Chen JH, Pashley DH, Tay FR (2014) Ability of new obturation materials to improve the seal of the root canal system: a review. Acta Biomater 10(3):1050–1063. https://doi.org/10.1016/j.actbio.2013.11.015
Siqueira JF Jr, Magalhães KM, Rôças IN (2007) Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J Endod 33(6):667–672. https://doi.org/10.1016/j.joen.2007.01.004
Mohammadi Z, Dummer PM (2011) Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J 44(8):697–730
Sirén EK, Kerosuo E, Lavonius E, Meurman JH, Haapasalo M (2014) Ca(OH)2 application modes: in vitro alkalinity and clinical effect on bacteria. Int Endod J 47(7):628–638. https://doi.org/10.1111/j.1365-2591.2011.01886
Lima RKP, Guerreiro-Tanomaru JM, Faria-Júnior NB, Tanomaru-Filho M (2012) Effectiveness of calcium hydroxide-based intracanal medicaments against enterococcus faecalis. Int Endod J 45(4):311–316. https://doi.org/10.1111/j.1365-2591.2011.01976.x
Camoes IC, Salles MR, Chevitarese O, Gomes LN (2004) Diffusion of Ca(OH)2 associated with different vehicles: chromatographic study (high-performance liquid chromatography). J Endod 30(1):30–34. https://doi.org/10.1097/00004770-200401000-00006
Wilson CE, Cathro PC, Rogers AH, Briggs N, Zilm PS (2015) Clonal diversity in biofilm formation by Enterococcus faecalis in response to environmental stress associated with endodontic irrigants and medicaments. Int Endod J 48(3):210–219
Tenner C, Fuhrmann M, Wittmer A, Karygianni L, Altenburger MJ, Pelz K, Hellwig E, Al-Ahmad A (2014) New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J Endod 40(5):670–677
Silva FB, Almeida JM, Sousa SM (2004) Natural medicaments in endodontics—a comparative study of the anti-inflammatory action. Braz Oral Res 18(2):174–179
Ferreira FB, Torres SA, Rosa OP, Ferreira CM, Garcia RB, Marcucci MC, Gomes BP (2007) Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104(5):709–716. https://doi.org/10.1016/j.tripleo.2007.05.019
Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, de Souza Filho FJ (2006) In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102(4):544–550. https://doi.org/10.1016/j.tripleo.2006.04.010
Siqueira JF Jr, de Uzeda M (1997) Intracanal medicaments: evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J Endod 23(3):167–169. https://doi.org/10.1016/S0099-2399(97)80268-3
Anthony DR, Gordon TM, del Rio CE (1982) The effect of three vehicles on the pH of calcium hydroxide. Oral Surg Oral Med Oral Pathol 54(5):560–565
Gahyva SM, Siqueira JF Jr (2005) Direct genotoxicity and mutagenicity of endodontic substances and materials as evaluated by two prokaryotic test systems. J Appl Oral Sci 13(4):387–392
Andrade FB, Arias MP, Maliza AG, Duarte MA, Graeff MS, Amoroso-Silva PA, Midena RZ, Moraes IG (2015) A new improved protocol for in vitro intratubular dentinal bacterial contamination for antimicrobial endodontic tests: standardization and validation by confocal laser scanning microscopy. J Appl Oral Sci 23(6):591–598
Moncla BJ, Guevara PW, Wallace JA, Marcucci MC, Nor JE, Bretz WA (2012) The inhibitory activity of typified propolis against Enterococcus species. Z Naturforsch C 67(5–6):249–256
