Abstract
Introduction
This clinical study was conducted to compare the effectiveness of single-file reciprocating systems and rotary systems in removing endotoxins and cultivable bacteria in endodontic retreatment.
Methods
Thirty endodontically treated teeth with post-treatment apical periodontitis were selected. The specimens were divided into three groups according to the system used: WaveOne (n = 10), Reciproc instrument (n = 10), and ProTaper Universal Retreatment system (n = 10). Samples were collected before and after chemomechanical preparation. The irrigation was performed by using 2.5 % sodium hypochlorite. A chromogenic limulus amebocyte lysate assay test was used to quantify endotoxins. Culture techniques were used to determine bacterial colony-forming unit counts.
Results
At baseline, endotoxins and cultivable bacteria were recovered from 100 % of the root canal samples in a median value of 5.84 EU/mL and 4.98 × 103 CFU/mL, respectively. After CMP, no differences were found in the median percentage values of endotoxin reduction achieved with reciprocating systems—WaveOne [94.11 %] and Reciproc [93.29 %] and with rotary systems—ProTaper [94.98 %] (P > 0.05). Both single-file reciprocating systems [WaveOne (98.27 %) and Reciproc (99.54 %)] and rotary system [ProTaper (98.73 %)] were effective in reducing bacterial load (P > 0.05). Moreover, no differences were found among the systems tested.
Conclusions
The Reciproc and WaveOne reciprocating systems were as effective as the ProTaper system for removal of endotoxins and bacteria in endodontic retreatment.
Clinical relevance
All systems tested were effective to remove cultivable bacteria and endotoxin in endodontic retreatment. As no differences among systems were observed, it is possible to suggest that clinicians should choose the preferred technique to perform endodontic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the main causes of post-treatment apical periodontitis is the remaining infection present in root canal systems after endodontic treatment [1]. Considering that chemomechanical preparation assumes a pivotal role in endodontic retreatments in order to promote a thorough cleaning, disinfection, and shaping of the root canal [2].
Previous studies have shown that, regardless of the instrumentation technique and instruments/irrigants used, chemomechanical procedures are unable to promote an optimal disinfection of the root canal systems [3–7]. Additionally, the limited efficacy of hand and rotary instrumentation for removing root-filling material from endodontically treated teeth is also demonstrated [8–10].
In order to circumvent such limitations and make cleaning, disinfection, and shaping more predictable, modifications in instruments and techniques have been devised. The single-file systems are an example of a modified instrument that has been designed to shape the root canal completely from start to finish with one single file [11–14]. Particularly, the Reciproc (VDW) and WaveOne (Dentsply Maillefer) systems, are 2-M-wire reciprocating systems [14] that alloy increases instrument flexibility and improves its resistance to cyclic fatigue [15]. Recently, the effectiveness of reciprocating systems—i.e., WaveOne and Reciproc—in removing gutta-percha and sealer during endodontic retreatment has been demonstrated [16, 17]. However, evidence on their cleaning and disinfecting abilities is only incipient [12, 18]. Thereby, no clinical study has compared the ability of single-file instruments to rotary systems in the disinfection of endodontically treated teeth.
Taking into account that endodontic retreatments are a challenge with a high level of difficulty and are time consuming [8, 16], together with the convenience simplification of single-file instruments [19, 20]—which may promote cleaning and shaping effects, which is less time consuming compared with full series of rotary instruments—this clinical study was conducted to compare the effectiveness of single-file reciprocating systems and rotary systems in removing endotoxins and bacteria in endodontic retreatment.
Material and methods
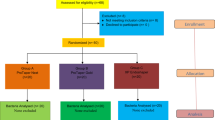
Thirty patients in need of endodontic retreatment were selected. Thirty teeth were included, all being previously root-filled and showing radiographic evidence of apical periodontitis. A detailed medical and dental history was obtained from each patient. None of the patients evaluated presented periodontal disease. Failure of root canal treatment was determined on the basis of clinical and radiographic examinations. The Human Research Ethics Committee of the São José dos Campos Dental School approved the research protocol describing the sample collection for this investigation, and all volunteer patients signed an informed consent form.
