Abstract
Objective
The aim of this study was to compare clinical outcomes between guided tissue regeneration (GTR) and access flap (AF) surgery in patients with aggressive periodontitis (AgP).
Methods
Eighteen AgP patients with similar bilateral intrabony defects were treated in this split-mouth, single-blinded, randomised, controlled clinical trial. All patients presented with ≥3 mm intrabony defects and ≥5 mm periodontal pocket depths (PPD). In each patient, one defect was treated with a polyglycolide membrane according to the GTR principle, whereas the contralateral side was treated with AF. For both sides, a simplified papilla preservation flap was used. At baseline, 6 and 12 months post-surgery, the clinical attachment levels (CAL) and PPD were evaluated.
Results
At 6 and 12 months, at the GTR sites, the mean [95 % CI] CAL gain was 1.7 mm [1.1, 2.3] and 1.6 mm [0.9, 2.1], respectively, while the mean [95 % CI] PPD reduction was 2.3 mm [1.9, 2.8] and 2.4 mm [1.9, 2.8], respectively. Similar CAL (1.6 mm [1.0, 2.2] and 2.1 mm [1.4, 2.7]) and PPD (2.0 mm [1.5, 2.4] and 2.5 mm [2.0, 3.0]) outcomes were observed at the control sites at 6 and 12 months, respectively. Notably, at the GTR-treated sites, 13 subjects presented with various degrees of membrane exposure.
Conclusions
Both therapies were effective in the treatment of intrabony defects in AgP patients, and no statistically significant differences between them could be demonstrated, possibly as a result of the differing degrees of membrane exposure at the GTR sites.
Clinical relevance
Both periodontal regeneration and conventional periodontal surgery are effective treatments for AgP patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main goal of periodontal therapy is to restore healthy and normal periodontal function. The initial treatment (non-surgical therapy), including establishing an effective oral hygiene programme, is aimed at removal of the root surface deposits causing inflammation and at controlling the bacterial infection that induces the pathologic and clinical changes in the periodontium [1]. However, if the initial phase of periodontal therapy does not resolve the residual periodontal pockets, which have been shown to be at higher risk of disease progression [2], the second phase is surgical treatment to further correct the diseased periodontal tissues.
There are several surgical techniques described in the literature where the healing is characterised by the down-growth of long-junctional epithelium (LJE)) which attaches to the root surface, leading to a ‘repair healing’ process [3]. More recently, access flap (AF) surgery with a simplified papillae preservation flap (SPPF) design has been proposed [4], having resulted in better vascularisation associated with improved clinical outcomes compared to a modified Widman flap (MWF) alone [5]. On the other hand, the objective of guided tissue regeneration (GTR) is also to restore the original architecture of the lost periodontal tissues. This procedure involves the placement of an occlusive barrier membrane onto an intrabony defect in such a manner that apical down-growth of the junctional epithelium along the root surface and growth of the gingival connective tissue are prevented (for review see Karring et al. 1993) [6]. At the same time, the occlusive barrier provides adequate space and time for the periodontal ligament (PDL) and alveolar bone cells to repopulate the root surface and the bony defect, respectively. In order to facilitate the complete closure of the wound and to prevent exposure of the membrane, the papillae preservation flap (PPF) techniques (i.e. modified papilla preservation (MPPF) [7] and SPPF [4]) have been commonly used with GTR procedures.
Systematic reviews have been published aimed at assessing the additional efficacy of GTR in the treatment of intrabony defects with respect to AF [8–10]. The reviewers concluded that GTR was consistently more effective than AF in term of clinical attachment gain and probing depth reduction in the treatment of intrabony defects. However, these reviews were limited to patients with the chronic periodontitis (CP) in subjects aged 21 years or older, and studies specifically treating AgP were excluded. Thus, there is only limited information regarding surgical therapy for AgP, and only few clinical trials comparing GTR and AF in AgP patients [11–15], mostly based on a limited number of subjects, comparing several defects within the same patient and utilising unclear randomisation procedures.
The aim of the present randomised controlled clinical trial was to compare the clinical outcomes at 6 and 12 months following GTR therapy utilising a bioresorbable membrane and AF with SPPF design in AgP patients.
