Abstract
Objectives
Site-specific suppression of bone remodelling has been implicated in bisphosphonate-(BP)-related osteonecrosis of the jaws (BRONJ). Due to the origin of jaw bone from cranial neural crest, osseous differentiation is regulated specifically by the antagonizing BMP-2-downstream-transcription factors Msx-1 and Dlx-5. Osteopontin has been implicated in bone remodelling and angiogenesis. The osteoblast and osteoclast progenitor proliferation mediating Msx-1 has been demonstrated to be suppressed in BRONJ. In vitro BPs were shown to increase Dlx-5 and to suppress osteopontin expression. This study targeted Dlx-5 and osteopontin in BRONJ-related and BP-exposed jaw bone compared with healthy jaw bone samples at protein- and messenger RNA (mRNA) level, since increased Dlx-5 and suppressed osteopontin might account for impaired bone turnover in BRONJ.
Materials and methods
Fifteen BRONJ-exposed, 15 BP-exposed and 20 healthy jaw bone samples were processed for real-time reverse transcription polymerase chain reaction (RT-PCR) and for immunohistochemistry. Targeting Dlx-5, osteopontin and glyceraldehyde 3-phosphate dehydrogenase mRNA was extracted, quantified by the LabChip-method, followed by quantitative RT-PCR. For immunohistochemistry, an autostaining-based alkaline phosphatase antialkaline phosphatase (APAPP) staining kit was used. Semiquantitative assessment was performed measuring the ratio of stained cells/total number of cells (labelling index, Bonferroni adjustment).
Results
The labelling index was significant decreased for osteopontin (p < 0.017) and significantly increased for Dlx-5 (p < 0.021) in BRONJ samples. In BRONJ specimens, a significant fivefold decrease in gene expression for osteopontin (p < 0.015) and a significant eightfold increase in Dlx-5 expression (p < 0.012) were found.
Conclusions
BRONJ-related suppression of bone turnover is consistent with increased Dlx-5 expression and with suppression of osteopontin. The BP-related impaired BMP-2–Msx-1–Dlx-5 axis might explain the jaw bone specific alteration by BP.
Clinical relevance
The findings of this study help to explain the restriction of RONJ to craniofacial bones. BRONJ might serve as a model of disease elucidating the specific signal transduction of neural crest cell-derived bone structures in health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2003, a previously not known disease occuring exclusively in the jaws was described for the first time: nitrogen containing bisphophonate (BP)-related osteonecrosis of the jaw (BRONJ) [26]. The clinical presentation of BRONJ includes non-healing osseous defects in the jaw bone and delayed reepithelisation and impaired intraoral soft tissue regeneration, accompanied by inflammation [35]. There is increasing evidence that BRONJ is caused by BP-related impairment of bone remodelling, by depletion of osteoclasts and by suppression of angiogenesis [25, 27, 28, 44, 48]. As BRONJ is restricted to jaw bone, a differential proliferation and osseous differentiation response to BP was described comparing osteoblasts and osteoclasts from jaw bone and extracranial bone. Due to their embryonic origin from the cranial neural crest derived tissue, a craniofacial-bone-restricted expression of the cell proliferation- and tissue regeneration-related BMP-2 downstream signalling intermediate Msx-1 has been described [7, 8]. The balanced expression of Msx-1 and the antagonistic acting Dlx-5 has been implicated in the regulation of alveolar bone remodelling [6, 37]. Msx-1 stimulates proliferation of osteoblasts but inhibits terminal differentiation and represses Dlx-5 [54]. Dlx-5 activates Runx-2 expression, followed by terminal differentiation of osteocytes [36]. Suppression of Msx-1 in BP-exposed and BRONJ-related jaw bone has been demonstrated [44, 46]. The site-specific suppression of jaw bone following BP exposition could be related to a relative overexpression of Dlx-5 caused by the BP-induced lack of Msx-1.
The cytokine osteopontin (OPN) is expressed in osteoblasts and osteoclasts and controls cell attachment, bone resorption and angiogenesis [14, 24, 43]. Absence of OPN has been described to be related to decreased angiogenesis and to impaired endothelial cell proliferation in vivo [4, 38]. Vascular OPN expression has been described to be increased during new bone formation in fracture healing [9]. Osteoclast accumulation and bone resorption is known to be deficient in OPN-lacking bone [4]. Experimentally topical administered zoledronic acid was shown to decrease OPN expression within the mineralised matrix of rat long bones [52]. OPN-activating Rank (L) was shown to be suppressed in BP- and BRONJ-related jaw bone [43, 44, 47]. Angiogenesis and expression of mucoperiosteal cell proliferation-related transforming growth factor beta in BRONJ- and BP-affected tissue were found to be diminished [46, 48].
