Abstract
Objectives
This study reports the radiographic analysis of a split-mouth, single-blinded, randomised controlled clinical trial which was designed to compare the efficacy of simplified papilla preservation flap (SPPF) with or without guided tissue regeneration (GTR) in patients with aggressive periodontitis (AgP).
Methods
Eighteen AgP patients who had similar bilateral intrabony defects were treated. In all patients, the defects presented with radiographic evidence of an intrabony defect ≥3 and ≥5 mm of periodontal pocket depths (PPD). The surgical procedures included access for root instrumentation using SPPF alone (control) or, after debridement, a placement of resorbable GTR membrane (test). The standardised radiographic assessments were carried out at pre-surgical baseline and at 6 and 12 month post-surgery. Radiographic linear measurements and subtraction radiography were used as the method of analysis.
Results
Both treatments showed significant improvements in linear radiographic bone fill and defect resolution at 6 and 12 months, compared to baseline. The 12-month subtraction radiography at the GTR sites showed a significant improvement compared to the 6-month outcomes.
Conclusions
Both therapies were effective in the treatment of intrabony defects in AgP patients although no significant differences between them could be demonstrated. The finding that the bone fill and resolution of the defect at the GTR sites were significantly higher at 12 months than at 6 months after treatment indicates that bone regeneration is still an ongoing process at 6 months post-surgery.
Clinical relevance
Radiographic assessment of periodontal regeneration should be carried out at 12 months post-surgery in order to evaluate the complete healing of the bony defect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The success of periodontal regeneration can be assessed by clinical, radiographical and histological evaluations. The measurements of clinical attachment level (CAL) and periodontal pocket depth (PPD) are the most frequently used examinations to assess the outcomes of regenerative procedures in a clinical practice. These clinical measurements, however, describe changes in soft tissue adaptation to the root surface but do not provide any accurate information about the type of attachment or hard tissue changes [1]. The direct measurement of bone sounding has therefore been used to assess bone topography without reflection of the soft tissue in which a mucoperiosteal flap is raised and the morphology of alveolar bone is directly measured during surgery as well as post-operatively (surgical re-entry) [2]. Radiographical examination provides a non-invasive, well-established method for the assessment of bone changes and also for monitoring outcomes following periodontal regeneration [1, 3]. Although histological examination is the ultimate methodology to determine the true extent of periodontal regeneration [1], the use of human biopsy is invasive and not possible for routine examination. Therefore, the probing measurements of PPD and CAL with radiographical assessment have become an acceptable approach for evaluation of the success of periodontal regeneration.
Radiographic assessments of periodontal regeneration compare changes in bone support from the time of surgery to the post-treatment intervals (determined by individual studies). In order to detect changes in bone support over time, two or more radiographs must be compared. Sequential radiographic assessments can be used to determine the regeneration of alveolar bone over time by measurements of bone gain/loss with or without quantification of the amount (densitometry) of alveolar bone [4]. Serial radiographs, however, may indicate apparent differences that do not reflect a true degree of regeneration of new tissues. Such differences may be the result of technical errors caused by geometric discrepancy (i.e. a different relationship between the x-ray beam and the structure of interest) and/or by differences in radiographic contrast due primarily to variation in processing [4]. Standardised radiographs have been utilised to minimise those variations in which the radiographs are taken using the parallel technique with a customised bite index (which made of cold-cure acrylic resin) and an aluminium step-wedge [5]. Subsequently, the radiographs may be analysed either by a direct measurement using a magnifying glass and a grid or a caliper [6], or by digitising the corresponding radiographs and analysis with computer software [6, 7]. Although both analytical techniques tend to underestimate the true linear distances, the computer assisted analysis has been found to have a lesser extent of discrepancy than the direct conventional measurements [6].
The present study reports the radiographic analysis of a randomised controlled clinical trial (RCT) which compared the clinical outcomes at 6 and 12 months following simplified papilla preservation flap (SPPF) with or without placement of a bioresorbable membrane (GTR) in the treatment of intrabony defects in aggressive periodontitis (AgP) patients. In this study, changes in alveolar bone structure following these two periodontal surgeries were assessed by a computer-assisted digital technique, which appears to be superior to conventional radiography and able to evaluate subtle periodontal bone changes by removing all anatomical structures other than those of immediate interest [8].