Materials and methods
Study design and subject population
This was a 12-month, randomised, single-blinded, split-mouth RCT in which the investigator assessing the clinical outcome was not aware of the identity of the treatment intervention. Each subject had similar bilateral intrabony defects that were simultaneously surgically treated by AF and GTR surgeries at the same visit. This study was reviewed and approved by the National Hospital for Neurology and Neurosurgery/Institute of Neurology and the Eastman Joint Research Ethics Committee, London, UK (study reference: 04/Q0512/93).
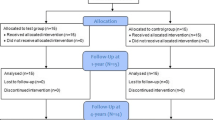
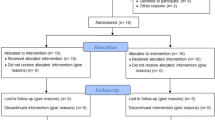
The subject population was recruited among patients referred by their general dentist to the Unit of Periodontology, Eastman Dental Institute/Hospital and had a provisional diagnosis of AgP at the relevant new patient clinic. The subjects were then re-examined at a dedicated clinic for AgP and, following the confirmation of the diagnosis, the subjects who had met the study’s criteria were allocated for initial treatment with staff hygienists. The initial treatment comprised oral hygiene instruction, scaling and root debridement. After 6 weeks of healing, the subjects were reviewed at the AgP clinic by the same examiner, and at this stage, patients who had residual periodontal pockets that fulfilled the study’s criteria were given a verbal and written explanation of the study and were invited to participate. Suitable subjects were advised on the nature and purpose of the study as well as their rights to withdraw at any time without affecting their future treatment. For those that agreed to take part, informed consent forms were obtained before any evaluations related to the study were performed. The subjects were then scheduled for a total of 10 visits over a 12-month period, which included the ‘baseline’, the surgical intervention and 8 follow-up appointments post-surgery (Fig. 1.)
Sample size
The sample size estimation was based on a previous study [16] that compared changes in CAL between AF and non-surgical treatment in juvenile periodontitis. The standard deviation (SD) of the differences between both treatments was calculated and then used for estimation of the sample size in the present study. A sample size of 16 subjects (i.e. pairs of results) was necessary in order to have an 80 % power to detect a difference in means of 0.75 mm of probing measurement (CAL), assuming a SD of CAL differences between treatments of 0.838, using a paired t test with a 0.05 two-sided significance level.
Inclusion and exclusion criteria
The subjects were confirmed with the diagnosis of AgP according to the periodontal disease classification of the International Workshop in 1999 [17] according to which the diagnosis of AgP is based on several ‘common criteria’ such as high occurrence in young adults, rapid periodontal attachment loss and bone destruction, familial aggregation of the disease and the patient being otherwise clinically healthy. In addition, the other key clinical features include: a level of plaque deposit which is not consistent with the severity of periodontal tissue destruction. Each subject must have also met all the following inclusion criteria: medically healthy, minimum age of 15 years old and over, presenting with similar bilateral vertical intrabony defects, the periodontal defects exhibiting a PPD at least 5 mm with radiographic evidence of alveolar bone loss of at least 3 mm and smoking ≤10 cigarettes/day. Subjects were excluded from the study if they were diagnosed with other forms of periodontal disease such as CP, had no improvement in oral hygiene after the initial therapy, refused to participate in the full requirements of the study, were pregnant or lactating, presented with any chronic illness, had contraindication to the surgical treatment and smoked >10 cigarettes/day.
Randomisation and allocation concealment
The sites receiving AF or GTR surgery were randomly allocated using a balanced random permuted block approach (4-unit block size) to prepare randomisation lists to avoid imbalance between the two treatments in terms of the unequal distribution of AF and GTR between right and left quadrants [18]. Since all defects had an equal chance of being allocated to either treatment, the differences between groups were due to chance and therefore any differences in outcomes could be attributed to the surgical intervention. Allocation to treatment intervention (AF or GTR) was concealed in an opaque envelope and revealed to the surgeon only on the day of treatment.
Clinical measurements and observations
All the clinical measurements pre- and post-surgery were carried out by one previously calibrated examiner (UD). The clinical parameters were evaluated at pre-surgical baseline, 6 and 12 months post-surgery which included PPD and CAL. PPD was defined as the distance from the gingival margin to the base of the periodontal pocket while CAL was defined as the distance from the cemento-enamel junction (CEJ) to the base of the pocket. If the CEJ was not detectable for anatomical or restorative reasons, the examiner adopted clinical landmarks such as base of the restoration and noted them on the study report form. In case that the gingival margin occurred coronally to CEJ, the distance from the gingival margin to CEJ (REC) was used to calculate CAL as PPD minus REC.