Arias MP, Maliza AG, Midena RZ, Graeff MS, Duarte MA, Andrade FB (2016) Effect of ultrasonic streaming on intra-dentinal disinfection and penetration of calcium hydroxide paste in endodontic treatment. J Appl Oral Sci 24(6):575–581. https://doi.org/10.1590/1678-775720150553
Marinho AC, Martinho FC, Goncalves LM, Rabang HR, Gomes BP (2015) Does the Reciproc file remove root canal bacteria and endotoxins as effectively as multifile rotary systems? Int Endod J 48(6):542–548. https://doi.org/10.1111/iej.12346
Ma J, Wang Z, Shen Y, Haapasalo M (2011) A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod 37(10):1380–1385. https://doi.org/10.1016/j.joen.2011.06.018
Chavez de Paz LE (2009) Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Appl Environ Microbiol 75(6):1734–1739. https://doi.org/10.1128/AEM.02000-08
Duarte MA, Midena RZ, Zeferino MA, Vivan RR, Weckwerth PH, Dos Santos F, Guerreiro-Tanomaru JM, Tanomaru-Filho M (2009) Evaluation of pH and calcium ion release of calcium hydroxide pastes containing different substances. J Endod 35(9):1274–1277. https://doi.org/10.1016/j.joen.2009.05.009
Zancan RF, Vivan RR, Milanda Lopes MR, Weckwerth PH, de Andrade FB, Ponce JB, Duarte MA (2016) Antimicrobial activity and physicochemical properties of calcium hydroxide pastes used as intracanal medication. J Endod 42(12):1822–1828. https://doi.org/10.1016/j.joen.2016.08.017
Haapasalo M, Orstavik D (1987) In vitro infection and disinfection of dentinal tubules. J Dent Res 66(8):1375–1379. https://doi.org/10.1177/00220345870660081801
Siqueira JF Jr, De Uzeda M, Fonseca ME (1996) A scanning electron microscopic evaluation of in vitro dentinal tubules penetration by selected anaerobic bacteria. J Endod 22(6):308–310. https://doi.org/10.1016/S0099-2399(96)80265-2
Evans M, Davies JK, Sundqvist G, Figdor D (2002) Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J 35(3):221–228
Outhwaite WC, Livingston MJ, Pashley DH (1976) Effects of changes in surface area, thickness, temperature and post-extraction time on human dentine permeability. Arch Oral Biol 21(10):599–603. https://doi.org/10.1016/0003-9969(76)90029-7
Sukawat C, Srisuwan T (2002) A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. J Endod 28(2):102–104. https://doi.org/10.1097/00004770-200202000-00013
Sjogren U, Figdor D, Spangberg L, Sundqvist G (1991) The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J 24(3):119–125
Grecca FS, Leonardo MR, da Silva LA, Tanomaru Filho M, Borges MA (2001) Radiographic evaluation of periradicular repair after endodontic treatment of dog’s teeth with induced periradicular periodontitis. J Endod 27(10):610–612
Siqueira JF Jr, Lopes HP (1999) Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J 32(5):361–369
Menezes MM, Valera MC, Jorge AO, Koga-Ito CY, Camargo CH, Mancini MN (2004) In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms within root canals. Int Endod J 37(5):311–319. https://doi.org/10.1111/j.0143-2885.2004.00799.x
Lo Giudice G, Cutroneo G, Centofanti A, Artemisia A, Bramanti E, Militi A, Rizzo G, Favaloro A, Irrera A, Lo Giudice R, Cicciù M (2015) Dentin morphology of root canal surface: a quantitative evaluation based on a scanning electronic microscopy study. Biomed Res Int 2015:164065. https://doi.org/10.1155/2015/164065.