Materials and methods
Experimental design and subject population
The standardised radiographs were taken as part of a 12-month, single-blind, split-mouth RCT. According to the split-mouth design, each subject had similar bilateral intrabony defects which were randomly surgically treated, at the same visit, by either an access flap (AF) using the simplified papilla preservation flap technique (SPPF) or with guided tissue regeneration (GTR) using a bioresorbable membrane (RESOLUT XT®, WL Gore & Associates Ltd., Flagstaff, Arizona, USA).
The subject population was recruited among AgP patients referred by their general dentist to the Unit of Periodontology, Eastman Dental Hospital. Subjects were then re-examined at a dedicated clinic for AgP, and, following the confirmation of the diagnosis, the subjects who had met the study’s criteria (as described below) were allocated for initial treatment with staff hygienists or graduate students. The initial treatment comprised oral hygiene instruction, scaling and root debridement. After 6 weeks of healing, the subjects were reviewed at the AgP clinic, and at this stage, patients who had residual periodontal pockets that fulfilled the study’s criteria were given a verbal and written explanation of the study and were invited to participate. Suitable subjects were advised on the nature and purpose of the study as well as their rights to withdraw at any time without affecting their future treatment. For those that agreed to take part, informed consent forms were obtained before any clinical data were collected at baseline visit. The subjects were then scheduled for a total of 10 visits over a 12-month period which included the ‘baseline’ visit, the surgical-intervention visit and eight follow-up appointments post-surgery. The standardised radiographs were taken at baseline and at 6 and 12 months post-surgery.
Inclusion and exclusion criteria
The subjects were confirmed with the diagnosis of AgP according to the periodontal disease classification of International Classification Workshop 1999 [9] which was based on several ‘common criteria’: high occurrence in young adults, rapid periodontal attachment loss and bone destruction, familial aggregation of the disease and the patient being otherwise clinically healthy. In addition, the other key clinical features included: a level of plaque deposit which is not consistent with the severity of periodontal tissue destruction. Each subject must also meet all the following inclusion criteria: medically healthy, minimum age of 15 years old, bilateral-vertical intrabony defects, periodontal defects of PPD at least 5 mm with radiographic evidence of an intrabony defect with a depth of at least 3 mm of alveolar bone loss and smoking ≤10 cigarettes/day. Subjects were excluded from the study if they were diagnosed with other forms of periodontal disease such as chronic periodontitis (CP), had no improvement in oral hygiene after the initial therapy, refused to participate in the full requirements of the study, were pregnant or lactating, presented with any chronic illness, had contraindication to the surgical treatment and smoke >10 cigarettes/day.
Radiographical analysis and standardised radiography
Standardised intraoral radiographs were taken at pre-surgical baseline and at 6 and 12 months post-operatively. The standardised radiographs were carried out using the parallel technique with a customised holder and an occlusal platform, which allowed a cold cure acrylic resin occlusal registration to be made (bite index), thus facilitating relocation of the holder and preserving the projection geometry in subsequent radiographs [5]. The aluminium step-wedges were used as a densitometric reference, in an attempt to minimise errors due to variation in exposure time and/or film processing which may result in false positive analysis [5]. The radiographs were digitised with a resolution of 300 dpi (dots per inch; high spatial resolution) using a high-definition flat scanner (Epson cover plus 1600 Pro; Epson, Hertfordshire, UK), and the images were stored in a personal computer until analysis. The radiographic analyses included linear measurements (i.e. baseline defect angle and defect bone fill) and subtraction radiography (analysis of change in bone density), using a semi-automated radiographic subtraction software programme (X-PoseIt, version 3.01; Torben Jorgensen, Lystrup, Denmark).