The probe tip (UCN-probe 15 mm) was gently inserted into the gingival pocket, and the depth of insertion read against the millimeter was recorded. Six points on each tooth were examined: mesio-buccal, mid-buccal, disto-buccal and the corresponding lingual sites. Although full-mouth probing measurements were recorded, only the measurements at the buccal and lingual sites of the treated periodontal defects were used to compare the clinical outcomes post-surgery.
Full-mouth plaque scores (FMPS) were recorded at baseline prior to surgery at 1, 1½, 3, 6 and 12 months post-operatively while full-mouth bleeding scores (FMBS) were recorded at the baseline, 6 and 12 months post-operatively. The FMPS was evaluated by assigning a binary score to each surface (1 for plaque present, 0 for absent) and calculating the percentage of total tooth surfaces that revealed the presence of plaque detected by the use of a periodontal probe [19]. Similarly, the FMBS was calculated after assessing dichotomously the presence of bleeding on probing from the bottom of the pocket when probing with a manual probe [19].
Surgical procedures
The surgical procedures were performed by one surgeon (GA) who is a specialist in periodontics at the Unit of Periodontology, Eastman Dental Hospital/Institute. The procedures were carried out under local anaesthesia at the Eastman Clinical Investigation Centre, Periodontology Unit.
At both sides of the oral cavity, an SPPF was used as previously described by Cortellini et al. [4]. Once both defects were completely debrided and treated to the same standard, an envelop containing the randomised treatment assignment was opened and the treatment intervention was then assigned as either repositioning and suturing of the flap without (SPPF control) or with a placement of the resorbable membrane (GTR/test) (RESOLUT XT®, WL Gore & Associates Ltd., Flagstaff, Arizona, USA).
At the GTR site, the intrabony defect was covered by the membrane overlapping the margins of the defect by 2–3 mm. The membrane was adapted and stabilised to the root surface by a sling suture (bioresorbable GORE-TEX® suture, WL Gore & Associates Ltd., Flagstaff, Arizona, USA) around the root trunk. The periosteum was then dissected at the base of the buccal flap to allow its tension-free adjustment, and the flap was coronally repositioned and sutured, as previously described [20].
Intra-surgical clinical measurements
Intra-surgical measurements were recorded for both SPPF- and GTR-treated sites, immediately after raising of the mucogingival flap and removal of the granulation tissue underneath, as noted above. These intra-surgical measurements were the following: (1) the distance from the CEJ to the most coronal extension of the alveolar bone crest (CEJ-BC); (2) the distance from the CEJ to the base of the defect (CEJ-BD); and (3) the defect depth (DD), defined as CEJ-BD minus CEJ-BC. The morphology of intrabony defects was also categorised into three groups according to the number of the surrounding bone walls, i.e. one-wall, two-wall and three-wall bony defects..
Post-surgical instruction and infection control
Following surgery, the subjects were instructed to rinse with 0.2 % chlorhexidine (Corsodyl, GlaxoSmithKline, Middlesex, UK) twice daily and avoid brushing or flossing at the surgical areas for a period of 6 weeks. Post-operative pain was relieved with tablets of either 600 mg Ibuprofen (Pfizer, Berkshire, UK) or 500 mg paracetamol (Panadol, GlaxoSmithKline, Middlesex, UK). Antibiotics were not prescribed in the beginning of the study unless patients presented with complications such as infection or suppuration. Smokers were asked to limit and possibly avoid smoking.
Post-surgical maintenance (the first 6 weeks, 3, 6 and 12 months)
The sutures were removed 2 weeks post-surgery. Post-surgical controls and professional tooth cleaning consisting of supragingival prophylaxis with a rubber cup and 1 % chlorhexidine gel (Corsodyl) were performed at 3, 7, 14, 28 and 48 days post-surgery. All patients were maintained in a periodontal supportive programme, and they received professional prophylaxis and calculus removal at 3, 6 and 12 months as previously described [21].
