Abstract
Objectives
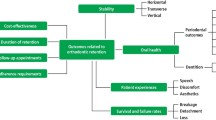
Orthodontic treatment is highly popular for restoring functional and facial esthetics in juveniles and adults. As a downside, prevalence of biofilm-related complications is high. Objectives of this review are to (1) identify special features of biofilm formation in orthodontic patients and (2) emphasize the need for strong concerted action to prevent biofilm-related complications during orthodontic treatment.
Materials and methods
Literature on biofilm formation in the oral cavity is reviewed to identify special features of biofilm formation in orthodontic patients. Estimates are made of juvenile and adult orthodontic patient population sizes, and biofilm-related complication rates are used to indicate the costs and clinical workload resulting from biofilm-related complications.
Results
Biofilm formation in orthodontic patients is governed by similar mechanisms as common in the oral cavity. However, orthodontic appliances hamper the maintenance of oral hygiene and provide numerous additional surfaces, with properties alien to the oral cavity, to which bacteria can adhere and form a biofilm. Biofilm formation may lead to gingivitis and white spot lesions, compromising facial esthetics. Whereas gingivitis after orthodontic treatment is often transient, white spot lesions may turn into cavities requiring professional restoration. Complications requiring professional care develop in 15 % of all orthodontic patients, implying an annual cost of over US$500,000,000 and a workload of 1,000 full-time dentists in the USA alone.
Conclusions
Improved preventive measures and antimicrobial materials are urgently required to prevent biofilm-related complications of orthodontic treatment from overshadowing its functional and esthetic advantages.
Clinical relevance
High treatment demand and occurrence of biofilm-related complications requiring professional care make orthodontic treatment a potential public health threat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthodontic treatment for restoring functional and facial esthetics is highly popular. Between 1982 and 2010, the number of orthodontic patients in North America has increased by 100 % (Fig. 1). Together, there are nearly four million juvenile, aged between 6 and 18 years, and more than one million adult patients in North America alone as reported by the American Association of Orthodontists [1]. The juvenile patients constitute about 7 % of the total population [2], which is much lower than the number of juvenile patients with an objective orthodontic treatment need, estimated to be between 17 and 43 % [3]. When subjective treatment need is taken into consideration, 50–75 % of the western population could benefit from orthodontic treatment [1]. Therefore, the number of potential orthodontic patients is much larger than that of currently treated patients, and further increase in the number of orthodontic patients over the coming years can be expected with increasing self-awareness of dental esthetics, oral health-related quality of life, and affordability of orthodontic treatment.
Number of orthodontic patients in North America over the past three decades. For 1982–1986, no data are available about the percentage of adult patients [1]
However, the downside of orthodontic treatment has not been much addressed. The region of the tooth surface around brackets is prone to adhesion of oral bacteria and subsequent biofilm formation or “dental plaque.” Oral biofilms on dental hard and soft tissues are the main cause of dental diseases, including caries and periodontal disease, and are difficult to remove. A single-time, self-performed manual brushing [4] is often insufficient and known to leave biofilm behind in retention sites, such as fissures, interproximal spaces, and gingival margins. Orthodontic appliances make effective biofilm removal even more difficult, and brushing nearly always leaves biofilm behind at the vulnerable bracket-adhesive-enamel junction and the sensitive region between brackets and gingival margin (Fig. 2), therewith contributing to the occurrence of dental diseases.
Orthodontic biofilm, visualized by staining with GUM Red-Cote, before (lower dentition) and after (upper dentition) removal of brackets. Stained areas, representing oral biofilm, can be clearly seen on the tooth surfaces around the area where the brackets have been bonded, around the brackets still present and along the gingival margins
In the current review, we identify the special features of biofilm formation in orthodontic patients, without aiming to fully describe mechanisms of oral biofilm formation in general, and provide an estimate of the occurrence of biofilm-related complications during orthodontic treatment, including consequences for dental health care in general.
Oral biofilm formation
Whereas it is beyond the scope of this review, to fully describe mechanisms of oral biofilm formation in general, we will briefly outline some important features. Oral biofilms form on all surfaces exposed to the human oral cavity, most notably on all oral hard and soft surfaces. Oral biofilms formed on tooth surfaces cause demineralization of enamel, which in its mildest form yields white spot lesions, indicative of subsurface decalcification. Biofilm formed below the gingival margin leads to inflammation of the gums, which in an extreme case can lead to periodontitis and tooth loss.
