Abstract
Objective
The objective of this study was to investigate serum and salivary levels of chemerin and MMP-9 as early diagnostic biomarkers for patients with oral premalignant lesions (OPMLs) and oral squamous cell carcinoma (OSCC).
Methods
This study included 45 individuals; 15 healthy control, 15 patients with OPMLs, and 15 patients with early stage OSCC. Chemerin and MMP-9 were determined in serum and saliva samples utilizing enzyme-linked immunosorbent assays.
Results
Serum and salivary levels of chemerin and MMP-9 in patients with OSCC were significantly higher than OPMLs and control group. Patients with OPMLs showed also elevated profiles for serum and salivary chemerin and MMP-9 compared to control group. Receiver operator characteristic curve analysis revealed that all tested biomarkers have 100 % sensitivity and 100 % specificity with area under the curve (AUC) of 1.00 in detecting early stage OSCC and OPMLs. In distinguishing OSCC from OPMLs, salivary MMP-9, serum chemerin, and salivary chemerin showed AUC of 0.99, 0.92, and 0.88, respectively, showing higher sensitivity and specificity compared with serum MMP-9 (AUC; 0.6) which failed to differentiate between the two conditions.
Conclusion
Chemerin and MMP-9 might be considered as salivary diagnostic biomarkers for OPMLs and early detection of OSCC and also for detecting early cancerization of OPMLs.
Clinical relevance
This research implied that salivary chemerin was a novel diagnostic factor for patients with OPML and early stage OSCC patients, and chemerin could be a new therapeutic target for regulating cancer angiogenesis and blocking malignization of OPMLs.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Oral cancer is the 6th most common cancer globally with approximately two thirds of all cases occurring in developing countries [1]. Oral squamous cell carcinoma (OSCC) is one of the ten most frequently diagnosed cancers in the world accounting for about 90 % of the oral cancer, with an annual estimated incidence approximately 275,000 [2].
Moreover, the fact that the survival of the patients with stages I–II of the disease ranges from 60 to 80 % suggests a high potential for treatment when oral cancer is early detected in high-risk patients [3]. Meanwhile, OSCC are mostly preceded by a period during which the affected epithelium shows histologic evidence of epithelial dysplasia, though not always clinically apparent. It has been reported that the annual rate of malignant transformation of oral premalignant lesions (OPMLs) varies from 0 to 20 % in 1–30 years [4]. While the risk of premalignant progression is associated with histological grade, it is currently impossible to predict accurately which lesions will progress into cancer. Moreover, some pre-cancerous lesions with unrecognized mild dysplasia might undergo malignant transformation and become severe dysplasia [5]. While histopathological analysis of oral biopsy is the only available gold standard for confirming oral cancer, however, it is time consuming, invasive, requires experts, and expensive to be used during screening programs [6]. Consequently, there is an unmet need for laboratory diagnostic tools to early detect oral cancer and to distinguish between the OPMLs and OSCC.
The molecular mechanisms involved in the cancerization of normal oral tissue and epithelial dysplasia are still poorly understood. Tumorigenesis depends on multiple steps including degradation of the basement membrane and extracellular matrix as well as angiogenesis [7]. In addition, the process of malignant transformation from OPMLs to OSCC is complicated and regulated by many factors [8]. Angiogenesis is one of the most important events which may play a role in evolution of OSCC from epithelial dysplasia [9]. Angiogenesis is a dynamic process regulated by pro- and anti-angiogenic molecules including chemerin [10] and extracellular matrix degradation proteins such as matrix metalloproteinase-9 (MMP-9) [11]. Thus, assessment of pro-angiogenic factors might be useful for the detection of early malignant changes in the oral cavity.
Chemerin is an 18 kDa novel member of adipokines, also referred to as tazarotene-induced gene 2, which was identified as the natural ligand of the previously orphan receptor ChemR23 [12]. Chemerin is widely expressed in adipose tissue, endothelium, fibroblasts, and keratinocytes [13] and circulates in human plasma as an inactive precursor (prochemerin) that is activated by extracellular proteases [12]. Increasing evidence has pointed to the importance of chemerin in various pathophysiological conditions [14]. Numerous studies have reported that chemerin is a multifunctional adipokine that plays important roles in regulating angiogenesis, cell proliferation, and inflammation [13, 15, 16]. In a recent review, Mariani and Roncucci [17] addressed the inflammatory role of chemerin through chemerin/chemR23 axis, this pathway may regulate immune responses by contributing to the pathogenesis of inflammatory diseases. As a potent leukocyte chemoattractant, chemerin specifically modulates chemotaxis and activation of macrophages, dendritic and natural killer cells to sites of inflammation through the ChemR23 receptor [18–20]. In addition, chemerin leads to the stimulation of intracellular signal path such as p38 and Erk ½, which in turn causes the induction and regulation of proinflammatory cytokines such as tumor necrosis factor alpha and interleukin-1β [15]. Moreover, active forms of chemerin have been isolated from human inflammatory exudates, including ovarian cancer ascites, rheumatoid arthritis, and osteoarthritis synovial fluids and serum [21, 22]. Recently, Özcan et al. [23] demonstrated that high levels of salivary chemerin in patients with periodontitis were correlated with the degree of tissue destruction. In addition, chemerin was widely overexpressed in several malignant tumors [24–28]. Although, Wang et al. [29] concluded that overexpression of chemerin mRNA and protein was associated with tumor angiogenesis and poor clinical outcomes in patients with squamous cell carcinoma of the tongue, yet, the impact of chemerin on carcinogenesis remains controversial and its role in OSCC is still unclear. Furthermore, the current literature lacks evidence concerning the specificity and sensitivity of salivary chemerin as biomarkers for detecting OSCC and OPMLs.
Advances in molecular biology have revealed that matrix metalloproteinases (MMPs) play an important role in cancer progression, invasion, and angiogenesis [30]. MMPs are a large gene family of zinc-dependent endopeptidases responsible for the degradation of all extracellular matrix and basement membrane components in physiological and pathological conditions. At least 26 members of the MMP family have been discovered to date; MMP-9 (gelatinase B) being the largest member of the gene family [31]. Recent literature provides well-established evidence regarding the overexpression of MMP-9 in cancer [32, 33]. It has also been shown that increased risk for developing oral cancer is associated with MMP-9 polymorphism [34]. MMP-9 has been demonstrated to participate in cancer pathogenesis as they degrade type IV collagen, elastin, and fibronectin and through the regulation of angiogenesis [35]. Although a considerable body of evidence has accumulated showing overexpression of MMP-9 in OSCC tissues [36–40], surprisingly, it is still unclear whether MMP-9 contributes to neoangiogenesis during the process of early tumorigenesis and what specific mechanisms regulate their production in OSCC [41].