Data management and statistical analysis
The data were entered in a microcomputer which was password protected. The statistical analysis of the clinical and radiographic outcomes was carried out using SPSS data analysis software (Ver.14.0; SPSS Inc.). Any p values of less than 0.05 (p < 0.05) were considered significant. The primary outcome variables of the trial were PPD and CAL. The probing measurements (PPD and CAL) of the control defects at baseline, 6 and 12 months were compared to the relevant results of the test defects, respectively, using a repeated measures analysis of variance and the Bonferroni post hoc test. When an analysis of variance was performed, its assumption was verified by confirming that the residuals were normally distributed with constant variance. The changes in the probing measurements (PPD and CAL) of the control group at 6 and 12 months (relatively to the baseline) were compared to those changes in the test group at 6 and 12 months, respectively, using a paired t test analysis. Within the same groups (AF or GTR), the change in the probing measurements at 6 months (relative to the baseline) was compared to the change at 12 months, using a paired t test. The relevant differences used in the paired t tests were approximately normally distributed.
Results
Subject accountability
Eighteen subjects were enrolled, and each was treated with both SPPF and GTR surgery on the same day (Table 1). Sixteen subjects completed the 6- and 12-month follow-up, and two subjects were lost due to unrelated treatment reasons. The mean FMPS and FMBS are described in Table 2.
Baseline clinical features of periodontal defects
Both treatment groups had similar baseline probing and intra-surgical measurements (Table 3). In addition, the majority of the treated defects in both groups was present in the mandibular molars and had ≥4-mm defect depth. The control group had predominantly two-wall defects (10/18), while most intrabony components in the test group (7/18) were categorised as three-wall.
Early wound healing observations
The healing at the control sites appeared to be uneventful at all times. At the GTR-treated sites, 13 subjects presented with membrane exposure. The exposed membrane area was classified as major exposure group if the diameter of the area was ≥4 mm bucco-lingually and as minor exposure group if ≤3 mm. In two subjects who were smokers, the GTR sites presented with major exposure during the first 3–5 days post-operatively and the interdental papilla presented with gingival flap necrosis. Due to the extensive membrane exposure, these two patients had the membrane removed at 1 and 4 weeks after surgery. One of them did not complete the follow-up after 3 months post-surgery. Two patients who had minor membrane exposure presented with slight suppuration at 1 and 4 weeks after surgery, and the infection was controlled following a course of antibiotics (i.e. metronidazole; 400 mg three times/day for 2 weeks). At the area of membrane exposure, irrigation with a 0.2 % chlorhexidine solution was performed at the follow-up visit and topical application of 1 % chlorhexidine gel was applied daily. In all minor membrane exposures, the membrane was degraded and the area became fully re-epithelialised within 2 weeks. Most common post-operative complaints were tenderness and discomfort at the GTR sites, which subsided 6–12 weeks post-surgery.
Clinical outcomes at 6 and 12 months
The clinical measurements of CAL and PPD at 6 and 12 months were carried out by the same previously calibrated examiner. A total of 16 out of 18 subjects completed the follow-up at 6 and 12 months, and no significant differences were observed between the SPPF and GTR sites at both healing periods post-surgery (p = 0.15, p = 0.74, respectively). However, compared to the baseline levels, the clinical outcomes at 6 and 12 months showed that both therapies resulted in a significant gain in CAL and a decrease in PPD following surgery although no significant differences between the treatments could be demonstrated (Table 4). Six months after therapy, the mean CAL gain (ΔCAL) was 1.6 mm [95 % CI 1.0, 2.2] for the control group and 1.7 mm [1.1, 2.3] for the test group (p = 0.12), while the mean PPD reduction (ΔPPD) was 2.0 mm [1.5, 2.4] for the control and 2.3 mm [1.9, 2.8] for the test group (p = 0.09). Twelve months post-surgery, the mean ΔCAL was 2.1 mm [1.4, 2.7] for the control group and 1.6 mm [0.9, 2.1] for the test (p = 0.14), while the mean ΔPPD was 2.5 mm [2.0, 3.0] for the control and 2.4 mm [1.9, 2.8] for the test (p = 0.09). In each group, there were no significant differences between the clinical changes at 6 and at 12 months, as described in Table 5.