Linear radiographic measurements
The radiographic angle of the intrabony defects was measured by identifying the following three anatomical landmarks on the digitised radiographs, as previously described by Tsitoura et al. (2004) (Fig. 1): (1) the cementoenamel junction (CEJ) of the tooth involved in the intrabony defect (A), (2) the most coronal position of the alveolar bone crest of the intrabony defect when it touches the root surface of the adjacent tooth before treatment (top of the crest) (B) and (3) the most apical extension of the intrabony destruction where the periodontal ligament space still remained at its normal width before treatment (the most apical part of the defect) (D). If dental restorations were present, the apical margin of the restoration was used instead of the CEJ as a fixed reference point. The radiographic angle was then defined by the two lines that represent the root surface of the involved tooth (AD) and the bone defect surface (BD), and thereby the baseline radiographic angle was expressed linearly as AD and BD. The AD distance (radiographic defect depth) was measured at baseline and at 6 and 12 months post-surgery; the differences observed at post-surgery, when compared to the baseline, represented linear bone fill [10].
Following the measurement of the radiographic defect angle, the baseline defect angle in each treatment group was then categorised as narrow, intermediate and wide, based upon the interquartile ranges as defined in the present study: angles ≤19° were defined as narrow and angles ≥31° were defined as wide, on the observed interquartile ranges; the defect angles that ranged from 20° to 30° were defined as intermediate [10].
Subtraction radiography
Following alignment of the radiographs (as noted below), the software programme analyses the grey shade values of the two radiographs in which the grey shade values of each pixel in one image are subtracted from the corresponding pixel value in the other image, resulting in a new ‘subtraction image’ which represents the differences in grey shade between the two radiographs. The differences in grey shade may be interpreted as differences in bone density [11].
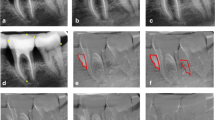
For the alignment of the images, seven to nine fixed (reference) points were defined in the baseline and post-surgical images, allowing the geometric differences to be corrected to some extent by the programme (Fig. 2). The region of control (RC) was selected to represent the areas expected not to be involved in bone changes (i.e. the aluminium step-wedges). The region of interest (ROI) corresponding to the intrabony defect was outlined by drawing a triangular area which was identified by the defect angle, as noted above, and two additional auxiliary lines as follows. The first auxiliary line (AUX 1) was drawn in the direction of the tooth axis. The second auxiliary line (AUX 2) perpendicular to the tooth axis was drawn through the most coronal extension of the lateral wall of the intrabony defect, completing a triangle for subtraction analysis (ROI) (Fig. 2) [12].
Subtraction radiography. a, d Baseline radiographs. Fixed points were marked (7 points in a and 8 points in d). The defect angle is shown in red. The first auxiliary line in light blue (AUX 1) was drawn as the tooth long axis, and then a second auxiliary line in the same colour (AUX 2) was drawn perpendicular to AUX 1 through the alveolar bone crest, creating a triangular area for the region of interest (ROI) (#2). The aluminium step-wedge was used as the region of control (RC) and drawn in dark blue (#1). b, e Radiographs at 6 months post-surgery. Identical numbers (to the baseline image) of the fixed points were marked (7 points in b and 8 points in e). c, f ‘Subtraction images’ in which the red and blue areas in the ROI illustrate the areas of bone loss and gain, respectively
The homogeneity of the images was evaluated by the number corresponding to the grey shades scale in the ‘subtraction image’ [13]. Pixels with a grey scale within the mean ± 2 of standard deviation (SD) of the RC were defined as unchanged [11]. Pixel values that appeared above this level were defined as hard tissue gain, while values below this level were defined as hard tissue loss. The programme operates with a dynamic range of 256 grey shades [11], and, by this definition, all pixels in a perfect ‘subtraction image’ of a site without bone change would have a mean grey level of 128 (or within ±2 SD of the RC). Bone gain was defined as pixels with a grey level >128 and appeared to be blue in the ‘subtraction image’ (Fig. 2). Bone loss was defined as pixels with a grey level <128 and appeared to be red in the ‘subtraction image’ (Fig. 2) [11].
The radiographs at baseline were subtracted from the 6- and 12-month images, resulting in the new ‘subtraction images’ at 6 and 12 months. The mean grey values and the size of the gain, loss and unchanged areas were analysed for significant differences between the access flap surgery (AF) and GTR surgical sites.