Considering the recent findings in BRONJ of site-specific actions of BP, local osteopetrotic bone morphology changes, impaired bone remodelling, interaction with the BMP-2 signalling downstream and diminished neoangiogenesis, altered expression of Dlx-5 and OPN could contribute to the explanation of the BRONJ- pathology.
The aim of this immunohistochemical and molecular study was to investigate if the expressions of Dlx-5 and OPN in jaw bone are influenced by intravenous BP therapy and if there is a differential impairment of expression between BP-exposed and BRONJ-related jaw bone.
Material and methods
Patients and material harvesting
Jaw bone specimens from 50 patients have been included in this study. The specimens used in this study measured on average 8 × 5 × 5 mm and were immediately separated into two equal parts. One part was immediately shock frozen at −80 °C, the other part was fixed in 4 % formalin.
The ethical aspects of the study were approved by the ethical committee of the University of Erlangen-Nuremberg (Ref. No. 4272). Fifteen bone specimens have been obtained from clinically and histologically evident BRONJ of 15 patients undergoing radical sequestrotomy. The specimens were harvested in 15 consecutively treated patients. The specimens used in this study were part of the tissue samples provided for routine histopathological diagnostics. Each specimen included in this investigation was confirmed to present the histopathological aspects of BRONJ. Besides histopathological characteristics of BRONJ, criteria for including the specimen in this investigation were (i) intravenous application of either pamidronate or zoledronate for at least 12 months and (ii) clinical evidence of exposed jaw bone for at least 8 weeks. Any former radiotherapy was excluded. The clinical data and the description of treatment procedures for the patients included in this study were documented previously [40]. Fifteen specimens of jaw bone were taken during dental extraction procedures from patients undergoing intravenous therapy of zoledronate in the absence of exposed jaw bone. The controls comprised 20 alveolar bone specimens that were collected during intraoral surgery procedures in patients with no BP history and no clinical signs of intraoral inflammation or periodontitis. Of the 20 control samples, 13 specimens were from the alveolar crest after a tooth extraction that required the removal of sharp bone ridges and adaptation of soft tissues, 4 specimens were obtained during orthognatic surgery in the lower jaw, and 3 specimens were from bone tissue that covered wisdom teeth that required removal from the lower jaw. Gender and age of the patients were matched in the BRONJ and control groups, except the four samples from the orthognatic surgery procedure. The average age of the patients in the BRONJ group was significantly higher than that in the four normal patients that underwent orthognatic surgery.
Immunohistochemical staining
Four per cent formalin fixed, decalcified (10 % EDTA, pH 7.4) paraffin-embedded tissue samples were prepared for immunohistochemical staining as consecutive sections using a microtome (Leica, Nussloch, Germany) and dewaxed in graded alcohol. Immunohistochemical staining was performed with the alkaline phosphatase–anti-alkaline phosphatase method and an automated staining device (Autostainer plus, DakoCytomation, Hamburg, Germany). We used the standard protocol recommended for the staining kit (Dako Real, Cat. No. K5005, DakoCytomation). Proteins were detected by incubating tissues in the autostainer (20 °C, 1 h) with specific antibodies. OPN was detected with a monoclonal mouse-IgG anti-human OPN antibody (anti-OPN; sc-73631, Santa-Cruz, Santa Cruz, USA; dilution 1:150). Dlx-5 was detected with a polyclonal goat-IgG (anti-human Dlx-5; sc-18152, Santa Cruz, USA; dilution, 1:100). The secondary antibodies were included in the staining kit; biotinylated polyclonal goat-anti-mouse was used for OPN; rabbit anti-goat IgG was used for Dlx-5 (E 0466, DAKO; dilutions, 1:100). Stains were visualised with the Fast Red Solution, localised by biotin-associated activation of the secondary antibodies (K5005, ChemMate-Kit, Dako). This was followed by incubation in haematoxylin for counterstaining the nucleus. Two consecutive tissue samples were processed per immunohistochemical stain; one served as a negative control in each case (identical treatment, but replacement of the primary antibody with an IgG-istotype of the primary antibody). A positive control sample that was known to stain positive for a given antibody was included in each series.