To date, the knowledge about specific molecules involved in malignant transformation of OPML and early detection of OSCC has not been satisfying. Evidence-based recommendations highlighted that sensitive and specific diagnostic biomarkers for screening of OPMLs and early detection of oral cancer must be identified and validated to maximize treatment efficacy for future patients [42, 43]. Although potential salivary biomarkers have been identified for the diagnosis of oral cancer [44–46], surprisingly, few studies have examined salivary tumor markers in patients with OSCC. Saliva harvesting is noninvasive, eliminate risks of contamination and easy to use, which may make it an attractive alternative to serum testing [47]. Salivary examination has been proposed to be an effective modality for early diagnosis of oral cancer because of the direct contact between the oral cancer lesion and saliva [5]. Moreover, it may provide a cost-effective approach for the screening of large high-risk populations, as patients with OPMLs [6]. Based on the above mentioned data, the aim of this study was to identify and compare the levels of chemerine and MMP-9 in serum and saliva of subjects with OPMLs, OSCC, and healthy controls. Based on the authors’ knowledge, this is the first investigation conducted in an attempt to explore the diagnostic potentials of salivary chemerin and MMP-9 as biomarkers which might be helpful in differentiating between OSCC and OPMLs and in early cancer detection.
Materials and methods
This clinical trial has been registered at ClinicalTrials.gov (identifier NCT02323672).
Study population
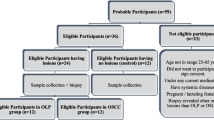
The entire study sample comprised 45 individual. Fifteen subjects with definitely diagnosed early stage OSCC (9 female and 6 male; range 22 to 67 years) and 15 subjects with OPML (10 female and 5 male; range 24 to 60 years) were enrolled in the study as were 15 age-sex matched control subjects (8 female and 7 male; range 26 to 58 years). All OSCC patients had recently been diagnosed with primary disease and had not received any prior treatment in the form of chemotherapy, radiotherapy, surgery, or alternative remedies. The group with OPML included patients with atrophic oral lichen planus, actinic keratosis, and speckled leukoplakia. The control group were healthy normal individuals free form any systemic disease or inflammatory oral lesions or periodontal disease.
Inclusion and exclusion criteria
All individuals enrolled in this study were selected from the Outpatient clinic, Department of Oral Medicine and Periodontology, Faculty of Oral and Dental Medicine, Cairo University and National cancer institute, Cairo University during the period from February 2014 to September 2014. A detailed medical history of each subject was obtained according to the detailed questionnaire of the modified Cornell Medical Index [48]. Body mass index (BMI) was measured based on World Health Organization guidelines. Inclusion criteria included patients diagnosed with early (stage I or II) OSCC or OPMLs based on oral clinical examination and confirmed by histopathological examination. All subjects included in this study had clinically healthy gingiva with zero plaque index, gingival index, and clinical attachment loss and ≤3 mm pocket depth. Exclusion criteria included patients with systemic disease, inflammatory oral lesions or periodontal disease; patient had a history of prior malignancy, immunodeficiency, autoimmune disorders, hepatitis, or human immunodeficiency virus infection; and prior treatment in the form of chemotherapy, radiotherapy, surgery or alternative medicine, pregnancy or lactation, and former or current smokers.
Comprehensive oral diagnosis was carried out for all individuals enrolled in the study using the Department of Oral Diagnosis chart. Following an explanation of the study as well as the information about the sampling procedures, each subject signed a written informed consent form approved by the Faculty Research Ethics committee (September 2013). After obtaining patients’ written consent, tissue biopsy specimens were harvested for the lesions clinically diagnosed as OPML and processed for further histopathologic examination in order to confirm the clinical diagnosis. The tissue specimens were processed by the Department of Oral Pathology, Faculty of Oral and Dental Medicine, Cairo University, and the histopathologic diagnosis of all specimens was confirmed by two oral pathologists. All of the OPMLs were found to have moderate to severe epithelial dysplasia in order to qualify for enrollment. Seven specimens were graded as moderate epithelial dysplasia, while eight were graded as severe epithelial dysplasia.
Collection of samples
Serum sample collection
Peripheral venous blood samples (5 ml) were taken by standard venipuncture from patients and controls using plain tubes. Samples were centrifuged. The clarifying supernatant was filtered and stored at −20 °C until assayed.
Salivary sample collection
Collection of whole unstimulated saliva (WUS) was done using standard techniques according to Navazesh [49]. At the time of saliva collection, lesions were actively symptomatic. Salivary samples were obtained in the morning and subjects were asked not to eat, brush their teeth, or use mouth rinse at least 2 h prior to salivary sample collection on that day. Samples were obtained by requesting subjects to swallow first, tilt their head forward, and expectorate 10 ml of unstimulated whole saliva into a sterile centrifuge tube. After collection, the saliva was immediately centrifuged for 2 min at 10,000×g and the clarified supernatant was filtered through a 0.45 μm low protein binding membrane, separated into 0.5-ml aliquots and frozen at −80 °C until assayed.
Detection of human MMP-9 levels in saliva and serum samples
Quantitation of MMP-9 levels was done using Quantikine ELISA kit provided by R&D system Inc., Minneapolis, USA (Catalog Number DMP900). This assay is a quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for MMP-9 has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any MMP-9 present is bound by the immobilized antibody. The unbound substances were removed by washing. The enzyme-linked monoclonal antibody specific for MMP-9 is added to all wells. A substrate solution is added to the wells after washing to remove any unbound antibody-enzyme reagent. Color was developed in proportion to the amount of MMP-9 bound in the initial step. The color development is stopped and the intensity of the color is measured by ELISA reader. MMP-9 levels were determined as nanogram per milliliter.