Love RM (2001 Jul) Enterococcus faecalis—a mechanism for its role in endodontic failure. Int Endod J 34(5):399–405
Bryce G, O'Donnell D, Ready D, Ng YL, Pratten J, Gulabivala K (2009 Sep) Contemporary root canal irrigants are able to disrupt and eradicate single- and dual-species biofilms. J Endod 35(9):1243–1248. https://doi.org/10.1016/j.joen.2009.05.034
Estrela C, Bammann LL, Pimenta FC, Pecora JD (2001) Control of microorganisms in vitro by calcium hydroxide pastes. Int Endod J 34(5):341–345
Mohammadi Z, Shalavi S (2012) Is chlorhexidine an ideal vehicle for calcium hydroxide? A microbiologic review. Iran Endod J 7(3):115–122
Barbin LE, Estrela C, Guedes DF, Spano JC, Sousa-Neto MD, Pecora JD (2013) Detection of parachloroaniline, reactive oxygen species, and 1-chloro-4-nitrobenzene in high concentrations of chlorhexidine and in a mixture of chlorhexidine and calcium hydroxide. J Endod 39(5):664–668. https://doi.org/10.1016/j.joen.2012.10.018
Yeung SY, Huang CS, Chan CP, Lin CP, Lin HN, Lee PH, Jia HW, Huang SK, Jeng JH, Chang MC (2007) Antioxidant and pro-oxidant properties of chlorhexidine and its interaction with calcium hydroxide solutions. Int Endod J 40(11):837–844. https://doi.org/10.1111/j.1365-2591.2007.01271.x
Waris G, Ahsan H (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5:14. https://doi.org/10.1186/1477-3163-5-143
Prabhakar AR, Hadakar SG, Raju OS (2012) Comparative evaluation of pH and antibacterial effect of various calcium hydroxide combinations on E. faecalis and its effect on root strength: an in vitro study. Contemp Clin Dent 3(1):42–47. https://doi.org/10.4103/0976-237X.94545
Saatchi M, Shokraneh A, Navaei H, Maracy MR, Shojaei H (2014) Antibacterial effect of calcium hydroxide combined with chlorhexidine on Enterococcus faecalis: a systematic review and meta-analysis. J Appl Oral Sci 22(5):356–365
Bhandari S, SA T, Patil CR (2014) An in vitro evaluation of antimicrobial efficacy of 2% chlorhexidine gel, propolis and calcium hydroxide against Enterococcus faecalis in human root dentin. J Clin Diagn Res 8(11):ZC60–ZC63. https://doi.org/10.7860/JCDR/2014/10359.5144
Takaisi-Kikuni NB, Schilcher H (1994) Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med 60(3):222–227. https://doi.org/10.1055/s-2006-959463
Grover C, Shetty N (2014) Evaluation of calcium ion release and change in pH on combining calcium hydroxide with different vehicles. Contemp Clin Dent 5(4):434–439. https://doi.org/10.4103/0976237X.142803
Ganesh MR, Chaurasia VR, Masamatti VK, Mujeeb A, Jhamb A, Agarwal JH (2014) In vitro evaluation of antibacterial efficacy of calcium hydroxide in different vehicles. J Int Soc Prev Commun Dent 4(1):56–60. https://doi.org/10.4103/2231-0762.131268
de Andrade Ferreira FB, Silva ESPA, do Vale MS, de Moraes IG, Granjeiro JM (2004) Evaluation of pH levels and calcium ion release in various calcium hydroxide endodontic dressings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97(3):388–392. https://doi.org/10.1016/S1079210403005237
Nerwich A, Figdor D, Messer HH (1993) pH changes in root dentin over a 4-week period following root canal dressing with calcium hydroxide. J Endod 19(6):302–306
Funding
This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo - FAPESP (2010/20186-3 and 2013/26120-2) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Thais Cristina Pereira received research grants from FAPESP and CAPES.
Author Layla Reginna Silva Munhoz de Vasconcelos declares that she has no conflict of interest.
Author Márcia Sirlene Zardin Graef declares that she has no conflict of interest.
Author Maria Cristina Marcucci Ribeiro declares that she has no conflict of interest.
Author Marco Antonio Hungaro Duarte declares that he has no conflict of interest.
Author Flaviana Bombarda de Andrade declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Evaluation and comparison of different calcium hydroxide pastes, with aqueous and viscous vehicles, beside some additives as propolis, chlorhexidine, and camphorated paramonochlorophenol, regarding antimicrobial effect (by confocal laser scanning microscopy and microbiological culture), penetrability (by confocal laser scanning microscopy), pH (pHmeter), solubility (microtomography), and calcium ion release (atomic absorption spectrophotometer).
Rights and permissions
About this article
Cite this article
Pereira, T.C., da Silva Munhoz Vasconcelos, L.R., Graeff, M.S.Z. et al. Intratubular decontamination ability and physicochemical properties of calcium hydroxide pastes. Clin Oral Invest 23, 1253–1262 (2019). https://doi.org/10.1007/s00784-018-2549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2549-0