Semiquantitative immunohistochemical analysis
The sections were examined qualitatively under a bright-field microscope (Axioskop, Zeiss, Jena, Germany) at ×100–400 magnification for changes in the number and localisation of stained cells (osteocytes, osteoblasts, osteoblast progenitor) in samples of BRONJ-related, BP-exposed and healthy bone. Subperiosteal bone tissue was observed, including bone trabecular and endosteal structures. In BRONJ samples, bone tissue attached to the necrotic zone was located for observation. Within these areas, three visual fields per section for each sample were digitized at ×200 magnification using a charge-coupled device camera (Axiocam 5, Zeiss, Jena, Germany) and the program Axiovision (Axiovison, Zeiss, Jena, Germany). For this purpose, randomised systematic subsampling was performed according to the method described by Weibel and in accordance with own pre-research [50]. Semiquantitative analysis of cytoplasmic expression of OPN and Dlx-5 was performed determining the labelling index as the ratio of positively stained cells to the total number of cells per visual field.
Quantitative mRNA analysis and real-time reverse transcriptase polymerase chain reaction
Frozen tissues were agitated (Mixer Mill, Quiagen, Hilden, Germany) in lysis buffer (RNeasy Kit, QIAGEN, Hilden, Germany), and total RNA from tissues was extracted using RNeasy Kit according to the manufacturer’s protocol. Quantitative measurement of messenger RNA (mRNA) in each sample was performed using a commercial microfluid Lab-on-a-Chip technology (Agilent RNA 6000 Pico Kit and the Agilent 2100 Bioanalyzer, Agilent, Waldbronn, Germany). Complementary DNAs (cDNAs) from total RNA were synthesised using the High Capacity cDNA Archive Kit (Cat. No. 4322171; Applied Biosystem, CA, USA) according to the manufacturer’s protocol. Real-time RT quantitative PCR analyses were done using QuantiTect Primer Assays [Hs_SPP1_1_SGQuantiTect Primer Assay (200) (Cat. No. QT01008798) for OPN and Hs_DLX51_SG QuantiTect Primer Assay (200) (Cat. No. GT00016898) for DLX-5. For normalisation, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used [Hs_GAPDH_1_SG QuantiTect Primer Assay (200) (Cat. No. QT00079247), QIAGEN)] on the ABI Prism 7300 Sequence Detection System (Applied Biosystems). The QuantiTect TM SYBR® green PCR kit (Cat. No. 204143; QIAGEN) was used for PCR amplification. In total, 40 ng of cDNA was used for each PCR reaction in a total volume of 25 μl. Each PCR run included a 15-min activation time at 95 °C. The three-step cycle was run as follows: denaturing at 94 °C for 15 s, annealing at 55 °C for 30 s and extension at 72 °C for 34 s. Formation of undesired side products during PCR that contribute to fluorescence was assessed by melting curve analysis after PCR. The relative quantification (RQ) of mRNA was performed by the ∆∆CT method. OPN and DLX-5 mRNA quantities were analysed in duplicate and normalised against GAPDH as endoneous control. GAPDH has been shown to represent stable expressions in differentiated mesenchymal cells [22]. The expression of the target genes was determined in relation to mRNA isolated from healthy jaw bone of healthy volunteer as control.
Statistical analysis
In order to analyse the immunohistochemical cytoplasmic staining and the spatial pattern of expression, the labelling index of positively stained cells per visual field was taken. Comparing the relative gene expressions, addressed by the real-time RT-PCR, the mean gene expression for OPN and Dlx-5 in the pool of healthy oral periosteum was set as 1. Gene expressions in both groups of investigation were stated as relative expressions compared to healthy periosteum expression. Multiple measurements per group of investigation were aggregated prior to analysis. Descriptive analysis of labelling index and relative gene expression data was performed using the median (ME) and the interquartile range (IQR). Graphical description was performed on diagrams representing the median, the interquartile range, minimum (Min) and maximum (Max). Confirmatory comparisons were made between treatment and control groups using generalised estimating equations (GEE) with “treatment modality” and “subject id” as independent factors for appropriate analysis of repeated measurements per individual. Multiple p values were adjusted according to Bonferroni by multiplying each p value obtained by the number of confirmatory tests performed (n = 10). Two-sided adjusted p values of p ≤ 0.05 were considered to be significant. All calculations were made using SPSS 17.0 for Windows (SPSS Inc., Chicago, USA).