Oral biofilms are diverse communities of adhering microorganisms, embedded in a self-produced matrix of extracellular polymeric substances and possessing a complex, spatially heterogeneous and dynamic structure [5]. The extracellular matrix acts not only as a glue for the biofilm, ensuring adhesion to a substratum and integrity of the biofilm itself [6], but also hampers penetration of antimicrobials into the biofilm to offer protection to organisms in a biofilm mode of growth. Although the bacterial diversity in the oral cavity is estimated to include at least 800 different species, consisting of a wide variety of gram-positive and gram-negative bacteria, oral biofilms accumulate through sequential and ordered colonization by different strains and species present in the oral cavity [7].
Bacterial adhesion depends on the properties of the bacterial cell and substratum surfaces. Under clinical conditions, surface roughness is the overruling property of any material placed in the oral cavity with respect to bacterial adhesion and biofilm formation, especially in supragingival regions where sizeable detachment forces are operative during the day [8]. Roughness is of less importance in relatively stagnant regions, such as in subgingival pockets, and here, substratum hydrophobicity plays a major role.
Oral biofilm in orthodontic patients
Placement of an orthodontic appliance consisting of metals and polymers is accompanied by the creation of surfaces with properties, alien to those of the natural oral hard and soft surfaces. In addition, the number of retention sites is much larger in orthodontic patients. These special features not only increase the amount of biofilm but also the prevalence of cariogenic bacteria such as mutans streptococci [9] and periodontopathic bacteria such as Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Tannerella forsythia, and Fusobacterium species [10]. Moreover, orthodontic appliances greatly reduce the efficacy of natural oral cleansing forces and of mechanical biofilm removal by toothbrushing [11].
The variety of alien surfaces introduced by orthodontic intervention provides numerous additional surfaces to which microorganisms can adhere and form a biofilm. Banding induced more biofilm formation mostly at the gingival margin, periodontal inflammation, and white spot lesions than bonding [12]. Composite bonding resins are prone to bacterial adhesion at the vulnerable bracket-adhesive-enamel junction, especially since polymerization shrinkage may yield a gap at the contact interface where bacteria find themselves protected against oral cleansing forces and antibacterial agents [13]. Moreover, bacterial adhesion forces to composite resin, often having a rougher surface than enamel or brackets, were stronger than those to brackets or saliva-coated enamel [14].
Initial biofilm formation in vivo has been observed on different bracket materials [15]. Brackets placed maxillary or at labial surfaces harvested more biofilm than those at mandibular or lingual ones [16]. Although more anaerobic and aerobic organisms have been found in self-ligating than in conventional bracket sites [17], the occurrence of white spot enamel lesions and gingival inflammation was similar in both patient groups [18], indicating that biofilm formation on the brackets themselves is less harmful than when formed at the bracket-adhesive-enamel junction.
No difference was found regarding biofilm weight or biofilm-related clinical indices between different ligating devices. However, use of elastomeric rings was related to a higher incidence of enamel demineralization [19]. In general, complicated auxiliaries create areas difficult to clean and enhancing biofilm formation [11].
Removable acrylic retainers stimulate early biofilm formation, harvesting different strains of Streptococci and Candida, and provide new retention sites favoring bacterial adhesion and growth [20]. Fixed retainers in direct contact with the enamel surface cannot be removed for extensive cleaning and may yield extensive biofilm formation [21]. No differences were found in the clinical plaque and gingivitis indices between fixed retainers made of multistrand or single-strand wires, but more biofilm was isolated from the multistrand wires having niches where biofilms can be easily formed and are protected against environmental attacks [22] (Fig. 3).
Complications arising from biofilms during orthodontic treatment
Enamel demineralization
Enamel demineralization surrounding brackets is the most common side effect in orthodontics and can range from white spot lesions to cavitation upon bracket removal (Fig. 4). This can occur on both vestibular and lingual surfaces, with the most affected sites being the bracket-adhesive-enamel junction on teeth at the esthetic region [14]. Enamel remineralization of white spot lesions can be achieved spontaneously by saliva or actively by fluoride or calcium-phosphate-based remineralization [23]. Whether complete remineralization occurs or not is related to the type and severity of the lesions [11]. White spot lesions can develop rapidly in susceptible individuals within the first month of treatment and can remain visible many years after debonding or in severe cases can appear as a permanent enamel scar [11]. Fast developing or soft lesions are mostly superficial enamel defects and may almost completely remineralize within a few weeks. In most patients, lesions develop gradually during treatment and remineralize extremely slowly. Micro-abrasion, in essence, an invasive method removing sound as well as diseased tissue, is an effective professional cosmetic measure to treat permanent enamel scarring [24], which may also take place spontaneously leading to a gradual regression of the white spot lesion. More severely, white spot lesions may turn into actual cavities, and not seldom, orthodontic appliances have to be removed before the treatment goal has been reached to prevent further demineralization. The long-term presence of white spot lesions or of composite restorations at labial surfaces of teeth, with the potential to turn into cavities or discolor, respectively, is the most prevalent biofilm-related complications in orthodontics, compromising facial esthetics after an often lengthy and costly orthodontic treatment.