Discussion
The results of the present study indicated that both SPFF (control) and GTR (test) treatment in AgP patients resulted in significant clinical improvements at 6 and 12 months post-surgery, comparing to the baseline. The clinical results of the present study are in agreement with other split-mouth studies comparing AF surgery with GTR treatment in intrabony defects in CP patients using non-resorbable membranes [22] and bioresorbable membranes [23, 24], in which no statistically significant difference in ΔCAL was found between the two treatment modalities. However, in another clinical study where a small number of AgP patients (6 AgP) were treated by AF surgery and non-resorbable GTR membranes [12], a significantly higher CAL and PPD improvement at the GTR-treated sites was found at 12 months post-surgery, in comparison to the present study. When a resorbable polyglycolide membrane (RESOLUT XT) was used in AgP patients, an average ΔCAL of 3.4 [2.3] mm and an ΔPPD of 4.0 [2.1] mm at 12 months post-operatively was reported [15], whereas in the present study, the ΔCAL and ΔPPD was 1.6 and 2.4 mm at 12 months, respectively.
Thus far, the present study is the only RCT that examined the effectiveness of SPPF with and without GTR therapy using a resorbable polyglycolide membrane (RESOLUT XT) in the treatment of intrabony defects in AgP patients. This type of flap design (SPPF) had been previously evaluated in a multicentre study where intrabony defects were treated with and without resorbable polylactide GTR membranes in severe CP. The 12-month results revealed a ΔCAL of 3.5 [2.1] mm and 2.6 [1.8] mm at the GTR and AF sites, respectively [25]. A similar ΔCAL was observed in the control group (SPPF alone) of the present study. The favourable results at the SPPF (control) sites may be explained, at least partly, by the influence of the design of the gingival flap (SPPF) that allows full preservation of the interdental tissues and thereby primary closure of the interproximal space, possibly enhancing wound protection and blood clot stability [26]. It has also been shown that an SPPF resulted in better vascularisation and ultimately improved clinical outcome post-surgery, compared to an MWF [5, 27, 28].
Despite a careful surgical procedure in which an SPPF was used, the present study found that interproximal wound dehiscence with membrane exposure occurred in the majority of the test sites during the first 4 weeks post-operatively. Moreover, the ΔCAL was also found to be reduced at the sites with membrane exposure, compared to those with the non-exposed membrane. Such exposure has previously been reported in several GTR studies using various types of non-resorbable and resorbable membranes [29, 30, 31, 32, 33, 34]. A meta-analysis of the effect of membrane exposure on the clinical outcome, analysing five studies where either resorbable or non-resorbable membranes were used, demonstrated that the sites with exposed membrane had a significantly lower ΔCAL (4.22 [0.15] mm) than the sites without membrane exposure (4.69 [0.13] mm) (p < 0.05) [35]. It has been suggested that such membrane exposure strongly increases the risk of bacterial contamination [36], which ultimately compromises the possible success of periodontal regeneration [34]. In addition, the early exposure to the oral environment can accelerate the degradation process of the membrane [15, 37, 38], which could have a deleterious effect on its barrier function and thereby result in the down-growth of gingival epithelium and ultimately a repair type of healing with the long junctional epithelium (LJE). Thus, the GTR procedure could have a morbidity effect on the clinical outcome, adversely affecting the potential success of periodontal regeneration by GTR treatment. In contrast, other periodontal regenerative methods such as the use of enamel matrix derivative (EMD) (i.e. Emdogain®), an extract of enamel proteins including amelgenins of various molecular weights, would generate fewer post-operative complications although the actual clinical advantages of using this material are still uncertain [39].