A sample size calculation was performed based on the article published by Martinho and colleagues [18]. It was considered an alpha of 95 % and power of 80 %, which resulted in nine samples per group totaling to 27 teeth required. Samples were collected from 30 single-rooted teeth, with their pulp chamber having no visual communication with oral fluid caused by extensive decay or failure in restoration and no periodontal disease. Teeth that could not be isolated with rubber dam were excluded. Patients who had received antibiotic treatment during the last 3 months or who had undergone previous root canal treatment in the last 2 years were excluded from the study.
The presence of persistent periapical radiolucent lesion, voids in or around the root canal filling examined at ×16 magnification with an operating microscope (Opto, São Carlos, São Paulo, Brazil), persistent symptoms such as pain on palpation and discomfort to percussion, and persistent sinus were considered to be reasons for retreatment [21]. Cases with suspicion of vertical root fracture were submitted to an operating microscope and computerized tomography analysis and were excluded if a fracture was verified. Undefined cases with persistent signs and symptoms of a possible root fracture and those where an exploratory surgery was required were also excluded.
Files, instruments, and all materials used in this study were treated with Co60 gamma radiation (20 kGy for 6 h) for sterilization and elimination of preexisting endotoxins (EMBRARAD; Empresa Brasileira de Radiação, Cotia, SP, Brazil). The method used for disinfection of the operative field has been previously described elsewhere [22]. Briefly, the teeth were isolated with a rubber dam. The crown and surrounding structures were disinfected with 30 % H2O2 (volume/volume for 30 s), followed by 2.5 % NaOCl for the same period of time and then inactivated with 5 % sodium thiosulfate. The sterility of the external surfaces of the crown was checked by taking a swab sample from the crown surface and streaking it onto blood agar plates, which were then incubated both aerobically and anaerobically. The microbiological procedures used in this study had been previously described by Endo et al. [7]. A two-stage access preparation was performed. The access cavity was made without the use of water spray but under manual irrigation with sterile/endotoxin-free saline and by using sterile/endotoxin-free high-speed diamond bur. This first stage was performed to promote a major removal of the contaminants (microorganisms and endotoxins). In the second stage, before entering the pulp chamber, the access cavity was disinfected according to the decontamination protocol described above. Sterility was checked by taking swab samples of the cavity surface and streaking them onto blood agar plates, with subsequent incubation at 37 °C under both aerobic and anaerobic conditions. A new sterile pyrogen-free bur was used under irrigation with sterile/endotoxin-free saline to access the canal. In order to achieve the full length of the canal for the first microbiological and endotoxins samplings (s1), a k-file (Dentsply Maillefer, Ballaigues, Switzerland) pathway was used through root-filling materials into the full length of the canal—determined by the pre-operative. The first endotoxin sampling was taken by introducing sterile/apyrogenic paper points (Dentsply Maillefer) into the full length of the canal, which was determined radiographically and retained in position for 60 s for sampling. Immediately afterward, the sample was placed in a pyrogen-free glass and immediately suspended in 1-mL limulus amebocyte lysate (LAL) water according to the endotoxin dosage by using a kinetic chromogenic LAL (Lonza, Walkersville, MD) assay. This sampling procedure was repeated with three paper points that were pooled in a sterile tube containing 1-mL Viability Medium Göteborg Agar III (VMGA III) transport medium [23] for microbial cultivation.
After accessing the pulp chamber and subsequent first endotoxin sampling, teeth were randomly divided into three groups: WaveOne (Dentsply Maillefer, Ballaigues, Switzerland) (n = 10); Reciproc instrument (VDW, Munich, Germany) (n = 10), and ProTaper Universal Retreatment system (Dentsply Maillefer) (n = 10). After the first sampling, the root canal length was determined from the pre-operative radiograph and confirmed using an apex locator (Novapex; Forum Technologies, Rishon le-Zion, Israel). The root canals were then prepared according to the group selection.