Detection of chemerin level in saliva and serum samples
The serum and salivary levels of chemerin were measured using the RD191136200R Human Chemerin ELISA which is a sandwich enzyme immunoassay for the quantitative measurement of human chemerin. The kit was provided by BioVendor–Laboratorní medicína, Guang Zhou, CHINA. In the Biovendor Human Chemerin ELISA, standards, quality controls, and samples are incubated in microtitration wells pre-coated with polyclonal anti-human chemerin antibody. After a 60-min incubation followed by washing, biotin labeled polyclonal anti-human chemerin antibody is added and incubated with the captured chemerin for 60 min. After another washing, streptavidin-HRP conjugate is added. After 30 min incubation and the last washing step, the remaining conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of chemerin. A standard curve is constructed by plotting absorbance values against chemerin concentrations of standards, and concentrations of unknown samples are determined using this standard curve. Chemerin levels were determined as nanogram per milliliter.
Statistical and power analysis
Using G power analysis program [50], sample size was determined by comparing the mean ± SD for chemerin nanogram per milliliter in the control and cancer groups. A total sample size of 16 patients was calculated to be sufficient to detect effect size (f = 1.39) by considering level of significance α = 0.05, with 80 % power. This number was increased to 30 patients (15 in each group) to increase the validity of the results. Data were presented as mean and standard deviation (SD) values. Wilcoxon-signed rank test was used to compare serum and salivary values of the tested biomarkers in the three studied groups. Analysis of variance (ANOVA) test was carried out for comparing the biochemical data in different groups at 5 % level of significance. To evaluate the diagnostic effectiveness of the studied biomarkers and the extent to which the obtained data could separate the different clinical settings, receiver operating characteristic (ROC) curve analysis was performed and the area under curve (AUC) presented a direct measure of the diagnostic accuracy of the test. The biomarker that has the largest AUC was identified as having the strongest predictive power for detecting OSCC or OPMLs. The best cut off value was defined for each biomarker as the test result with the highest sensitivity and specificity and that lied closest to the left upper corner of the curve. All data were processed with a computerized statistical package (SPSS 15.0 for Windows, SPSS Inc., Chicago, IL).
Results
Table 1 shows demographic data for all subjects included in this study and the tumor clinicopathological parameters of patients with OPMLs and OSCC. All included subjects had a normal BMI (<25 > 18.5 kg/m2). No statistical differences were found between the three groups regarding age and BMI (P > 0.05). The results of this investigation indicated that serum and salivary levels of chemerin and MMP-9 in OSCC patients were significantly elevated as compared to their levels in patients with OPMLs as well as to their levels in the control healthy group (P < 0.05). Furthermore, serum and salivary levels of chemerin and MMP-9 in patients with OPMLs were significantly elevated as compared to their levels in the control healthy group (P < 0.05). In addition, a significant difference was observed when comparing between serum levels and salivary levels of both chemerine and MMP-9 in OPMLs and OSCC patients (P < 0.05), where the highest values for both chemerin and MMP-9 were detected in the serum samples. Regarding the control group, there was a significant difference between serum and salivary levels of chemerin (P < 0.05), yet, no significant difference was evident when comparing between serum and salivary values of MMP-9 (P = 0.1363). Table 2 demonstrates the comparison between serum and salivary levels of chemerin and MMP-9 in all studied groups.
In order to characterize these potential biomarkers for OSCC early prediction, ROC curve analysis was performed. The areas under curve (AUC) values of salivary chemerin, serum chemerin, salivary MMP-9, and serum MMP-9 were 1.00, with a 100 % sensitivity, specificity, and diagnostic accuracy. Based on the distribution of sensitivities and specificities, cut off/thresholds of the tested biomarkers were chosen for detecting early stage OSCC. The current data showed a cut off value of >6.29, 307, 260.3, and 184.5 ng/ml for salivary chemerin, serum chemerin, salivary MMP-9, and serum MMP-9, respectively. Therefore, cases above these cut off values are diagnosed as having OSCC, whereas cases below these values are diagnosed as negative (healthy) cases. Another ROC curve was plotted to evaluate the diagnostic value of the tested biomarkers in patients with OPMLs. Likewise, salivary chemerin, serum chemerin, salivary MMP-9, and serum MMP-9 revealed AUC of 1.00 with a 100 % sensitivity, specificity, and diagnostic accuracy in discriminating patients with OPMLs from healthy control. The clinically relevant cut off values of chemerin in saliva and serum were >5.87 and >223.04 ng/ml, respectively, while the cut off points of MMP-9 in saliva and serum were >214.35 and >197.63 ng/ml, respectively, i.e., patients with levels exceeding these values are diagnosed with OPMLs, while patients having lower levels are diagnosed as healthy subjects. Collectively, this study demonstrated that all the tested biomarkers have the ability to differentiate the presence or absence of both early stage OSCC and OPMLs.
In addition, to distinguish patients with OSCC from those with OPMLs, the ROC curve analysis revealed an AUC of 0.88, 0.92, 0.99, and 0.6 for salivary chemerin, serum chemerin, salivary MMP-9, and serum MMP-9, respectively, (Fig. 1). Salivary MMP-9 had the highest diagnostic accuracy (93 %), whereas a cut off value of >318.74 ng/ml was detected with 100 % sensitivity and 93 % specificity. This was followed by serum chemerin, revealing a good diagnostic accuracy (80 %), 87 % sensitivity, and 93.3 % specificity with a cut off value of >507.06 ng/ml. Salivary chemerin also provided a good diagnostic accuracy (73 %), where a cut off value of >9.19 ng/ml showed high levels of sensitivity (93 %) and specificity (80 %). However, ROC curve analysis showed that serum MMP-9 had the least diagnostic accuracy (46 %) for classifying cases into malignant and premalignant, a cut off value of 429.27 ng/ml was observed with low levels of sensitivity and specificity, 53.3 and 93.3 %, respectively. The cases above these cut off values were considered as malignant and below these cut off values were considered as premalignant cases.
The detailed statistics of AUC, cut off values, corresponding sensitivities and specificities, and diagnostic accuracy (Youden index value) for all of the tested biomarkers in diagnosing OSCC, OPMLs, and distinguishing them from each other are listed in the Tables 3–4.