Calibration (repeatability) of radiographic linear measurements
The radiographic linear measurements were carried out by one previously calibrated examiner (TR). The examiner was assessed for repeatability using 50 randomly selected radiographs of intrabony defects. Defect depth and defect angle were measured twice at different time points within a week interval. The repeatability was analysed by Stata 10 statistical software (StataCorp LP). The examiner was found to have good repeatability for the measurements of the defect depth, with a Lin’s concordance correlation coefficient equal to 0.99, the maximum likely difference between a pair of readings being 0.75 mm (p > 0.05). When assessing defect angulations, there was also very good repeatability with a Lin’s concordance correlation coefficient equal to 0.90, the maximum likely difference between a pair of readings being 8° (p > 0.05).
Data management and statistical analysis
The data were entered in a microcomputer which was password protected. The statistical analysis of the clinical and radiographic outcomes was carried out using SPSS data analysis software (Ver.14.0; SPSS Inc.). For all statistical comparisons, p-values of less than 0.05 (p < 0.05) were considered significant.
Statistical analysis of the radiographic linear measurements
The linear radiographic measurements (i.e. CEJ-BD distance, CEJ-BC distance and defect depth) of the control and the test groups were compared between baseline and at 6 and 12 months post-surgery, using a repeated measures analysis of variance and the Bonferroni post hoc tests comparing baseline with each of 6 and 12 months post-surgery. The baseline radiographic angle affecting the probability of obtaining CAL gain >2 mm with the use of GTR technique was analysed using a logistic regression model. The assumptions underlying the analysis of variance were checked by a study of the residuals and were satisfied.
Statistical analysis of the subtraction radiography
The results of subtraction radiography at 6 and 12 months were divided into the levels of grey values (i.e. gain, loss and no-change) and size of these areas in pixels. The subtraction radiographic outcomes of the control group were compared to those relevant results in the test group separately at 6 and 12 months, using a Wilcoxon signed-rank test. Within the same groups (AF or GTR), the difference between the 6- and 12-month outcomes were compared using a Wilcoxon signed-rank test.
Results
Subject accountability
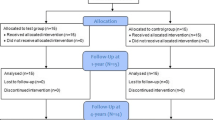
Eighteen subjects were enrolled and each was treated with both AF and GTR surgeries at the same day (Table 1). Sixteen subjects completed the 6- and 12-month follow-up and two subjects were lost due to reasons unrelated to the treatment. The clinical assessments at 6 and 12 months were reported in a separated article submitted to Clinical Oral Investigations.
Radiographic data were collected at baseline pre-surgery and at 6 and 12 months post-operatively. A total of 36 standardised radiographs were taken at baseline (18 radiographs each from the AF and GTR sites), 32 radiographs at 6 months and 32 radiographs at 12 months post-operatively (16 radiographs each from the AF and GTR sites). The radiographs were digitised and used for the analysis of baseline defect angle, radiographic bone fill (linear measurement) and change in bone density (subtraction radiography).
Baseline clinical features of periodontal defects
Table 2 shows that both surgical groups had similar baseline probing and intra-surgical measurements. The majority of the treated defects in both groups was present in the mandibular molars and had ≥4 mm defect depth. The control group had predominantly 2-wall defects (10/18), while most intrabony components in the test group (7/18) were categorised as 3-wall.
Radiographic linear outcomes at baseline
Table 3 shows that the mean CEJ-BD distance was 7.2 mm [95 % confident interval (CI) 6.2, 8.2] for the control defects and 8.2 mm [6.9, 9.4] for the test defects (p = 0.10). The mean CEJ-BC distance was 2.2 mm [1.4, 2.9] for the control and 2.2 mm [1.4, 2.8] for the test sites (p = 0.13), whereas the radiographic defect depth was 5.1 mm [4.5, 6.1] for the control sites and 6.0 mm [5.0, 7.0] for the test sites (p = 0.09).
Baseline defect angle
The baseline radiographic defect angle in each group was measured and then categorised as narrow, intermediate and wide based upon interquartile ranges, as noted above [10]. The baseline defect angles were found to be similar in both surgical groups in which a mean of 26.7° [23.7°, 29.8°] was observed in the control group and 24.2° [21.1°, 27.2°] in the test group; the mean difference was 2.1° [1.2°, 5.1°]. The defect angles were found to be narrow in 16.7 % of the control group and 33.3 % of the test group. In both groups, 22 % of the defect angles were wide, and the rest of the defect angles were categorised as intermediate.