All instruments were set into permanent rotation with a 6:1 contra-angle handpiece (Sirona, Bensheim, Germany) powered by a torque-limited electric motor (VDW.Silver Reciproc motor, VDW). The preparation were as follows: in WaveOne group, the primary instrument of the WaveOne system was used with the VDW Silver motor in an in-and-out pecking motion of about 3 mm in amplitude with very light apical pressure in the “WaveOne” mode. After three pecking motions, the instrument was removed from the canal and cleaned. This procedure was repeated until reaching the working length. In Reciproc group, the Reciproc system was used with the VDW Silver motor (VDW) in an in-and-out pecking motion of about 3 mm in amplitude with very light apical pressure in the “Reciproc all” mode. After three pecking motions, the instrument was removed from the canal and cleaned. It was repeated until reaching the working length. The D1, D2, and D3 files of the ProTaper Universal Retreatment system were used sequentially in a pecking motion toward the apex until reaching the working length with D3 for group 3. All instruments were used with the VDW Silver motor at a constant speed of 500 rpm for D1 and of 400 rpm for both D2 and D3 with a torque of 3 Ncm.
Irrigation was performed with disposable syringes and 30-G NaviTip needles (Ultradent, South Jordan, UT) by using 5 mL 2.5 % NaOCl solution between the pecking sequences (WaveOne and Reciproc groups) and between files (ProTaper).
Before the second sampling after instrumentation, NaOCl was inactivated with 5-mL sterile 0.5 % sodium thiosulfate during a 1-min period, which was then removed with 5-mL sterile/ apyrogenic water. Next, a new sampling procedure (s2) was performed as described previously at s1.
Determination of endotoxin concentration (kinetic chromogenic LAL assay)
The kinetic chromogenic LAL assay (Lonza) used for quantification of endotoxins had been previously reported by the author [24]. Briefly, for the test, 100-mL apyrogenic water (reaction blank), the five standard endotoxin solutions (0.005–50 endotoxin units [EU]/mL), root canal samples, and positive controls (each root canal sample contaminated with a known concentration of endotoxin [10 EU/mL]) were added to a 96-well apyrogenic plate. The tests were carried out in quadruplicate. The plate was incubated at 37 ± 1 °C for 10 min in a Kinetic-QCL (Lonza) reader, which was coupled to a microcomputer by means of the WinKQCL software. Next, 100 mL of chromogenic reagent were added to each well. After the beginning of the kinetic test, the software continuously monitored absorbance at 405 nm in each microplate well and automatically calculated the log/log linear correlation between reaction time of each standard solution and the corresponding endotoxin concentration.
Determination of cultivable bacterial counts (culturing procedure)
The method used for culture procedures in the present study had been previously reported by the author [24]. Briefly, the transport media containing the root canal samples were thoroughly shaken for 60 s (Vortex; Marconi, Piracicaba, São Paulo, Brazil). Serial 10-fold dilutions were made up to 10−4 in tubes containing fastidious anaerobe broth (FAB; Lab M, Bury, UK). Fifty microliters of the serial dilutions were plated onto 5 % defibrinated sheep blood fastidious anaerobe agar (FAA; Lab M) by using sterile plastic spreaders to culture non-selectively obligate anaerobes and facultative anaerobes. The plates were incubated at 37 °C in anaerobic atmosphere for up to 14 days. After this period, colony-forming units (CFUs) were visually quantified for each plate.
Statistical analysis
The data collected (endotoxin concentrations and CFU counts) were statistically analyzed by using the SPSS for Windows (SPSS, Inc., Chicago, IL, USA). The Kolmogorov–Smirnov’s test showed that the distributions of the studied variables deviated from the normality. Wilcoxon test was used when significant differences were found between different sampling times. Comparison between the root canal treatment groups (WaveOne, Reciproc, and ProTaper Universal Retreatment systems) was performed by using the Kruskal–Wallis test. Significance level was always set at 5 % (P < 0.05).
Results
Sterility samples taken from the external and internal surfaces of the crown and its surrounding structures, tested before and after entering the pulp chamber, showed no microbial growth.