Discussion
Most OSCC is not diagnosed until an advanced stage, which has been one of the major reasons for minimally improved survival rate over the years. Therefore, early oral cancer detection is imperative where treatment in the pre-invasive stage offers the best prognosis and even the chance of cure [47]. The gold standard for definitive diagnosis of oral cancer is based on traditional stage predicted indices (TNM system) and histopathological grading [51]. Unfortunately, these predictors are subjective and relatively unreliable, with inter and intra-observer variability [6]. Consequently, there has been an ever-growing effort dedicated to the basic research of oral cancer, focusing on identifying biological indicators as alternative diagnostic methods for early cancer detection [3, 43, 52]. To the authors’ knowledge from indexed literature, this is the first study in which pro-angiogenic factors like chemrine and MMP-9 are investigated in the saliva of patients with OSCC and OPMLs. This investigation showed that serum and salivary chemerin and MMP-9 profiles were significantly elevated in patients with OSCC compared to OPMLs and healthy subjects as well as in patients with OPMLs compared to control subjects. The studied biomarkers showed no correlation with neither age nor BMI, thus, it can be assumed that the differences in chemerine and MMP-9 levels between the groups in this study do not arise from age or BMI [23].
In this study, the OPMLs group included patients having oral lesions with a recognized tendency for malignant transformation as atrophic oral lichen planus (OLP) and speckled leukoplakia [53, 54]. Paroloni et al. [18] hypothesized that chemerin local production in the endothelium may orchestrate the colocalization of dendritic and natural killer cells in OLP lesions, which might partly explain the currently elevated levels of chemerin observed in OPMLs like atrophic OLP. In addition, the current findings were similar to those reported by Chang et al. [55]. The authors observed elevated serum levels of MMP-9 in patients with OSCC and oral leukoplakia compared to control subjects, suggesting that this biomarker could identify patients at risk of oral cancer. Furthermore, other investigations showed that MMP-9 was expressed in tissues of oral leukoplakia [56] and that this expression was up-regulated during cancerization of these lesions [57]. Consistent with these data, previous studies demonstrated that MMP-9 was overexpressed in oral dysplastic lesions suggesting that it might serve as a biomarker of malignant transformation [58] and even increased in more severe dysplasia compared to those with lower degrees of dysplasia [41]. In support with this study, Chen et al. [54] observed that MMP-9 expression was significantly higher in both OSCC from OLP and atrophic OLP compared to non-atrophic OLP and healthy tissue, suggesting that MMP-9 might be a useful predictive marker for determining the early stages of OLP cancerization. Interestingly, recent reports concluded that salivary MMP-9 was higher in habitual gutka (pre-cancerous) chewers compared with non-chewers [59] and could be considered as a sensitive and specific diagnostic biomarker for OLP [60]. Based on the above mentioned studies, this investigation recommends that OLP should be carefully followed due to the possibility of malignant transformation and definitive diagnosis should be established as early as possible [54].
The present study observed that serum and salivary chemerin was highly elevated in patients with OSCC compared to OPMLs and control groups, which is in agreement with others reporting elevated serum chemerin levels in patients with gastric cancer [28, 61]. Moreover, chemerin has been suggested as a potential biomarker for cancer diagnosis [24, 27]. Although chemerin expression was up-regulated in cancer tissues [25, 26], chemerin was showed to be suppressed in other tumors [62, 63]. A probable explanation for these inconsistencies is that the expression of chemerin may vary in different types of cancers, which are not rare phenomena in tumor biology [64, 65]. In accordance with the present data, Wang et al. [29] demonstrated that chemerin was overexpressed in poorly differentiated squamous cell carcinoma of the tongue (SCCOT) and was associated with tumor angiogenesis as well as poor clinical outcomes. The authors proposed that chemerin might be a novel prognostic indicator for patients with SCCOT and targeting chemerin may serve as a novel approach in cancer treatment.
Nearly, all biological activities of tumors depend on tumor angiogenesis, where adipokine signaling plays an important role in the development of cancer. However, the relationship between chemerin levels and tumor angiogenesis has not yet been explored in OSCC lesions. This study suggests that the highly elevated serum and salivary levels of chemerine in patients with OSCC might be attributed to its angiogenic potentials. This assumption is supported by the pro-angiogenic function of chemerin revealed by in vitro angiogenic assays [15, 19]. Chemerin was shown to promote angiogenesis in adipose tissue and enhance adipocyte differentiation [10]. Moreover, chemerin has been associated with markers of endothelial activation [16] and had a direct association with the vascular endothelial growth factor (VEGF) in diabetic patients with peripheral vascular disease [66]. A recent review suggested that chemerin might play a distinct role in tumor pathogenesis through the regulation of angiogenesis and that suppression of chemerin in these specific tissues might be beneficial in suppressing the tumor spread [20].
The data presented herein are in line with previous reports showing elevated levels of serum MMP-9 in patients with OSCC [39, 67]. Similarly, numerous studies demonstrated that MMP-9 was overexpressed in head and neck cancer as well as OSCC [11, 39–41, 68]. Although Kousen et al. [69] reported that the expression of MMP-9 in OSCC cells had no prognostic role, yet the strong stromal signal of MMP-9 was associated with poor tumor differentiation. The results of this study are supported by previous reports suggesting that MMP-9 might be a useful marker for predicting early stage OSCC as well as a good target for intervention therapy [36–38]. Recently, Monteiro et al. [40] also suggested that MMP-9 could be regarded as an important prognostic biomarker in OSCC. As for the current demonstrated increase in salivary MMP-9, it is worth noting that it was shown earlier to be elevated in saliva of patients with OSCC [44, 45].
There is enough evidence that enhanced production of MMP-9 might play a significant role in the induction of early angiogenic events during carcinogenesis [70]. Vilen et al. [71] suggested that MMP-9 may play a dual role, switching from a pro-angiogenic to an anti-angiogenic molecule in cancer angiogenesis. MMP-9 can act as a pro-angiogenic factor during tumorigenesis via increasing VEGF activity [35, 41, 72]. In contrast, other studies were unable to confirm these results [7, 30, 73] which might be explained by the anti-angiogenic role of MMP-9 proposed by Vilen et al. [71]. The variety of methodologies and the low sample sizes might also explain the discrepancy in these results.