Results
Numbers of Dlx-5 expressing cells and Dlx-5 mRNA are increased in BRONJ-related and BP-exposed jaw bone
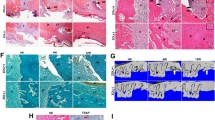
Dlx-5 expression was found in osteoblasts and osteocytes of healthy jaw bone (Fig. 1a), of BP-exposed jaw bone (Fig. 1b) and the BRONJ samples (Fig. 1c). Dlx-5-expressing osteocytes showed a higher cellular density in the BP-exposed bone and the BRONJ group than in normal jaw bone. The median labelling index of Dlx-5-expressing osteoblasts and osteocytes was significantly increased in the BP-exposed (ME, 46; IQR, 6.5) and in the BRONJ-related bone (p < 0.02; ME, 51; IQR, 7) compared to control jaw bone (ME, 19; IQR, 4; Fig. 2a, Table 1). There was no significant difference of labelling index of Dlx-5 expression between the BP-exposed and the BRONJ-affected jaw bone (Fig. 2a). The Dlx-5 mRNA was found to be significantly elevated in the BP-exposed jaw bone (ME, 8.14; IQR, 0.99) and BRONJ bone (p < 0.012; ME, 8.49; IQR, 1.39) compared to the control jaw bone (ME, 1; IQR, 0.22; Fig. 3a). No significant difference of mRNA expression for Dlx-5 was seen between BP-exposed and BRONJ-related jaw bone (Fig. 3a) In BRONJ- and BP-exposed bone, Dlx-5 mRNA was found to be expressed approximately eightfold higher than in normal jaw bone.
Immunohistochemistry presents increased cellular staining of Dlx-5 (a–c) and reduced osteopontin (d–f) in BP- and BRONJ-related jaw bone. Scale bars represent 100 μm. Original magnification is ×200 immunohistochemistry shows Dlx-5 staining preferentially in bone adhering endosteal cells of control jaw bone samples (black arrows) (a). Dlx-5 expression lacking endosteal regions are present in control jaw bone (white arrows). In BP-exposed jaw bone cellular Dlx-5 staining is visible in the endosteal parts and within the mineralized bone tissue (black arrows) (b). In BRONJ-related bone, endosteal and periosteal tissue presented high intensity staining for Dlx-5 accompanied by cellular Dlx-5 staining of bone embedded cells (black arrows) (c). Osteopontin staining is pronounced at the endosteal bone margins and in endosteal cells (black arrows) (d) in control jaw bone. In BP-exposed jaw bone the endosteal expression of osteopontin is nearly absent (white arrows). Only scattered single cells express osteopontin within endosteal or periosteal tissue (black arrows) (e). In BRONJ-related tissue, osteopontin was expressed in single cells only within the bone matrix (black arrow) (f)
Relative cellular staining (labelling index) is significantly increased for Dlx-5 (a) and significantly decreased for osteopontin (b) in BP-exposed and BRONJ-related jaw bone. The boxplots represent the median, the maximum and minimum values as well as the 25th and the 75th percentile. Compared with control jaw bone the relative cellular staining (labelling index) of Dlx-5 in BP-exposed and in BRONJ-related jaw bone is significantly increased (p < 0.021) (a). There is no significant difference of expression for Dlx-5 between BP-exposed and BRONJ-related jaw bone (a). The labelling index of the osteopontin expression issignificantly decreased in BP-exposed and BRONJ-related jaw bone compared with control bone (p < 0.017) (b). No significant difference of labelling index for osteopontin is seen between BP-exposed and BRONJ-related jaw bone (b)
Increased gene expression of Dlx-5 in BP- and BRONJ-related bone (a) and suppression of osteopontin mRNA (b). The mRNA analysis is quantitatively normalized to GAPDH relative gene expression for Dlx-5 is eightfold increased at mRNA level in BP-exposed and BRONJ-related jaw bone samples (p < 0.012) (a). There is no significant difference in gene expression of Dlx-5 between BP-exposed and BRONJ-related bone tissue (a). Relative osteopontin mRNA was fivefold suppressed in BP-exposed and BRONJ-related jaw bone (p < 0.015) (b). No significant difference was seen between BP-exposed and BRONJ-related jaw bone describing the relative mRNA expression of osteopontin (b)
Number of OPN-expressing cells is reduced in the BP-affected and the BRONJ-related jaw bone, and OPN mRNA in BRONJ- and BP-exposed bone is suppressed
The pattern of OPN expression differed between the specimens of normal jaw bone (Fig. 1d), the BP-exposed (Fig. 1e) and the BRONJ-related jaw bone (Fig. 1f). Expression of OPN was present throughout the entire bone sections in normal jaw bone, pronounced at endosteal and periosteal bone margins (Fig. 1d). In the BP-exposed jaw bone, OPN was expressed only sparely in endosteal cells and cells along the bone–periosteal transitional zone (Fig. 1e). Only single cells expressed OPN in BRONJ-related bone samples (Fig. 1f). Endosteal expression of BRONJ was absent in BRONJ-associated jaw bone (Fig. 1f). The number of OPN-expressing cells was significantly reduced in BP-affected jaw bone, as reflected by the significantly reduced labelling index for OPN staining in BP-exposed bone samples (p < 0.017; ME, 11; IQR, 4) and in BRONJ-related jaw bone samples (p < 0.017; ME, 11; IQR, 3) compared to jaw bone (ME, 42.5; IQR, 6.5; Fig. 2b, Table 2). Measuring relative, normalised mRNA ratios for OPN in BP-exposed jaw bone, BRONJ-samples and healthy jaw bone, OPN mRNA expression was significantly suppressed (p < 0.015) in BP-exposed bone (p < 0.015; ME, 0.21; IQR, 0.05) and BRONJ-related jaw bone (Table 3; ME, 0.1; IQR, 0.02) compared to healthy jaw bone (ME, 1; IQR, 0.25; Fig. 3b, Table 4). These data indicated a fivefold suppression of OPN mRNA expression in BP-exposed and BRONJ-related bone compared to jaw bone. There was no significant difference of OPN mRNA expression between BP-exposed and BRONJ-affected jaw bone (Fig. 3b).