Determination of endotoxin concentration (LAL assay)
The standard curve for detection of endotoxins fulfilled the criteria of linearity (r = 1). At the baseline (s1), the LAL assay indicated that endotoxins were detected in 100 % of the root canals with a median value of 5.84 EU/mL (range, 0.093–9.15 EU/mL) (30/30). Individual median and ranging values of endotoxins found in all groups at different sampling times are shown in Table 1. At s2, regardless of the instrumentation systems tested, endotoxins were still detected in all root canal samples (30/30). After CMP (s2), no differences were found in the median percentage values of endotoxin reduction achieved with reciprocating systems—WaveOne [94.11 %] and Reciproc [93.29 %] and with rotary systems—ProTaper Universal Retreatment [94.98 %] (P > 0.05).
Determination of cultivable bacterial counts (culturing procedure)
Bacteria were found in all initial samples of the 30 root canals investigated. In the baseline samples, cultivable bacteria were recovered from 100 % of the root canals tested with a median value of 4.98 × 103 CFU/mL (range, 2.6 × 102 to 1.6 × 105 CFU/mL) (30/30). Regarding bacterial reduction, no differences were found comparing WaveOne [98.27 %], Reciproc [99.54 %], and ProTaper Universal Retreatment [98.73 %] systems were effective in reducing the bacteria (P > 0.05). At s2, cultivable bacteria was recovered in 3 of 10 (30 %) for WaveOne, 4 of 10 (40 %) for Reciproc, and 4 of 10 (40 %) for ProTaper Universal Retreatment. Table 1 provides an overview of the endotoxin concentrations (EU/mL) and amount of cultivable bacteria (CFU/mL). The percentage values of bacterial and endotoxin reductions found in all groups tested at different sampling times are shown in Fig. 1.
Discussion
Data obtained in the present study have indicated that the Reciproc and WaveOne reciprocating systems were as effective as the ProTaper Universal retreatment system for removal of endotoxins and bacteria in endodontic retreatment. Thus, none of the instrumentation systems were effective on eliminating endotoxins and bacteria in 100 % of the root canals investigated.
It is generally acknowledged that in most cases in which endodontic treatment fails, the failure occurs when treatment procedures have not met a satisfactory standard for control and elimination of infection [25]. In consonance, at the baseline samples, the present study revealed the presence of cultivable bacteria in 100 % of the samples from endodontically treated teeth. The initial number of viable bacteria in post-treatment apical periodontitis ranged from 102 to 105 CFU/mL, which is comparable to previous investigations [7, 26, 27].
In the present study, Reciproc and WaveOne reciprocating instruments and ProTaper Universal Retreatment rotary instruments were used without the use of solvent. Controversies, exists on the use of solvent during endodontic retreatment [16, 17, 26]. Although solvents such as chloroform have been use to facilitate the process, their side-effects—i.e., cytotoxic potential and potential for forming a residual film of softened gutta-percha on the dentin walls—can overcome their benefits [17, 26].
The LAL assay used for quantification of Gram-negative bacterial endotoxins in the root canal samples collected before and after chemomechanical preparation is based on a serine protease catalytic coagulation cascade activated by the presence of endotoxins [26]. Because of its extreme sensitivity to endotoxins [28], LAL assay is the most widely used method for analysis of endodontic contents [7, 18, 22, 24].
The results of the kinetic chromogenic LAL assay indicated the presence of Gram-negative bacteria endotoxins in all root canal samples from endodontically treated teeth analyzed. This finding is supported by molecular studies that indicate Gram-negative bacterial species as candidate pathogens in post-treatment disease [7, 29]. Our study revealed the median value of endotoxins of 5.84 EU/mL (range, 0.093–9.15 EU/mL). Endo et al. [7] investigating the levels of endotoxins in endodontically treated teeth, revealed a median value of 3.96 EU/mL by the Turbidimetric Kinetic LAL assay. Such difference might be attributed the LAL test selection [30]. Particularly, the KQCL-test (Kinetic Chromogenic LAL test) used in the present study is one of the LAL tests that best fits for analysis of endotoxins present in root canal infection because of its better precision and better reproducibility compared to other tests [30].