Meanwhile, previous observations which indicate that chemerin could induce functional angiogenesis along with concurrent increases in MMP-9, might suggest a potential causal relationship between chemerin-induced MMP-9 activity and angiogenesis. For instance, chemerin has been shown to induce MMP-9 in human endothelial cells and other MMPs in human chondrocytes [15, 19]. Kaur et al. [15] demonstrated that chemerin leads to the formation of new blood vessels through various signaling pathways, including MAPK, Akt, and activation of endothelial gelatinases (MMP-2/-9), promoting endothelial cell proliferation, migration, and capillary tube formation. Considering that enhanced gelatinase (MMP-2/-9) production is a feature of early vascular remodeling and dysregulated angiogenesis that contributes to endothelial barrier dysfunction [74], hence, a chemerin-induced increase in MMP-9 activity suggests a potential functional association between the chemerin/chemR23 system and angiogenesis [15, 17]. In addition, Wang et al. [61] found that chemerin stimulated cancer cell invasion in vitro through induction of MMPs. However, the current literature lacks studies correlating the relation between chemerin and MMP-9 whether in saliva or in serum, thus future research is required to elucidate the precise role of MMP-9 in chemerin-induced angiogenesis. Still, whether the increased chemerin observed in this study might regulate OSCC angiogenesis through increasing MMP-9 levels, needs to be further explored.
In a recent systematic review, Guerra et al. [52] concluded that only few salivary biomarkers used to early diagnose head and neck cancer were diagnostically accurate. An ideal diagnostic biomarker should have high disease sensitivity and specificity, mandatory presence in all affected patients, and provide a cut off value with minimal overlap between normal and disease states. In view of that, all the tested biomarkers in this investigation revealed an excellent diagnostic performance (100 %) with 100 % sensitivity and specificity in discriminating OSCC patients as well as patients with OPMLs from healthy controls. In an attempt to differentiate between patients with OSCC and those with OPMLs, results of the ROC curve analysis for salivary MMP-9 and serum chemerin revealed an AUC of 0.99 and 0.92, with high levels of sensitivity (100 and 87 %), specificity (93.3 %), and diagnostic accuracy 93 and 80 %, respectively. This was followed by salivary chemerin with an AUC of 0.8 with high levels of sensitivity (93 %) and specificity (80 %). The present study suggests that these biomarkers might have a diagnostic utility as surrogate indicators of carcinogenic transformation from premalignant to malignant condition in oral cancer. This agrees with other reports in the literature showing that early angiogenic activity may play a role in promoting tumor progression in epithelial dysplasia [8, 9]. However, this investigation showed that sensitivity and specificity were very low for serum MMP-9 with an AUC of 0.6 bringing to light the limits of this marker to distinguish OSCC from OPMLs.
Adipokines are believed to act through their effects on insulin sensitivity and hence altered levels of these hormones were associated with diabetes mellitus. In recent years, researchers have examined the potential link between chemerin and the pathogenesis of insulin resistance, obesity, and metabolic syndrome. Chemerin serum levels were discovered to be higher within the serum of patients with type 2 diabetes mellitus than in the normal-glucose-tolerant groups [75]. The increasing research in this area is gradually revealing the complex adipokine-mediated interplay among adipose tissue and metabolic disorders. Accordingly, the preliminary results presented in this study encourage future research to evaluate the prognostic potentials of chemerin as biomarker for oral cancer detection in patients suffering from diabetes mellitus. The current investigation paves the way for further clinical studies to examine the discriminatory power of salivary chemerin as an oral screening test, in the flesh patients with OPML and OSCC who might also have impaired glucose tolerance, diabetes, and obesity.
In conclusion, within the limitations of this study, salivary chemerin and MMP-9 might be considered as biomarkers for detecting malignant transformation of OPMLs and diagnosing early stage OSCC and OPMLs. This investigation proposes highly sensitive salivary diagnostic biomarkers that may be applied as a potential technique for oral cancer screening and early detection of OSCC [76]. Nevertheless, it must be noted that this salivary analysis may be regarded as an aid and not as a replacement for other well-established diagnostic tools available for oral cancer [6]. In addition, the efficacy of these biomarkers can only be determined through well-designed, large, prospective multi-institutional trials, where identification of early cancer changes may be useful in selecting patients for early interventional therapies. Further studies are also warranted to elucidate the molecular mechanisms regulating MMP-9 in chemerin-induced angiogenesis of oral cancer and premalignant conditions.
References
Yu T, Wu Y, Helman JI, Wen Y, Wang C, Li L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Mol Cancer Res 2011 9(2):161–172. Epub 2011/01/06. doi:10.1158/1541-7786.mcr-10-0386. PubMed PMID: 21205837.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009 45(4–5):309–316. Epub 2008/09/23. doi:10.1016/j.oraloncology.2008.06.002. PubMed PMID: 18804401.
Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 2011 3:33. Epub 2011/07/30. doi:10.1186/1758–3284–3-33. PubMed PMID: 21798030; PubMed Central PMCID: PMCPmc3170277.
Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med 2008 37(1):1–10. Epub 2007/12/25. doi:10.1111/j.1600-0714.2007.00579.x. PubMed PMID: 18154571.
Wu JY, Yi C, Chung HR, Wang DJ, Chang WC, Lee SY, et al. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol 2010 46(4):226–231. Epub 2010/02/09. doi:10.1016/j.oraloncology.2010.01.007. PubMed PMID: 20138569.
Nagler RM. Saliva as a tool for oral cancer diagnosis and prognosis. Oral Oncol 2009 45(12):1006–1010. Epub 2009/10/16. doi:10.1016/j.oraloncology.2009.07.005. PubMed PMID: 19828359.
Kim SH, Cho NH, Kim K, Lee JS, Koo BS, Kim JH, et al. Correlations of oral tongue cancer invasion with matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) expression. J Surg Oncol 2006 93(4):330–337. Epub 2006/02/24. doi:10.1002/jso.20461. PubMed PMID: 16496371.
Michailidou EZ, Markopoulos AK, Antoniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008 2:126–32. Epub 2008/01/01. doi: 10.2174/1874210600802010126. PubMed PMID: 19444318; PubMed Central PMCID: PMCPMC2606660.