Using logistic regression model analysis, the baseline defect angle affecting the probability of obtaining ΔCAL >2 mm with the use of GTR technique showed that the probability of obtaining ΔCAL >2 mm was 2.1 times higher (95 % CI 1.75, 5.33) when the radiographic defect angle was narrow (≤19°), than when the radiographic defect angle was wide (≥31°) (p = 0.04); however, no significant association was found with the intermediate group.
Radiographic linear measurements at 6 and 12 months
The mean radiographic linear measurements included the following parameters: (1) CEJ-BD distance, (2) CEJ-BC distance and (3) radiographic defect depth at 6 and 12 months post-surgery, compared to the baseline. The results show that there were no significant differences between the mean AF control and the GTR test sites in any of the parameters at 6 and 12 months post-surgery (Table 3). However, compared to the baseline, both treatments significantly reduced the CEJ-BD distances and the defect depth at 6 and 12 months post-operatively, as shown in (Table 4).
Subtraction radiography at 6 and 12 months
The results of subtraction radiography in Table 5 demonstrate that no statistically significant differences were observed between the groups at 6 or 12 months post-surgery. However, at 6 months, the control group presented with a slightly increased bone density (corresponding to a higher grey value) and a larger area of new bone formation (corresponding to a larger size of bone gain) than the test group. At 12 months, the control group presented with a slightly increased bone density at the new bone area (corresponding to a higher grey value of bone gain) and showed slightly lesser area of bone loss than the test group. At the same time, the area of new bone formation (corresponding to size of bone gain) in the test group appeared to be greater than the control, and areas of no changes were also similar in the control and test groups at 12 months. The comparison between 6- and 12-month outcomes indicated that bone density (grey value) at the GTR sites and the area of new bone formation (size of bone gain) significantly increased at 12 months (p = 0.03).
Discussion
Radiographic assessment is an essential tool, along with clinical measurements which depict the soft tissue changes, to determine the success of periodontal regeneration. The radiographic assessments in the present study supported the clinical findings where both therapies (AF and GTR) resulted in significant improvements at 6 and 12 months post-surgery, compared to the baseline, although there were no significant differences between both treatments at any time points. Moreover, compared to the baseline, both treatments resulted in statistically significant bone fill at 6 and 12 months post-surgery, and as a result, the radiographic defect depth was also significantly reduced in both treatments. The linear bone changes (CEJ-BD distance and defect depth) observed in the present study at the GTR (test) group were comparable to previous investigations that evaluated the hard tissue changes by radiographic assessment following the use of various types of membrane [12, 14].
The amounts of bone fill in GTR therapy observed in the present study were significantly more favourable in narrow (≤19°) and deep defects than in wide (≥31°) and shallow defects, which is in agreement with other previous studies [12, 15, 16]. A study by Tonetti et al. (1993) reported that radiographic defect angle of intrabony defects had a significant impact on treatment outcomes following GTR therapy. Moreover, narrow defects (≤25°) presented with CAL gains of 1.5 mm more than wide defects (≥37°) following GTR treatment [15]. The decreased amounts of clinical outcome in defects with wide angle following GTR treatment may be due to the collapse of the membrane, which results in a decrease in the space available for the regeneration of the periodontium [15]. In contrast, a recent multicentre study has reported a lack of relationship between baseline defect angle and clinical outcomes following GTR/deproteinised bovine bone mineral (Bio-Oss, Bio-guide) with a papilla preservation flap [17]. However, the results of this study [17] may not be comparable to those of the present study because the use of the additional bone fillers is likely to have had a substantial influence on the post-surgical outcomes of this study. The possible diagnostic value of defect angle for the clinical outcome post-treatment has also been evaluated where enamel matrix derivative protein (EMD) was used in the treatment of intrabony defects [10], and it demonstrated that the probability of obtaining CAL gain ≥4 mm was 2.5 times higher when the radiographic defect angle was ≤22° than when it was ≥36°. It is notable that the different cut-off values, which defined narrow/wide defects, between all the studies mentioned above were based on interquartile ranges of the data distribution in each study, and thereby the cut-off points were found to vary between studies. In conclusion, the results of the present study suggest that the baseline defect angle could be of value for clinicians for estimating the potential ΔCAL in intrabony defects treated by GTR therapy.