Several factors including the initial defect depth and morphology of the intrabony defects have been suggested to influence the ΔCAL and ΔPPD following GTR therapy [40–42]. The GTR sites of the present study had an average baseline PPD of 6.1 mm [5.7, 6.5], baseline CAL of 6.6 mm [6.3, 7.0], and most intrabony components were categorised as three-wall defect with intrabony defect depth of 3.8 mm [3.0, 12.0]. Compared to the present study, Cortellini et al. [25] and Mengel et al. [15] reported a relatively higher baseline PPD of 8.2 [1.9] mm and 8.0 [1.4] mm and the baseline CAL of 9.5 [1.2] mm and 9.4[1.4] mm, respectively. In addition, the multicentre study by Cortellini et al. [25] also found a deeper intrabony component of 6.3 [1.7] mm than the present study. It has previously been demonstrated that greater amounts of clinical attachment and bone can be gained in deeper intrabony defects [40, 43]. Moreover, defects deeper than 3 mm have been found to result consistently in greater probing attachment gain than defects of 3 mm or less [44]. In addition, a previous study showed that an ΔCAL was associated with the depth of the three-wall intrabony components of the defect [20]. The findings of this study thus also support the suggestion that levels of ΔCAL and ΔPPD following periodontal regeneration maybe influenced, at least partly, by the morphology of the initial defects.
Another factor that has shown to influence the clinical outcomes following GTR therapy is the oral hygiene of the patients throughout the healing period [40]. The average FMPS in the present study were low from the beginning since all patients had received non-surgical therapy with a session of oral hygiene instruction and reinforcement as necessary. This FMPS remained relatively low throughout the follow-up period but still relatively higher than other GTR studies [20, 45]. Such differences may partly contribute to the reduced clinical improvements in terms of ΔCAL and ΔPPD of the present study when compared to other investigations [20, 45].
Conclusion
The present study showed that both AF and GTR were effective in the treatment of intrabony defects in patients affected by AgP, although no significant differences between the two treatments could be demonstrated possibly because of the varying extent of membrane exposure observed in this trial.
Based on the results of the present study, it can be concluded that:
-
both SPPF alone and GTR with an SPPF flap design can be effectively used for the treatment of intrabony defects of AgP patients;
-
both SPPF and GTR treatments resulted in significant gain in CAL and reduction in PPD at 6 and 12 months;
-
the ΔCAL was found to be reduced at the sites with membrane exposure, compared to those with the non-exposed membrane.
References
Garrett S (1996) Periodontal regeneration around natural teeth. Ann Periodontol/Am Acad Periodontology 1:621–666. doi:10.1902/annals.1996.1.1.621
Papapanou PN, Wennstrom JL (1991) The angular bony defect as indicator of further alveolar bone loss. J Clin Periodontol 18:317–322
Caton J, Nyman S, Zander H (1980) Histometric evaluation of periodontal surgery. II Connective tissue attachment levels after four regenerative procedures. J Clin Periodontology 7:224–231
Cortellini P, Prato GP, Tonetti MS (1999) The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int J Periodontics Restorative Dent 19:589–599
Retzepi M, Tonetti M, Donos N (2007) Comparison of gingival blood flow during healing of simplified papilla preservation and modified Widman flap surgery: a clinical trial using laser Doppler flowmetry. J Clin Periodontol 34:903–911. doi:10.1111/j.1600-051X.2007.01119.x
Karring T, Nyman S, Gottlow J, Laurell L (1993) Development of the biological concept of guided tissue regeneration—animal and human studies. Periodontology 2000 1:26–35
Cortellini P, Pini Prato G, Tonetti MS (1996) The modified papilla preservation technique with bioresorbable barrier membranes in the treatment of intrabony defects. Case reports. Int J Periodontics Restorative Dent 16:546–559
Needleman I, Tucker R, Giedrys-Leeper E, Worthington H (2002) A systematic review of guided tissue regeneration for periodontal infrabony defects. J Periodontal Res 37:380–388