Mohtasham N, Babakoohi S, Salehinejad J, Montaser-Kouhsari L, Shakeri MT, Shojaee S, et al. Mast cell density and angiogenesis in oral dysplastic epithelium and low- and high-grade oral squamous cell carcinoma. Acta Odontol Scand 2010 68(5):300–304. Epub 2010/07/01. doi: 10.3109/00016357.2010.494622. PubMed PMID: 20586672.
Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010 95(5):2476–85. Epub 2010/03/20. doi: 10.1210/jc.2010–0042. PubMed PMID: 20237162; PubMed Central PMCID: PMCPmc2869547.
Patel BP, Shah PM, Rawal UM, Desai AA, Shah SV, Rawal RM, et al. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J Surg Oncol 2005 90(2):81–88. Epub 2005/04/22. doi: 10.1002/jso.20240. PubMed PMID: 15844188.
Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem 2005 280(41):34661–34666. Epub 2005/08/13. doi: 10.1074/jbc.M504868200. PubMed PMID: 16096270.
Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev 2011 22(5–6):331–338. Epub 2011/11/29. doi: 10.1016/j.cytogfr.2011.11.004. PubMed PMID: 22119008.
Zabel BA, Kwitniewski M, Banas M, Zabieglo K, Murzyn K, Cichy J. Chemerin regulation and role in host defense. Am J Clin Exp Immunol. 2014 3(1):1–19. Epub 2014/03/25. PubMed PMID: 24660117; PubMed Central PMCID: PMCPmc3960757.
Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 2010 391(4):1762–1768. Epub 2010/01/05. doi: 10.1016/j.bbrc.2009.12.150. PubMed PMID: 20044979.
Landgraf K, Friebe D, Ullrich T, Kratzsch J, Dittrich K, Herberth G, et al. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab 2012 97(4):E556–E564. Epub 2012/03/23. doi: 10.1210/jc.2011-2937. PubMed PMID: 22438234.
Mariani F, Roncucci L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm Res 2015 64(2):85–95. Epub 2014/12/31. doi: 10.1007/s00011-014-0792-7. PubMed PMID: 25548799.
Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007 109(9):3625–3632. Epub 2007/01/05. doi: 10.1182/blood-2006-08-038844. PubMed PMID: 17202316.
Berg V, Sveinbjornsson B, Bendiksen S, Brox J, Meknas K, Figenschau Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21–157). Arthritis Res Ther. 2010 12(6):R228. Epub 2011/01/05. doi: 10.1186/ar3215. PubMed PMID: 21192818; PubMed Central PMCID: PMCPmc3046541.
Fatima SS, Rehman R, Baig M, Khan TA. New roles of the multidimensional adipokine: chemerin. Peptides 2014 62:15–20. Epub 2014/10/04. doi: 10.1016/j.peptides.2014.09.019. PubMed PMID: 25278490.
Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003 198(7):977–85. Epub 2003/10/08. doi: 10.1084/jem.20030382. PubMed PMID: 14530373; PubMed Central PMCID: PMCPmc2194212.
Huang K, Du G, Li L, Liang H, Zhang B. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers 2012 17(1):16–20. Epub 2011/11/15. doi: 10.3109/1354750x.2011.634028. PubMed PMID: 22080616.
Ozcan E, Saygun NI, Serdar MA, Kurt N. Evaluation of the salivary levels of visfatin, chemerin, and progranulin in periodontal inflammation. Clin Oral Investig. 2014 Epub 2014/08/29. doi: 10.1007/s00784–014–1308-0. PubMed PMID: 25164155.
Fernandez-Ranvier GG, Weng J, Yeh RF, Khanafshar E, Suh I, Barker C, et al. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg 2008 143(9):841–846; discussion 6. Epub 2008/09/17. doi: 10.1001/archsurg.143.9.841. PubMed PMID: 18794420.
Yamaguchi Y, Du XY, Zhao L, Morser J, Leung LL. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J Biol Chem. 2011 286(45):39510–9. Epub 2011/09/29. doi: 10.1074/jbc.M111.258921. PubMed PMID: 21949124; PubMed Central PMCID: PMCPmc3234774.
Kumar JD, Holmberg C, Kandola S, Steele I, Hegyi P, Tiszlavicz L, et al. Increased expression of chemerin in squamous esophageal cancer myofibroblasts and role in recruitment of mesenchymal stromal cells. PLoS One. 2014 9(7):e104877. Epub 2014/08/16. doi: 10.1371/journal.pone.0104877. PubMed PMID: 25127029; PubMed Central PMCID: PMCPmc4134237.
Ntikoudi E, Kiagia M, Boura P, Syrigos KN. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev 2014 40(1):22–30. Epub 2013/07/23. doi: 10.1016/j.ctrv.2013.06.005. PubMed PMID: 23870486.
Zhang J, Jin HC, Zhu AK, Ying RC, Wei W, Zhang FJ. Prognostic significance of plasma chemerin levels in patients with gastric cancer. Peptides 2014 61:7–11. Epub 2014/08/26. doi: 10.1016/j.peptides.2014.08.007. PubMed PMID: 25152503.
Wang N, Wang QJ, Feng YY, Shang W, Cai M. Overexpression of chemerin was associated with tumor angiogenesis and poor clinical outcome in squamous cell carcinoma of the oral tongue. Clin Oral Investig 2014 18(3):997–1004. Epub 2013/07/23. doi: 10.1007/s00784-013-1046-8. PubMed PMID: 23868294.
Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, et al. Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck. 2006 28(1):31–39. Epub 2005/11/03. doi: 10.1002/hed.20298. PubMed PMID: 16265652.
Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg 1997 84(2):160–166. Epub 1997/02/01. PubMed PMID: 9052425.
Ren F, Tang R, Zhang X, Madushi WM, Luo D, Dang Y, et al. Overexpression of MMP Family Members Functions as Prognostic Biomarker for Breast Cancer Patients: A Systematic Review and Meta-Analysis. PLoS One. 2015 10(8):e0135544. Epub 2015/08/14. doi: 10.1371/journal.pone.0135544. PubMed PMID: 26270045; PubMed Central PMCID: PMCPMC4535920.
Liao D, Huang H, Zhu Z, Li T, Li H, Huang GL, et al. Prognostic value of matrix metalloproteinase 9 in nasopharyngeal carcinoma: a meta-analysis. Minerva Med. 2016 Epub 2016/01/23. PubMed PMID: 26799856.