In the present study, subtraction radiography was used to assess the density changes in intrabony defects following AF and GTR therapy. In both treatments, the subtraction images showed that the majority of bone loss appeared at the alveolar bone crest while bone formation took place in the middle and bottom part of the defects. This healing pattern has also been observed in previous studies of intrabony defects following periodontal surgery [6] as well as alveolar bone sockets following tooth extraction [11]. The alveolar bone loss at the regenerating (GTR) sites may be explained at least partly by membrane exposure, which has shown to negatively affect alveolar bone regeneration [18]. It has been suggested that SPPF (which was the flap design used in the current study) may improve vascularisation during periodontal wound healing, resulting in greater amounts of clinical attachment level gain [19–21]. Despite a careful surgical procedure where an SPPF was used, the present study found that interproximal wound dehiscence with membrane exposure occurred in the majority of the test sites during the first 4 weeks post-operatively presenting reduced ∆CAL (Rakmanee et al. 2015, in press), an observation that may explain the radiographic findings in these sites as well. At the same time though, an interesting finding was that at the GTR sites, the bone fill and resolution of the defect were significantly higher at 12 months than at 6 months post-surgery, indicating that bone regeneration is still an ongoing process at 6 months post-surgery [22].
Radiographic assessment is used to evaluate changes in hard tissue following periodontal therapy in which comparison of the radiographs, which are taken at time intervals post-treatment, is only possible when the projection geometry is identical [1]. The conventional non-standardised radiography technique resulted in distortion between the pre- and post-treatment radiographs [17]. It was therefore suggested that the calculation of a correction factor for assessing levels of distortion between pre- and post-treatment radiographs may be used to minimise this distortion during analysis [17], although this is time consuming and increases technical errors. In contrast, the present study utilised standardised radiography to diminish the variations of the projection geometry between pre- and post-surgical radiographs using the parallel technique with a customised bite index and aluminium step-wedges [5]. Despite the use of standardised radiography, some degree of magnification or distortion of the radiographs was inevitable, although the high reproducibility of the standardised technique and the use of computer analysis could minimise the discrepancies that may occur.
Conclusion
Based on the results of the present study, it was concluded that
-
the AF and GTR treatments both resulted in significant radiographic bone fill and resolution of the intrabony components at 6 and 12 months,
-
the GTR sites presented ongoing radiographic resolution and bone fill of the intrabony defects from 6 to 12 months,
-
the radiographic defect angle could be of value for clinicians to assess the potential clinical success in treatment of intrabony defects by GTR therapy and
-
subtraction radiography could be utilised to determine subtle bone changes following periodontal surgery.
References
Reddy MS, Jeffcoat MK (1999) Methods of assessing periodontal regeneration. Periodontology 2000(19):87–103
Cortellini P, Pini Prato G, Tonetti MS (1993) Periodontal regeneration of human infrabony defects. II. Re-entry procedures and bone measures. J Periodontol 64:261–8. doi:10.1902/jop.1993.64.4.261
Lynch SE (1992) Methods for evaluation of regenerative procedures. J Periodontol 63:1085–92. doi:10.1902/jop.1992.63.12s.1085
Hausmann E (2000) Radiographic and digital imaging in periodontal practice. J Periodontol 71:497–503. doi:10.1902/jop.2000.71.3.497
Griffiths GS, Bragger U, Fourmousis I, Sterne JA (1996) Use of an internal standard in subtraction radiography to assess initial periodontal bone changes. Dentomaxillo Facial Radiol 25:76–81
Eickholz P, Hausmann E (1998) Evidence for healing of interproximal intrabony defects after conventional and regenerative therapy: digital radiography and clinical measurements. J Periodontal Res 33:156–65