Discussion
The present study showed a significantly increased expression of the osseous differentiation mediating transcription factor Dlx-5 in cells of BP-exposed and BRONJ-related jaw bone in vivo for the first time. The cytokine osteopontin, related to bone formation, bone resorption and bone remodelling, was found to be suppressed in BP-exposed and BRONJ-related jaw bone. The immunohistochemical and molecular biology results of this study are consistent with the findings of other groups, describing BRONJ as local osteopetrotic alteration of the jaw bone [16, 27]. The data of this study substantiate the histopathological description of BRONJ as drug-induced non-inflammatory oversuppression of bone renewal in jaw bones [28].
Dlx-5 expression is known to be increased following BP exposition of osteoblasts in vitro [23]. The significantly increased labelling index of Dlx-5 (p < 0.021) and the significantly increased mRNA level (p < 0.017) in BP-exposed and in BRONJ-related jaw bone of our in vivo study is in accordance to these in vitro findings. Dlx-5 has been implicated in differentiation and remodelling processes of mineralised tissues [36]. Overexpression of Dlx-5 in craniofacial osteoblast precursors has ben demonstrated to accelerate osteoblastic differentiation [2, 29, 41]. Furthermore, Dlx-5 was demonstrated to induce osteoblastic differentiation of craniofacial periosteal cells in vitro [41]. Lack of Dlx-5 has been identified to be related to impaired alveolar bone formation [13]. Indeed, lack of Dlx-5 has been demonstrated to affect the development and differentiation of craniofacial bone structures, whereas extracranial bone structures were shown to be unaffected by the lack of Dlx-5 [2, 13, 19, 55]. The known characteristic histomorphological changes of BRONJ-related jaw bone are consistent with the findings of elevated Dlx-5 expression within the affected jaw bone in our study [28].
Osteopontin, which was found to be suppressed within the BRONJ-related jaw bone in our study, has been described to be critically involved in the regulation of bone turnover and angiogenesis in bone repair [3, 15]. Deficency of osteopontin in animal models of bone regeneration was shown to be related to similar impairment of tissue repair processes as BRONJ-affected tissue [15]. Specifically, the reduction of callus volume in early fracture repair of OPN-lacking mice corresponds to the known non-healing extraction sockets following dental extraction in BP-exposed jaw bone [1, 15]. Furthermore, OPN-deficient mice presented a higher mineral content within their bone tissue consistent with the hypermineralisation and sclerosis of jaw bone following BP treatment [1, 10, 21]. The reduction of neovascularisation within the first week of fracture repair in OPN-deficient mice remarkably corresponds to the suppression of neoangiogenesis in BRONJ-affected tissue, whereas density of mature vessels is not impaired in BRONJ- or BP-related tissue [15, 49]. Since OPN has been described to be critically involved in bone resorption, the lack of OPN expression in BP-exposed and BRONJ-affected bone is in accordance with action of BP [4].