After chemomechanical preparation (s2), both single-file reciprocating systems and rotary systems were effective in reducing more than 93 % of endotoxin contents from endodontically treated teeth, with no statistical differences between them. Martinho et al. [18] reported the ability of WaveOne and Reciproc systems in reducing endotoxin contents in 95.15 and 96.21 % from primarily infected root canals, respectively. Thereby, at s2, regardless of the instrumentation systems tested, endotoxins were still detected in all root canal samples from endodontically treated teeth. The limited ability of root canal procedures on eliminating endotoxins has also been reported [7, 22]. It is important to highlight whether this residual of endotoxins is capable of perpetuating an inflammatory process in periapical tissues, is a yet unknown issue. However, it is known that little amounts of endotoxins can exhibit a potent inflammatory response.
Currently, the culture analysis revealed that both single-file reciprocating instrumentation—WaveOne [98.27 %], Reciproc [99.54 %]—and ProTaper Universal Retreatment [98.73 %] showed to be similarly effective in reducing bacterial load. The effectiveness of reciprocating systems in reducing in reducing bacterial load in >94 % has been demonstrated by previous in vitro studies [11, 12]. Moreover, the efficacy of WaveOne, Reciproc, and ProTaper Universal Retreatment system for removing filling material from endodontically treated teeth—an important aspect for disinfection—has been elucidated [10, 16, 17].
Regardless of the instrumentation technique selected, our findings indicated that cultivable bacteria were still detected in 30–40 % of the root canal samples. Previous studies have shown that, regardless of the instrumentation technique and instruments/irrigants used, chemomechanical procedures are unable to promote an optimal disinfection of the root canal systems [3–5, 7]. The clinical implications of bacterial persistence in infected root canals after endodontic treatment have been discussed in endodontic literature [6, 31].
It is important to highlight that some differences can be found between the file systems selected. The Reciproc and WaveOne files used in the present study are made of a special NiTi alloy called M-Wire, created by means of an innovative thermal treatment process [32–34]. The benefits of this M-Wire alloy are increased flexibility and improved resistance to cyclic fatigue of the instruments [32–34]. This reciprocating system produces a wider motion in the counter-clockwise direction but shorter in the clockwise course, resulting in a more centered file in the canal. Associated with the marked taper of these files, this condition creates a greater contact area between the instrument and gutta-percha, allowing filling removal as effective as that obtained with continuous rotation [17]. The Reciproc and WaveOne files are used in a reciprocal motion, which requires special automated devices. Rotary instruments convey debris toward the cervical portion of the canal because of their cross-sectional shape in combination with the rotary motion that acts like a screw-conveyor [17]. Thus, ProTaper Universal Retreatment system is able to remove large amounts of gutta-percha through spirals running around the instruments, which produce both cutting and softening actions. Regarding the tip diameter of the systems tested, the D3 of the ProTaper Universal Retreatment system is the final instrument recommended by the manufacturer, whereas the Reciproc and the primary of the WaveOne systems were elected for being the reciprocating instruments whose tips are the closest equivalent to the tip of the D3 instrument. Controversy exists on whether the size of apical enlargement can significantly influence the outcome of root canal disinfection [35, 14, 11]. Despite the different apical preparation diameter as well as the differences in the design features of the file systems evaluated in the present study tested, our results showed no differences in the median percentage values of bacterial and endotoxin reduction.
Overall, the present investigation demonstrated that although in the groups of WaveOne and Reciproc systems, only one file was fully used for root canal retreatment, there was no impact on the root canal disinfection, particularly against endotoxins as well as on cultivable bacteria present in endodontically treated teeth compared to full-sequence ProTaper Universal Retreatment system. In conclusion, the Reciproc and WaveOne reciprocating systems were as effective as the ProTaper Universal retreatment system for removal of endotoxins and bacteria in endodontic retreatment.