Murphy KG, Gunsolley JC (2003) Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol/Am Acad Periodontology 8:266–302. doi:10.1902/annals.2003.8.1.266
Needleman I, Tucker R, Giedrys-Leeper E, Worthington H (2005) Guided tissue regeneration for periodontal intrabony defects—a Cochrane systematic review. Periodontology 2000 37:106–123. doi:10.1111/j.1600-0757.2004.37101.x
DiBattista P, Bissada NF, Ricchetti PA (1995) Comparative effectiveness of various regenerative modalities for the treatment of localized juvenile periodontitis. J Periodontol 66:673–678. doi:10.1902/jop.1995.66.8.673
Sirirat M, Kasetsuwan J, Jeffcoat MK (1996) Comparison between 2 surgical techniques for the treatment of early-onset periodontitis. J Periodontol 67:603–607. doi:10.1902/jop.1996.67.6.603
Benque E, Zahedi S, Brocard D, Oscaby F, Justumus P, Brunel G (1997) Combined collagen membrane and hydroxyapatite/collagen chondroitin-sulfate spacer placement in the treatment of 2-wall intrabony defects in chronic adult and rapidly progressive periodontitis patients. J Clin Periodontol 24:550–556
Benque E, Zahedi S, Brocard D, Oscaby F, Justumus P, Brunel G (1997) Guided tissue regeneration using a collagen membrane in chronic adult and rapidly progressive periodontitis patients in the treatment of 3-wall intrabony defects. J Clin Periodontol 24:544–549
Mengel R, Soffner M, Flores-de-Jacoby L (2003) Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: results of a 12-month clinical and radiological study. J Periodontol 74:899–908. doi:10.1902/jop.2003.74.6.899
Wennstrom A, Wennstrom J, Lindhe J (1986) Healing following surgical and non-surgical treatment of juvenile periodontitis. A 5-year longitudinal study. J Clin Periodontol 13:869–882
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol/Am Acad Periodontology 4:1–6. doi:10.1902/annals.1999.4.1.1
Altman DG, Bland JM (2005) Treatment allocation by minimisation. BMJ 330:843. doi:10.1136/bmj.330.7495.843
Tonetti MS, Lang NP, Cortellini P, Suvan JE, Adriaens P, Dubravec D, Fonzar A, Fourmousis I, Mayfield L, Rossi R, Silvestri M, Tiedemann C, Topoll H, Vangsted T, Wallkamm B (2002) Enamel matrix proteins in the regenerative therapy of deep intrabony defects. J Clin Periodontol 29:317–325
Cortellini P, Tonetti MS (2000) Focus on intrabony defects: guided tissue regeneration. Periodontology 2000(22):104–132
Tonetti MS, Cortellini P, Suvan JE, Adriaens P, Baldi C, Dubravec D, Fonzar A, Fourmousis I, Magnani C, Muller-Campanile V, Patroni S, Sanz M, Vangsted T, Zabalegui I, Pini Prato G, Lang NP (1998) Generalizability of the added benefits of guided tissue regeneration in the treatment of deep intrabonydefects. Evaluation in a multi-center randomized controlled clinical trial. J Periodontol 69:1183–1192. doi:10.1902/jop.1998.69.11.1183
Pritlove-Carson S, Palmer RM, Floyd PD (1995) Evaluation of guided tissue regeneration in the treatment of paired periodontal defects. Br Dent J 179:388–394
Bratthall G, Soderholm G, Neiderud AM, Kullendorff B, Edwardsson S, Attstrom R (1998) Guided tissue regeneration in the treatment of human infrabony defects. Clinical, radiographical and microbiological results: a pilot study. J Clin Periodontol 25:908–914
Ratka-Kruger P, Neukranz E, Raetzke P (2000) Guided tissue regeneration procedure with bioresorbable membranes versus conventional flap surgery in the treatment of infrabony periodontal defects. J Clin Periodontol 27:120–127
Cortellini P, Tonetti MS, Lang NP, Suvan JE, Zucchelli G, Vangsted T, Silvestri M, Rossi R, McClain P, Fonzar A, Dubravec D, Adriaens P (2001) The simplified papilla preservation flap in the regenerative treatment of deep intrabony defects: clinical outcomes and postoperative morbidity. J Periodontol 72:1702–1712. doi:10.1902/jop.2001.72.12.1702
Wikesjo UM, Selvig KA (1999) Periodontal wound healing and regeneration. Periodontology 2000(19):21–39
Retzepi M, Tonetti M, Donos N (2007) Gingival blood flow changes following periodontal access flap surgery using laser Doppler flowmetry. J Clin Periodontol 34:437–443. doi:10.1111/j.1600-051X.2007.01062.x
Donos N, D’Aiuto F, Retzepi M, Tonetti M (2005) Evaluation of gingival blood flow by the use of laser Doppler flowmetry following periodontal surgery. A pilot study. J Periodontal Res 40:129–137. doi:10.1111/j.1600-0765.2005.00777.x