Vairaktaris E, Vassiliou S, Nkenke E, Serefoglou Z, Derka S, Tsigris C, et al. A metalloproteinase-9 polymorphism which affects its expression is associated with increased risk for oral squamous cell carcinoma. Eur J Surg Oncol 2008 34(4):450–455. Epub 2007/05/15. doi: 10.1016/j.ejso.2007.03.024. PubMed PMID: 17498910.
Henriques AC, de Matos FR, Galvao HC, Freitas Rde A. Immunohistochemical expression of MMP-9 and VEGF in squamous cell carcinoma of the tongue. J Oral Sci 2012 54(1):105–111. Epub 2012/04/03. PubMed PMID: 22466894.
Katayama A, Bandoh N, Kishibe K, Takahara M, Ogino T, Nonaka S, et al. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res 2004 10(2):634–640. Epub 2004/02/05. PubMed PMID: 14760086.
Impola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H, et al. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol 2004 202(1):14–22. Epub 2003/12/25. doi: 10.1002/path.1479. PubMed PMID: 14694517.
de Vicente JC, Fresno MF, Villalain L, Vega JA, Hernandez Vallejo G. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol 2005 41(3):283–293. Epub 2005/03/04. doi: 10.1016/j.oraloncology.2004.08.013. PubMed PMID: 15743691.
Patel BP, Shah SV, Shukla SN, Shah PM, Patel PS. Clinical significance of MMP-2 and MMP-9 in patients with oral cancer. Head Neck. 2007 29(6):564–572. Epub 2007/01/26. doi: 10.1002/hed.20561. PubMed PMID: 17252594.
Monteiro LS, Delgado ML, Ricardo S, Amaral BD, Salazar F, Pacheco J, et al. Prognostic significance of CD44v6, p63, Podoplanin, and MMP-9 in oral squamous cell carcinomas. Oral Dis 2016. Epub 2016/01/21. doi: 10.1111/odi.12442. PubMed PMID: 26788715.
de Carvalho Fraga CA, Farias LC, de Oliveira MV, Domingos PL, Pereira CS, Silva TF, et al. Increased VEGFR2 and MMP9 protein levels are associated with epithelial dysplasia grading. Pathol Res Pract 2014 210(12):959–964. Epub 2014/12/03. doi: 10.1016/j.prp.2014.06.020. PubMed PMID: 25441661.
Smith J, Rattay T, McConkey C, Helliwell T, Mehanna H. Biomarkers in dysplasia of the oral cavity: a systematic review. Oral Oncol 2009 45(8):647–653. Epub 2009/05/16. doi: 10.1016/j.oraloncology.2009.02.006. PubMed PMID: 19442563.
Gualtero DF, Suarez Castillo A. Biomarkers in saliva for the detection of oral squamous cell carcinoma and their potential use for early diagnosis: a systematic review. Acta Odontol Scand 2016 74(3):170–177. Epub 2015/11/19. doi: 10.3109/00016357.2015.1110249. PubMed PMID: 26577643.
Shpitzer T, Hamzany Y, Bahar G, Feinmesser R, Savulescu D, Borovoi I, et al. Salivary analysis of oral cancer biomarkers. Br J Cancer. 2009 101(7):1194–8. Epub 2009/10/01. doi: 10.1038/sj.bjc.6605290. PubMed PMID: 19789535; PubMed Central PMCID: PMCPmc2768098.
Shpitzer T, Bahar G, Feinmesser R, Nagler RM. A comprehensive salivary analysis for oral cancer diagnosis. J Cancer Res Clin Oncol 2007 133(9):613–617. Epub 2007/05/05. doi: 10.1007/s00432-007-0207-z. PubMed PMID: 17479291.
Nagler R, Bahar G, Shpitzer T, Feinmesser R. Concomitant analysis of salivary tumor markers—a new diagnostic tool for oral cancer. Clin Cancer Res 2006 12(13):3979–3984. Epub 2006/07/05. doi: 10.1158/1078-0432.ccr-05-2412. PubMed PMID: 16818695.
Wang Q, Gao P, Wang X, Duan Y. Investigation and identification of potential biomarkers in human saliva for the early diagnosis of oral squamous cell carcinoma. Clin Chim Acta 2014 427:79–85. Epub 2013/10/23. doi: 10.1016/j.cca.2013.10.004. PubMed PMID: 24144867.
Abramson JH. The cornell medical index as an epidemiological tool. Am J Public Health Nations Health. 1966 56(2):287–98. Epub 1966/02/01. PubMed PMID: 5948222; PubMed Central PMCID: PMCPmc1256865.
Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993 694:72–77. Epub 1993/09/20. PubMed PMID: 8215087.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007 39(2):175–191. Epub 2007/08/19. PubMed PMID: 17695343.
Takes RP, Rinaldo A, Silver CE, Piccirillo JF, Haigentz M, Jr., Suarez C, et al. Future of the TNM classification and staging system in head and neck cancer. Head Neck 2010 32(12):1693–1711. Epub 2010/03/02. doi: 10.1002/hed.21361. PubMed PMID: 20191627.
Guerra EN, Acevedo AC, Leite AF, Gozal D, Chardin H, De Luca Canto G. Diagnostic capability of salivary biomarkers in the assessment of head and neck cancer: a systematic review and meta-analysis. Oral Oncol 2015 51(9):805–818. Epub 2015/07/15. doi: 10.1016/j.oraloncology.2015.06.010. PubMed PMID: 26170140.
Silverman S, Jr., Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 1984 53(3):563–568. Epub 1984/02/01. PubMed PMID: 6537892.
Chen Y, Zhang W, Geng N, Tian K, Jack Windsor L. MMPs, TIMP-2, and TGF-beta1 in the cancerization of oral lichen planus. Head Neck. 2008 30(9):1237–1245. Epub 2008/07/22. doi: 10.1002/hed.20869. PubMed PMID: 18642282.
Chang PY, Kuo YB, Wu TL, Liao CT, Sun YC, Yen TC, et al. Association and prognostic value of serum inflammation markers in patients with leukoplakia and oral cavity cancer. Clin Chem Lab Med 2013 51(6):1291–1300. Epub 2012/11/17. doi: 10.1515/cclm-2012-0504. PubMed PMID: 23154424.