References
Siqueira JF Jr, Lopes HP (2011) Chemomechanical preparation. In: Siqueira JF Jr (ed) Treatment of endodontic infections. Quintessence Publishing, London
Siqueira JF Jr, Alves FR, Versiani MA, Rôças IN, Almeida BM, Neves MA, Sousa-Neto MD (2013) Correlative bacteriologic and micro-computed tomographic analysis of mandibular molar mesial canals prepared by self-adjusting file, reciproc, and twisted file systems. J Endod 39:1044–1050. doi:10.1016/j.joen.2013.04.034
Barbizam JV, Fariniuk LF, Marchesan MA, Pecora JD, Sousa-Neto MD (2002) Effectiveness of manual and rotary instrumentation techniques for cleaning flattened root canals. J Endod 28:365–366
Siqueira JF Jr, Araújo MC, Garcia PF, Fraga RC, Dantas CJ (1997) Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod 23:499–502. doi:10.1016/S0099-2399(97)80309-3
Peters OA, Schönenberger K, Laib A (2001) Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 34:221–230
Siqueira JF Jr, Rôças IN (2008) Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 34(1291–1301):e1293. doi:10.1016/j.joen.2008.07.028
Endo MS, Martinho FC, Zaia AA, Ferraz CC, Almeida JF, Gomes BP (2012) Quantification of cultivable bacteria and endotoxin in post-treatment apical periodontitis before and after chemo-mechanical preparation. Eur J Clin Microbiol Infect Dis 31:2575–2583. doi:10.1007/s10096-012-1598-6
Bramante CM, Fidelis NS, Assumpção TS, Bernardineli N, Garcia RB, Bramante AS, de Moraes IG (2010) Heat release, time required, and cleaning ability of MTwo R and ProTaper universal retreatment systems in the removal of filling material. J Endod 36:1870–1873. doi:10.1016/j.joen.2010.08.013
Hammad M, Qualtrough A, Silikas N (2008) Three-dimensional evaluation of effectiveness of hand and rotary instrumentation for retreatment of canals filled with different materials. J Endod 34:1370–1373. doi:10.1016/j.joen.2008.07.024
Ma J, Al-Ashaw AJ, Shen Y, Gao Y, Yang Y, Zhang C, Haapasalo M (2012) Efficacy of ProTaper Universal Rotary Retreatment system for gutta-percha removal from oval root canals: a micro-computed tomography study. J Endod 38:1516–1520. doi:10.1016/j.joen.2012.08.001
Machado ME, Nabeshima CK, Leonardo MF, Reis FA, Britto ML, Cai S (2013) Influence of reciprocating single-file and rotary instrumentation on bacterial reduction on infected root canals. Int Endod J 46:1083–1087. doi:10.1111/iej.12108
Alves FR, Rôças IN, Almeida BM, Neves MA, Zoffoli J, Siqueira JF Jr (2012) Quantitative molecular and culture analyses of bacterial elimination in oval-shaped root canals by a single-file instrumentation technique. Int Endod J 45:871–877. doi:10.1111/j.1365-2591.2012.02045.x
Arias A, Perez-Higueras JJ, de la Macorra JC (2012) Differences in cyclic fatigue resistance at apical and coronal levels of Reciproc and WaveOne new files. J Endod 38:1244–1248. doi:10.1016/j.joen.2012.05.022
Bürklein S, Schäfer E (2012) Apically extruded debris with reciprocating single-file and full-sequence rotary instrumentation systems. J Endod 38:850–852. doi:10.1016/j.joen.2012.02.017
Shen Y, Cheung GS, Bian Z, Peng B (2006) Comparison of defects in ProFile and ProTaper systems after clinical use. J Endod 32:61–65. doi:10.1016/j.joen.2005.10.017
Fruchi Lde C, Ordinola-Zapata R, Cavenago BC, Hungaro Duarte MA, da Silveira Bueno CE, De Martin AS (2014) Efficacy of reciprocating instruments for removing filling material in curved canals obturated with a single-cone technique: a micro-computed tomographic analysis. J Endod 40:1000–1004. doi:10.1016/j.joen.2013.12.011
Rios Mde A, Villela AM, Cunha RS, Velasco RC, De Martin AS, Kato AS, Bueno CE (2014) Efficacy of 2 reciprocating systems compared with a rotary retreatment system for gutta-percha removal. J Endod 40:543–546. doi:10.1016/j.joen.2013.11.013