Both Dlx-5 and OPN have been implicated in the regulation of balanced bone formation and resorption by mediating the process of osteoblast–osteoclast coupling [4, 18, 37]. This lack of OPN in BP- and BRONJ-related jaw bone in our study is consistent with the histomorphological and cell biology alterations following BP exposition. Since the expression of osteoblast–osteoclast coupling-related genes has been described to be site specific, a possible explanation of the restriction of BRONJ to the jaws could be resembled by the jaw-specific oversuppression of the bone turnover and remodelling. The site-specific, more profound impact of zoledronic acid on bone remodelling in the mandibula compared to the tibia in an experimental model of fracture repair substantiates this suggestion [53]. Even single systemic doses of zoledronic acid were shown to differentially affect bone remodelling and inflammation in craniofacial bone compared to tibia bone, resulting in a suppression of osteogenic and bone remodelling-related genes in jaw bone [11]. Indeed, the differential expression of the BMP-2–Msx-1/Msx-2–Dlx-5 signalling downstream axis in jaw bone compared with extracranial bones has been described [5, 7, 29, 31, 32]. In particular, due to the origin of jaw bone from cranial neural crest drived tissue, the expression of the Dlx-5 antagonising transcription factor Msx-1 and the essentially osteoclast activating Rank (L) have been described to be higher in jaw bone compared with extracranial bone [33, 34]. Overexpression of Msx-1 and Rank (L) has been implicated in the pathology of jaw-bone-restricted giant cell granuloma, characterised by local inflammation and hyperproliferation of premature bone tissue [20]. Both Msx-1 and Rank (L) were demonstrated to be suppressed in BP-exposed jaw bone and in BRONJ-affected mucoperiosteal tissue and jaw bone [44, 47]. Therefore, a relative increase in Dlx-5 expression in jaw bone following BP exposition, induced by a loss of Msx-1, could contribute to the explanation of the specific oversuppression of BP-related jaw bone. The suggestion of reciprocal impairment of Dlx-5 and Msx-1 due to BP exposition in jaw bone is supported by the finding of our studies presenting an eightfold increase in Dlx-5 expression accompanied by an eightfold decrease in Msx-1 in BP-affected jaw bone [45, 47].
The results of this study help to understand the alteration of osteoblast–osteoclast signal transduction in BRONJ since in vivo data, corresponding to the clinicopathological features of BRONJ, are presented. The analysis of in vivo data is of specific importance, since the expression of bisphosphonate-sensitive genes in osteoblasts and osteoclasts has been described to differ between in vitro cell culture conditions and bone tissue. For example, Rank (L) has been reported to be differentially induced in vitro, depending on the BP concentration [39]. Despite this in vitro finding, suppression of Rank (L) is known to be an important antiresorptive mechanism of BP in the clinical in vivo use [51].
Furthermore, the expression of housekeeping genes itself, used for normalisation of mRNA measurement, was shown to be differentially regulated by BP in vitro and in vivo [42]. Wheras the use of GAPDH as appropriate housekeeping gene is widely accepted for in vivo analysis of BP-related bone metabolism and turnover, primary cell cultures of osteoblast progenitor cells and cell lines were shown to regulate GAPDH expression sensitive to BP exposition [12, 17, 42] [22, 30]. Therefore, the selection of appropriate housekeeping genes should be performed carefully according to the targeted cell type and according to the experimental setting.
Thus, from our study and the literature, it appears that the localised suppression of bone remodelling and the osteopetrotic histomorphological changes of jaw bone due to BRONJ and BP treatment could be explained by the BP-related impairment of the site-specific regulated BMP-2–Msx-1/Dlx-5 downstream and by suppression of the bone regeneration and angiogenesis-related OPN. The data of our study described an identic alteration of expression of Dlx-5 and OPN in BRONJ and BP-related jaw bone. This observation indicates a primary affection of the BMP-2 downstream by BP treatment, independently from secondary inflammation seen in BRONJ. Further research is needed to address a potential interaction of Dlx-5 and OPN in osteoblast–osteoclast coupling. Understanding the differences of regulation of bone turnover in cranial and extracranial bone due to their different embryonic origin might elucidate the formal pathology of BRONJ, explaining the impaired jaw bone remodelling.
References
Abu-Id MH, Warnke PH, Gottschalk J et al (2008) “Bis-phossy jaws”—high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg 36:95–103
Acampora D, Merlo GR, Paleari L et al (1999) Craniofacial, vestibular and bone defects in mice lacking the distal-less-related gene Dlx5. Development 126:3795–3809
Alford AI, Hankenson KD (2006) Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone 38:749–757