Soft tissue inflammation
Almost all orthodontic patients experience some degree of soft tissue inflammation (Fig. 4). Gingivitis during orthodontic treatment is often temporary and rarely progresses to periodontitis, although biofilms on retention sites increase the risk for periodontitis. Biofilms on temporary anchorage devices (Fig. 5), such as mini-screws, micro-implants, or mini-plates, can cause inflammation of surrounding soft tissues similar to peri-implantitis, especially on transgingival parts of the devices. These inflammations are associated with a 30 % increase in failure rate of the devices [25]. In addition, biofilms on the head of a temporary anchorage device may infect adjacent contacting mucosa resulting in aphthous ulceration forewarning a greater soft tissue inflammation [26]. Treatment of gingivitis or peri-implantitis in orthodontics includes local cleaning, application of antimicrobial-containing products, such as chlorhexidine, cetylpyridinium chloride, or triclosan preferably combined with brushing with a fluoridated toothpaste [26].
Other consequences of orthodontic biofilms
Bacteremia caused by trauma during appliance placement or removal is usually transient and occurs with an incidence of up to 10 % during fixed appliance treatment [27] and 30 % at removal of fixed expansion appliances [28]. Biofilms may also affect the appliance itself and cause pitting and crevice corrosion of metallic biomaterials, affecting mechanical properties, surface roughness, or topographies of composite adhesives [29]. Increase in roughness of the appliance materials due to biofilm is especially troublesome, since rougher surfaces promote biofilm formation [30], providing protective niches against environmental challenges. Hence, a vicious cycle develops in which biofilm formation amplifies itself and may eventually compromise the efficiency of clinical mechanics [31].
Occurrence of biofilm-related complications
Table 1 summarizes the occurrence of biofilm-related complications during orthodontic treatment. Noticeably, large differences exist in reported occurrences of the major complications possibly relating to the various patient compliances that will greatly affect the study outcome but are not systematically recorded in all studies. In a study on the prevalence of white spot lesions in 19-year-olds, only 23 % of all participants showed good compliance with oral hygiene instructions, while 77 % had moderate or poor compliance [32].
Based on Table 1, it can be concluded that white spot lesions are a very common biofilm-related complication during orthodontic treatment, with a conservative estimate of the occurrence of 60 %. Severe lesions requiring professional attention develop in up to 15 % of all patients [33]. Interproximal caries development is not significantly different from untreated controls [34], and periodontitis is virtually absent [35]. Gingivitis, often combined with gingival hyperplasia, is very common after orthodontic treatment but normally requires no treatment because of its transient nature [36].
The number of biofilm-related complications developing during orthodontic treatment is high. Considering the size of the current patient population, the results of this review indicate that three million orthodontic patients in the USA alone develop white spot lesions as a result of the treatment. Up to 750,000 of these patients require professional care after orthodontic treatment. We estimate that basic treatment of white spot lesion on teeth in the esthetic region costs at least US$650 per patient [37] adding up to nearly US$500,000,000 for all patients requiring professional care after orthodontic treatment. Since at least 2–3 h are needed per patient, the total amount of man hours involved in these restorative treatments is estimated to be around 2,000,000. This means that every year, around 1,000 dentists have to work full time in order to treat the consequences of biofilm-related complications after orthodontic treatment. Although most orthodontists are aware of these problems, effective preventive programs and focussed research efforts are lacking.
Traditional and current preventive measures
Mechanical removal
Effective manual or powered brushing and use of interdental brushes are still by far the most important measure for oral hygiene control in orthodontic patients. Manual toothbrushes with a special head design for orthodontics, such as staged, v-shaped, or triple-headed, are more efficient than brushes with a conventional planar bristle field [38]. Powered toothbrushes for removing biofilms are difficult to compare because of the diversity of frequencies or types of vibration, areas or types of bristle, and criteria or methods for assessment [39] but are generally accepted to perform better than manual brushing. However, the use of powered toothbrushes demonstrating noncontact removal (“cleaning beyond the bristles”) of oral biofilm [40] up to brushing distances of 6 mm, depending on the energy output and frequency of the brush [41], may be advisable for orthodontic patients, although a thorough evaluation of the use of such brushes has never been made.