Eickholz P, Kim TS, Steinbrenner H, Dorfer C, Holle R (2000) Guided tissue regeneration with bioabsorbable barriers: intrabony defects and class II furcations. J Periodontol 71:999–1008. doi:10.1902/jop.2000.71.6.999
Christgau M, Schmalz G, Reich E, Wenzel A (1995) Clinical and radiographical split-mouth-study on resorbable versus non-resorbable GTR-membranes. J Clin Periodontol 22:306–15
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol / Am Acad Periodontol 4:1–6. doi:10.1902/annals.1999.4.1.1
Tsitoura E, Tucker R, Suvan J, Laurell L, Cortellini P, Tonetti M (2004) Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J Clin Periodontol 31:643–7. doi:10.1111/j.1600-051X.2004.00555.x
Schropp L, Wenzel A, Kostopoulos L, Karring T (2003) Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restor Dent 23:313–23
Eickholz P, Horr T, Klein F, Hassfeld S, Kim TS (2004) Radiographic parameters for prognosis of periodontal healing of infrabony defects: two different definitions of defect depth. J Periodontol 75:399–407. doi:10.1902/jop.2004.75.3.399
Lehmann TM, Grondahl HG, Benn DK (2000) Computer-based registration for digital subtraction in dental radiology. Dentomaxillo Facial Radiol 29:323–46. doi:10.1038/sj/dmfr/4600558
Klein F, Kim TS, Hassfeld S, Staehle HJ, Reitmeir P, Holle R, Eickholz P (2001) Radiographic defect depth and width for prognosis and description of periodontal healing of infrabony defects. J Periodontol 72:1639–46. doi:10.1902/jop.2001.72.12.1639
Cortellini P, Tonetti M (1999) Radiographic defect angle influences the outcomes of GTR therapy in intrabony defects. In: 77th General session of the IADR, Vancouver, Canada, 10–13 March
Tonetti MS, Pini-Prato G, Cortellini P (1993) Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J Periodontol 64:934–40. doi:10.1902/jop.1993.64.10.934
Linares A, Cortellini P, Lang NP, Suvan J, Tonetti MS, European Research Group on P (2006) Guided tissue regeneration/deproteinized bovine bone mineral or papilla preservation flaps alone for treatment of intrabony defects. II: radiographic predictors and outcomes. J Clin Periodontol 33:351–8. doi:10.1111/j.1600-051X.2006.00911.x
Nowzari H, London R and Slots J (1995) The importance of periodontal pathogens in guided periodontal tissue regeneration and guided bone regeneration. Compendium of continuing education in dentistry 16:1042, 1044, 1046 passim; quiz 1058.
Graziani F, Gennai S, Cei S, Cairo F, Baggiani A, Miccoli M, Gabriele M, Tonetti M (2012) Clinical performance of access flap surgery in the treatment of the intrabony defect. A systematic review and meta-analysis of randomized clinical trials. J Clin Periodontol 39:145–56. doi:10.1111/j.1600-051X.2011.01815.x
Retzepi M, Tonetti M, Donos N (2007) Gingival blood flow changes following periodontal access flap surgery using laser Doppler flowmetry. J Clin Periodontol 34:437–43. doi:10.1111/j.1600-051X.2007.01062.x
Donos N, D’Aiuto F, Retzepi M, Tonetti M (2005) Evaluation of gingival blood flow by the use of laser Doppler flowmetry following periodontal surgery. A pilot study. J Periodontal Res 40:129–37. doi:10.1111/j.1600-0765.2005.00777.x
Wenzel A, Warrer K, Karring T (1992) Digital subtraction radiography in assessing bone changes in periodontal defects following guided tissue regeneration. J Clin Periodontol 19:208–13
Acknowledgments
This study was partly supported by the Periodontal Research Fund of the Unit of Periodontology at the Eastman Dental Institute, University College London, UK, and a scholarship from the Royal Thai Government. The authors acknowledge supports from staff at the Eastman Clinical Investigation Centre, Dr. Jean Suvan for randomisation processes, Dr. Francesco D’Aiuto for technical support in the subtraction radiography, Ms. Bambai Hirani and Ms. Donna Moskal-Fritzpatrick for chair-side clinical assistance and trial coordination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was reviewed and approved by the National Hospital for Neurology and Neurosurgery/Institute of Neurology and the Eastman Joint Research Ethics Committee, London, UK (study reference: 04/Q0512/93).
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Rakmanee, T., Griffiths, G.S., Auplish, G. et al. Radiographic outcomes following treatment of intrabony defect with guided tissue regeneration in aggressive periodontitis. Clin Oral Invest 20, 1227–1235 (2016). https://doi.org/10.1007/s00784-015-1609-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1609-y