Martinho FC, Gomes AP, Fernandes AM, Ferreira NS, Endo MS, Freitas LF, Camões IC (2014) Clinical comparison of the effectiveness of single-file reciprocating systems and rotary systems for removal of endotoxins and cultivable bacteria from primarily infected root canals. J Endod 40:625–629. doi:10.1016/j.joen.2013.12.006
Buchanan LS (2000) The standardized-taper root canal preparation–Part 1. Concepts for variably tapered shaping instruments. Int Endod J 33:516–529
Yared G (2008) Canal preparation using only one Ni-Ti rotary instrument: preliminary observations. Int Endod J 41:339–344. doi:10.1111/j.1365-2591.2007.01351.x
Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ (2003) Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 36:1–11. doi:10.1046/j.1365-2591.2003.00603.x
Martinho FC, Chiesa WM, Marinho AC, Zaia AA, Ferraz CC, Almeida JF, Souza-Filho FJ, Gomes BP (2010) Clinical investigation of the efficacy of chemomechanical preparation with rotary nickel-titanium files for removal of endotoxin from primarily infected root canals. J Endod 36:1766–1769. doi:10.1016/j.joen.2010.08.019
Möller AJR (1966) Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 74:1–380
Xavier AC, Martinho FC, Chung A, Oliveira LD, Jorge AO, Valera MC, Carvalho CA (2013) One-visit versus two-visit root canal treatment: Effectiveness in the removal of endotoxins and cultivable bacteria. J Endod 39:959–964. doi:10.1016/j.joen.2013.04.027
Nair PN, Sjögren U, Figdor D, Sundqvist G (1999) Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 87:617–627
Cheung GS, Ho MW (2001) Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol 16:332–337
Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M (2001) Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J 34:429–434
Gu LS, Ling JQ, Wei X, Huang XY (2008) Efficacy of ProTaper Universal rotary retreatment system for gutta-percha removal from root canals. Int Endod J 41:288–295. doi:10.1111/j.1365-2591.2007.01350.x
Rôças IN, Siqueira JF Jr (2012) Characterization of microbiota of root canal-treated teeth with posttreatment disease. J Clin Microbiol 50:1721–1724. doi:10.1128/JCM. 00531-12
Martinho FC, Chiesa WM, Zaia AA, Ferraz CC, Almeida JF, Souza-Filho FJ, Gomes BP (2011) Comparison of endotoxin levels in previous studies on primary endodontic infections. J Endod 37:163–167. doi:10.1016/j.joen.2010.11.020
Fabricius L, Dáhlen G, Sundqvist G, Happonen RP, Möller AJ (2006) Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. Eur J Oral Sci 114:278–285. doi:10.1111/j.1600-0722.2006.00380.x
Al-Hadlaq SM, Aljarbou FA, AlThumairy RI (2010) Evaluation of cyclic flexural fatigue of M-wire nickel-titanium rotary instruments. J Endod 36:305–307. doi:10.1016/j.joen.2009.10.032
Gutmann JL, Gao Y (2012) Alteration in the inherent metallic and surface properties of nickel-titanium root canal instruments to enhance performance, durability and safety: a focused review. Int Endod J 45:113–128. doi:10.1111/j.1365-2591.2011.01957.x
Bürklein S, Hinschitza K, Dammaschke T, Schäfer E (2012) Shaping ability and cleaning effectiveness of two single-file systems in severely curved root canals of extracted teeth: Reciproc and WaveOne versus Mtwo and ProTaper. Int Endod J 45:449–461. doi:10.1111/j.1365-2591.2011.01996.x
Marinho AC, Martinho FC, Zaia AA, Ferraz CC, Gomes BP (2012) Influence of the apical enlargement size on the endotoxin level reduction of dental root canals. J Appl Oral Sci 20:661–666
Acknowledgments
This work was supported by the Brazilian agencies FAPESP, CAPES and CNPq.
Conflict of interest
The authors deny any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinho, F.C., Freitas, L.F., Nascimento, G.G. et al. Endodontic retreatment: clinical comparison of reciprocating systems versus rotary system in disinfecting root canals. Clin Oral Invest 19, 1411–1417 (2015). https://doi.org/10.1007/s00784-014-1360-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1360-9