Lundgren D, Laurell L, Gottlow J, Rylander H, Mathisen T, Nyman S, Rask M (1995) The influence of the design of two different bioresorbable barriers on the results of guided tissue regeneration therapy. An intra-individual comparative study in the monkey. J Periodontol 66:605–612. doi:10.1902/jop.1995.66.7.605
Christgau M, Bader N, Schmalz G, Hiller KA, Wenzel A (1998) GTR therapy of intrabony defects using 2 different bioresorbable membranes: 12-month results. J Clin Periodontol 25:499–509
Dorfer CE, Kim TS, Steinbrenner H, Holle R, Eickholz P (2000) Regenerative periodontal surgery in interproximal intrabony defects with biodegradable barriers. J Clin Periodontol 27:162–168
Eickholz P, Kim TS, Steinbrenner H, Dorfer C, Holle R (2000) Guided tissue regeneration with bioabsorbable barriers: intrabony defects and class II furcations. J Periodontol 71:999–1008. doi:10.1902/jop.2000.71.6.999
Christgau M, Bader N, Felden A, Gradl J, Wenzel A, Schmalz G (2002) Guided tissue regeneration in intrabony defects using an experimental bioresorbablepolydioxanon (PDS) membrane. A 24-month split-mouth study. J Clin Periodontol 29:710–723
Ling LJ, Hung SL, Lee CF, Chen YT, Wu KM (2003) The influence of membrane exposure on the outcomes of guided tissue regeneration: clinical and microbiological aspects. J Periodontal Res 38:57–63
Machtei EE (2001) The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol 72:512–516. doi:10.1902/jop.2001.72.4.512
Chen YT, Wang HL, Lopatin DE, O’Neal R, MacNeil RL (1997) Bacterial adherence to guided tissue regeneration barrier membranes exposed to the oral environment. J Periodontol 68:172–179. doi:10.1902/jop.1997.68.2.172
Cortellini P, Clauser C, Prato GP (1993) Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol 64:387–391. doi:10.1902/jop.1993.64.5.387
Cortellini P, Pini Prato G, Tonetti MS (1993) Periodontal regeneration of human infrabony defects. II Re-entry procedures and bone measures. J Periodontol 64:261–268. doi:10.1902/jop.1993.64.4.261
Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV (2009) Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur J Oral Implantol 2:247–266
Tonetti MS, Prato GP, Cortellini P (1996) Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol 23:548–556
Falk H, Laurell L, Ravald N, Teiwik A, Persson R (1997) Guided tissue regeneration therapy of 203 consecutively treated intrabony defects using a bioabsorbable matrix barrier. Clinical and radiographic findings. J Periodontol 68:571–581. doi:10.1902/jop.1997.68.6.571
Trombelli L, Kim CK, Zimmerman GJ, Wikesjo UM (1997) Retrospective analysis of factors related to clinical outcome of guided tissue regeneration procedures in intrabony defects. J Clin Periodontol 24:366–371
Tonetti MS, Pini-Prato G, Cortellini P (1993) Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J Periodontol 64:934–940. doi:10.1902/jop.1993.64.10.934
Cortellini P, Carnevale G, Sanz M, Tonetti MS (1998) Treatment of deep and shallow intrabony defects. A multicenter randomized controlled clinical trial. J Clin Periodontol 25:981–987
Tonetti MS, Cortellini P, Lang NP, Suvan JE, Adriaens P, Dubravec D, Fonzar A, Fourmousis I, Rasperini G, Rossi R, Silvestri M, Topoll H, Wallkamm B, Zybutz M (2004) Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J Clin Periodontol 31:770–776. doi:10.1111/j.1600-051X.2004.00562.x
Acknowledgments
This study was partly supported by the Periodontal Research Fund of the Unit of Periodontology at the Eastman Dental Institute, University College London, UK and a Scholarship of The Royal Thai Government. The authors acknowledge support from staff of the Periodontology Unit, Dr Jean Suvan for randomisation processes, Ms Bambai Hirani for chair-side clinical assistance and Ms Donna Moskal-Fritzpatrick for trial coordination.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rakmanee, T., Griffiths, G.S., Auplish, G. et al. Treatment of intrabony defects with guided tissue regeneration in aggressive periodontitis: clinical outcomes at 6 and 12 months. Clin Oral Invest 20, 1217–1225 (2016). https://doi.org/10.1007/s00784-015-1608-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1608-z