Tortorici S, Mauro A, Burruano F, Difalco P, Leone A, Gerbino A, et al. Matrix metalloproteinase-2 matrix metalloproteinase-9 and inducible nitric oxide synthase in oral leukoplakia: immunohistochemistry and RT-PCR analysis. J Biol Regul Homeost Agents 2008 22(2):125–130. Epub 2008/07/04. PubMed PMID: 18597705.
Cai H-X, Pan C-B, Li H-G (2002) Expression of matrix metalloproteinases in cancerization of oral mucosa. Acad J SUMS 23:477–429
Jordan RC, Macabeo-Ong M, Shiboski CH, Dekker N, Ginzinger DG, Wong DT, et al. Overexpression of matrix metalloproteinase-1 and -9 mRNA is associated with progression of oral dysplasia to cancer. Clin Cancer Res 2004;10(19):6460–6465. Epub 2004/10/12. doi: 10.1158/1078-0432.ccr-04-0656. PubMed PMID: 15475433.
Javed F, Al-Kheraif AA, Al Amri MD, Mikami T, Vohra F, Warnakulasuriya S, et al. Periodontal parameters and whole salivary cytokine profiles among habitual gutka chewers and non-chewers. J Periodontol 2015 86(5):689–695. Epub 2015/01/24. doi: 10.1902/jop.2015.140556. PubMed PMID: 25612632.
Fathi MS, El Dessouky HF, Breni HA. CD4 + CD25+ T regulatory cells and MMP-9 as diagnostic salivary biomarkers in oral lichen planus. Egypt J Immunol 2013 20(2):39–53. Epub 2013/01/01. PubMed PMID: 24617046.
Wang C, Wu WK, Liu X, To KF, Chen GG, Yu J, et al. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: a clinical and experimental study. Peptides 2014 51:131–138. Epub 2013/11/28. doi: 10.1016/j.peptides.2013.10.009. PubMed PMID: 24274970.
Lin W, Chen YL, Jiang L, Chen JK. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clin Lab 2011 57(11–12):879–885. Epub 2012/01/14. PubMed PMID: 22239017.
Pachynski RK, Zabel BA, Kohrt HE, Tejeda NM, Monnier J, Swanson CD, et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med. 2012 209(8):1427–35. Epub 2012/07/04. doi: 10.1084/jem.20112124. PubMed PMID: 22753924; PubMed Central PMCID: PMCPmc3409495.
Thies A, Moll I, Berger J, Wagener C, Brummer J, Schulze HJ, et al. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol 2002 20(10):2530–2536. Epub 2002/05/16. PubMed PMID: 12011132.
Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, et al. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res 2004 64(24):8932–8938. Epub 2004/12/18. doi: 10.1158/0008-5472.can-04-0505. PubMed PMID: 15604255.
Zakareia FA. Correlation of peripheral arterial blood flow with plasma chemerin and VEGF in diabetic peripheral vascular disease. Biomark Med 2012 6(1):81–87. Epub 2012/02/03. doi: 10.2217/bmm.11.85. PubMed PMID: 22296200.
Liu CJ, Chang KW, Lin SC, Cheng HW. Presurgical serum levels of matrix metalloproteinase-9 and vascular endothelial growth factor in oral squamous cell carcinoma. Oral Oncol 2009 45(10):920–925. Epub 2009/06/09. doi: 10.1016/j.oraloncology.2009.04.007. PubMed PMID: 19502103.
Fan HX, Wang S, Zhao H, Liu N, Chen D, Sun M, et al. Sonic hedgehog signaling may promote invasion and metastasis of oral squamous cell carcinoma by activating MMP-9 and E-cadherin expression. Med Oncol 2014 31(7):41. Epub 2014/06/12. doi: 10.1007/s12032-014-0041-5. PubMed PMID: 24915900.
Kosunen A, Pirinen R, Ropponen K, Pukkila M, Kellokoski J, Virtaniemi J, et al. CD44 expression and its relationship with MMP-9, clinicopathological factors and survival in oral squamous cell carcinoma. Oral Oncol 2007;43(1):51–59. Epub 2006/06/27. doi: 10.1016/j.oraloncology.2006.01.003. PubMed PMID: 16798062.
Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002 2(4):289–300. Epub 2002/10/26. PubMed PMID: 12398893.
Vilen ST, Salo T, Sorsa T, Nyberg P. Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. ScientificWorldJournal. 2013 2013:920595. Epub 2013/02/01. doi: 10.1155/2013/920595. PubMed PMID: 23365550; PubMed Central PMCID: PMCPMC3556887.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000 2(10):737–44. Epub 2000/10/12. doi: 10.1038/35036374. PubMed PMID: 11025665; PubMed Central PMCID: PMCPMC2852586.
Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, Gallo O. Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer 2002 95(9):1902–1910. Epub 2002/10/31. doi: 10.1002/cncr.10916. PubMed PMID: 12404284.
Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation 2000 102(23):2861–2866. Epub 2000/01/11. PubMed PMID: 11104745.
Yang M, Yang G, Dong J, Liu Y, Zong H, Liu H, et al. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med. 2010 58(7):883–6. Epub 2010/07/06. doi: 10.231/JIM.0b013e3181ec5db2. PubMed PMID: 20601896.
Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem 2011 57(5):675–687. Epub 2011/03/09. doi: 10.1373/clinchem.2010.153767. PubMed PMID: 21383043.
Acknowledgments
The authors would like to thank Dr. Nayroz Tarrad Lecturer of Oral Medicine and Periodontology, Faculty of Dentistry, Fayoum University for her help in the recruitment of patients during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The study was funded by personal resources to be refunded later by the Ministry of Higher Education, Cairo, Egypt on international publishing.
Ethical approval
This article was approved by the Faculty of Oral and Dental Medicine Research Ethics committee, Cairo University in September 2013.
Informed consent
Following an explanation of the study as well as information about the sampling procedures, each subject signed a written informed consent form approved by the Faculty Research Ethics committee.
Rights and permissions
About this article
Cite this article
Ghallab, N.A., Shaker, O.G. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin Oral Invest 21, 937–947 (2017). https://doi.org/10.1007/s00784-016-1846-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1846-8