Asou Y, Rittling SR, Yoshitake H et al (2001) Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology 142:1325–1332
Babajko S, Meary F, Petit S et al (2011) Transcriptional regulation of MSX1 natural antisense transcript. Cells Tissues Organs 194:151–155
Babajko S, Petit S, Fernandes I et al (2009) Msx1 expression regulation by its own antisense RNA: consequence on tooth development and bone regeneration. Cells Tissues Organs 189:115–121
Berdal A, Lezot F, Nefussi JR et al (2000) Mineralized dental tissues: a unique example of skeletal biodiversity derived from cephaic neural crest. Morphologie 84:5–10
Berdal A, Molla M, Hotton D et al (2009) Differential impact of MSX1 and MSX2 homeogenes on mouse maxillofacial skeleton. Cells Tissues Organs 189:126–132
Blum A, Zarqh O, Peleg A et al (2012) Vascular inflammation and endothelial dysfunction in fracture healing. Am J Orthop (Belle Mead NJ) 41:87–91
Boskey AL, Spevak L, Paschalis E et al (2002) Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int 71:145–154
Cardemil C, Omar OM, Norlindh B et al (2013) The effects of a systemic single dose of zoledronic acid on post-implantation bone remodelling and inflammation in an ovariectomised rat model. Biomaterials 34:1546–1561
Das S, Edwards PA, Crockett JC et al (2014) Upregulation of endogenous farnesyl diphosphate synthase overcomes the inhibitory effect of bisphosphonate on protein prenylation in hela cells. Biochim Biophys Acta 1841:569–573
Depew MJ, Liu JK, Long JE et al (1999) Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126:3831–3846
Dodds RA, Connor JR, James IE et al (1995) Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: an in vitro and ex vivo study of remodeling bone. J Bone Miner Res 10:1666–1680
Duvall CL, Taylor WR, Weiss D et al (2007) Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res 22:286–297
Favia G, Pilolli GP, Maiorano E (2009) Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone 45(3):406-13. doi:10.1016/j.bone.2009.05.008
Fazzalari NL, Kuliwaba JS, Atkins GJ et al (2001) The ratio of messenger RNA levels of receptor activator of nuclear factor kappaB ligand to osteoprotegerin correlates with bone remodeling indices in normal human cancellous bone but not in osteoarthritis. J Bone Miner Res 16:1015–1027
Hassan MQ, Tare RS, Lee SH et al (2006) BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J Biol Chem 281:40515–40526
Holleville N, Quilhac A, Bontoux M et al (2003) BMP signals regulate Dlx5 during early avian skull development. Dev Biol 257:177–189
Houpis CH, Tosios KI, Papavasileiou D et al (2010) Parathyroid hormone-related peptide (PTHrP), parathyroid hormone/parathyroid hormone-related peptide receptor 1 (PTHR1), and MSX1 protein are expressed in central and peripheral giant cell granulomas of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109(3):415-24. doi:10.1016/j.tripleo.2009.09.026
Hunter GK, Hauschka PV, Poole AR et al (1996) Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochemical j 317(1):59–64
Jacobs C, Walter C, Ziebart T et al. (2014) Mechanical loading influences the effects of bisphosphonates on human periodontal ligament fibroblasts. Clin Oral Investig doi:10.1007/s00784-014-1284-4
Jeong HM, Jin YH, Choi YH et al (2013) Risedronate increases osteoblastic differentiation and function through connexin43. Biochem Biophys Res Commun 432:152–156
Kaji H, Sugimoto T, Miyauchi A et al (1994) Calcitonin inhibits osteopontin mRNA expression in isolated rabbit osteoclasts. Endocrinology 135:484–487
Lesclous P (2009) Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone 45(5):843-52. doi:10.1016/j.bone.2009.07.011
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Marx RE, Sawatari Y, Fortin M et al (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63:1567–1575
Marx RE, Tursun R (2012) Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: a blinded histopathologic comparison and its implications for the mechanism of each disease. Int J Oral Maxillofac Surg 41(3):283-9. doi:10.1016/j.ijom.2011.12.016
Newberry EP, Latifi T, Towler DA (1998) Reciprocal regulation of osteocalcin transcription by the homeodomain proteins Msx2 and Dlx5. Biochemistry 37:16360–16368
Ohe JY, Kwon YD, Lee HW (2012) Bisphosphonates modulate the expression of OPG and M-CSF in hMSC-derived osteoblasts. Clin Oral Investig 16:1153–1159
Orestes-Cardoso S, Nefussi JR, Lezot F et al (2002) Msx1 is a regulator of bone formation during development and postnatal growth: in vivo investigations in a transgenic mouse model. Connect Tissue Res 43:153–160
Orio F Jr, Palomba S, Cascella T et al (2005) Improvement in endothelial structure and function after metformin treatment in young normal-weight women with polycystic ovary syndrome: results of a 6-month study. J Clin Endocrinol Metab 90:6072–6076
Rawlinson SC, Mckay IJ, Ghuman M et al (2009) Adult rat bones maintain distinct regionalized expression of markers associated with their development. PLoS One 4:e8358
Reichert JC, Gohlke J, Friis TE et al (2013) Mesodermal and neural crest derived ovine tibial and mandibular osteoblasts display distinct molecular differences. Gene 525:99–106
Ruggiero SL (2011) Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci 1218:38–46
Ryoo HM, Hoffmann HM, Beumer T et al (1997) Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol 11:1681–1694
Samee N, Geoffroy V, Marty C et al (2008) Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am J Pathol 173:773–780
Scatena M, Almeida M, Chaisson ML et al (1998) NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol 141:1083–1093
Stefanik D, Sarin J, Lam T et al (2008) Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Dis 14:465–471
Stockmann P, Vairaktaris E, Wehrhan F et al. (2009) Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: a prospective clinical study with 12 months follow-up. Support Care Cancer 18(4):449-60. doi:10.1007/s00520-009-0688-1
Tadic T, Dodig M, Erceg I et al (2002) Overexpression of Dlx5 in chicken calvarial cells accelerates osteoblastic differentiation. J Bone Miner Res 17:1008–1014
Valenti MT, Bertoldo F, Dalle Carbonare L et al (2006) The effect of bisphosphonates on gene expression: GAPDH as a housekeeping or a new target gene? BMC Cancer 6:49
Walker CG, Dangaria S, Ito Y et al (2010) Osteopontin is required for unloading-induced osteoclast recruitment and modulation of RANKL expression during tooth drift-associated bone remodeling, but not for super-eruption. Bone 47:1020–1029
Wehrhan F, Hyckel P, Amann K et al. (2011) Msx-1 is suppressed in bisphosphonate-exposed jaw bone analysis of bone turnover-related cell signalling after bisphosphonate treatment. Oral Dis 17(4):433-42. doi:10.1111/j.1601-0825.2010.01778.x
Wehrhan F, Hyckel P, Amann K et al (2011) Msx-1 is suppressed in bisphosphonate-exposed jaw bone analysis of bone turnover-related cell signalling after bisphosphonate treatment. Oral Dis 17:433–442
Wehrhan F, Hyckel P, Guentsch A et al. (2011) Bisphosphonate-associated osteonecrosis of the jaw is linked to suppressed TGFbet1-signaling and increased Galectin-3 expression: a histological study on biopsies. J Transl Med 9:102. doi:10.1186/1479-5876-9-102
Wehrhan F, Hyckel P, Ries J et al. (2010) Expression of Msx-1 is suppressed in bisphosphonate associated osteonecrosis related jaw tissue—etiopathology considerations respecting jaw developmental biology-related unique features. J Transl Med 8:96. doi:10.1186/1479-5876-8-96
Wehrhan F, Stockmann P, Nkenke E et al. (2011) Differential impairment of vascularisation and angiogenesis in bisphosphonate-associated osteonecrosis of the jaw (BONJ) related mucoperiosteal tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112(2):216-21. doi:10.1016/j.tripleo.2011.02.028
Wehrhan F, Stockmann P, Nkenke E et al (2011) Differential impairment of vascularization and angiogenesis in bisphosphonate-associated osteonecrosis of the jaw-related mucoperiosteal tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112:216–221
Weibel ER (1989) Measuring through the microscope: development and evolution of stereological methods. J Microsc 155:393–403
Williams DW, Lee C, Kim T et al. (2014) Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am J Pathol doi:10.1016/j.ajpath.2014.07.010
Wu CC, Wang CC, Lu DH et al (2012) Calcium phosphate cement delivering zoledronate decreases bone turnover rate and restores bone architecture in ovariectomized rats. Biomed Mater 7:035009
Yu YY, Lieu S, Hu D et al (2012) Site specific effects of zoledronic acid during tibial and mandibular fracture repair. PLoS One 7:e31771
Zhang H, Hu G, Wang H et al (1997) Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol 17:2920–2932
Zhang Z, Song Y, Zhang X et al (2003) Msx1/Bmp4 genetic pathway regulates mammalian alveolar bone formation via induction of Dlx5 and Cbfa1. Mech Dev 120:1469–1479
Acknowledgements
This study was financially supported by the ELAN-Fonds of the University Erlangen-Nuremberg. The authors thank Andrea Krautheim-Zenk, Susanne Schoenherr and Elke Diebel for technical assistance, processing the tissue specimens and operating the immunohistochemistry autostainer apparatus.
Authors’ contributions
The authors’ initials are used. FW applied for grant support (ELAN-Fonds, University of Erlangen), initiated and conducted the study, formulated the hypothesis, established and conducted the methods and analytic procedures and wrote the manuscript. KA established immunohistochemistry, interpreted the data and was responsible for BRONJ-pathohistological assessment. PM and MW performed the immunohistochemical analysis and critically reviewed the manussript. JR established the mRNA analysis and wrote the manuscript section of the RT-PCR description. RP critically reviewed the manuscript and wrote part of the discussion. PS provided the patient’s data and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wehrhan, F., Amann, K., Möbius, P. et al. BRONJ-related jaw bone is associated with increased Dlx-5 and suppressed osteopontin—implication in the site-specific alteration of angiogenesis and bone turnover by bisphosphonates. Clin Oral Invest 19, 1289–1298 (2015). https://doi.org/10.1007/s00784-014-1354-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1354-7