Chemical biofilm control
A variety of chemical biofilm control measures including incorporation of antimicrobials in toothpastes, mouthrinses, varnishes, and adhesives are currently used. Chlorhexidine, however, still remains the most effective antimicrobial in reducing biofilm-related complications in orthodontic patients [42], although compliance may not be optimal in many patients since long-term use of chlorhexidine is known to stain teeth and tongue and affects taste sensation. Cetylpyridinium chloride is also an effective oral antimicrobial, but in many formulations, its bioavailability is low. The benefits of fluoride-containing toothpastes and mouthrinses in preventing caries have been well established, and besides aiding enamel remineralization, fluoride acts as a buffer to neutralize acids produced by bacteria and suppresses their growth [30]. Stannous fluoride provides dual benefits with respect to caries and biofilm prevention by stannous ions [43]. The combination of an amine fluoride/stannous fluoride-containing toothpaste or mouthrinse showed greater inhibition of biofilms, less white spot lesions, and gingivitis during orthodontic treatment than sodium fluoride-containing products [11]. Laser irradiation in addition to fluoride treatment has been suggested to prevent formation of white spot lesions both in vitro and in vivo [44].
Recently, it has been demonstrated that oral biofilm left behind after brushing absorbs antibacterial components from mouthrinses used after brushing to act as a reservoir for antibacterial components that are subsequently slowly released in bioactive concentrations [45]. Importantly, biofilm is always left behind where it appears most harmful to the enamel surface, in case of orthodontic treatment around brackets. Consequently, slow release of absorbed antibacterial components from biofilm left behind occurs where it matters most.
Modification of orthodontic materials
Fluoride has been incorporated into various orthodontic adhesives [46] to yield a slow release system with direct, beneficial clinical effects on enamel demineralization and remineralization. Other fluoride applications, which have not yet found their way to extensive clinical use, include coating of brackets and wires, e.g., titanium tetrafluoride or calcium fluoride [47], demonstrating sustained release of fluoride and associated reductions in lesion depths and total mineral loss around the bracket-adhesive-enamel junction. Fluoride-containing elastomeric rings have also been demonstrated to release significant amounts of fluoride with a concurrent clinical reduction in the degree of decalcification around brackets [48], although the number of Streptococcus mutans or anaerobic bacterial growth in saliva or biofilms surrounding the brackets remained the same [49].
Incorporation of antimicrobial agents in adhesives is more directly aimed at biofilm prevention. Antimicrobial release kinetics depend on the solubility of the antimicrobial in water, while the buildup of sufficiently high concentrations preventing microbial growth in saliva may be impossible due to washout in vivo. The solubility of chlorhexidine and triclosan in water, for instance, is low, and their release from adhesives may be less than required to reach a minimal inhibitory concentration preventing microbial growth [50]. The release of cetylpyridinium chloride in water from adhesives showed a burst release during the first 2 weeks, followed by a much lower tail release, and in vitro caused an inhibition zone on bacterially inoculated agar. Other antimicrobials such as benzalkonium chloride are only effective for 2 weeks after an initial burst release. Silver nanoparticles and quaternary ammonium polyethylenimine nanoparticles mixed into adhesives with an antibacterial activity upon contact are preferred since they are long-lasting [51], but the safety of nanoparticles for human use is still a matter of controversy.
Efforts required to prevent biofilm-related complications
Orthodontists should first of all inform patients adequately about the potential risks of treatment and emphasize preventive programs. Especially adult patients can be made aware, better than juveniles, of the importance of oral hygiene. As an essential part of a preventive program, patients should be encouraged toward a more intensive oral hygiene control and use of powered toothbrushes, in combination with fluoridated antibacterial toothpastes and antimicrobially effective mouthrinses, not solely aimed at creating fresh breath. Efforts to determine the possible clinical importance of noncontact, powered brushing in orthodontic patients should be undertaken.
Material-related efforts currently focus on the development of antimicrobial releasing adhesives to fix brackets to tooth surfaces which will protect the vulnerable bracket-adhesive-enamel junction against biofilm formation, but it is doubtful whether clinically, the small volume of adhesive applied to fix a bracket will be an effective reservoir for any antimicrobial over the duration of an average orthodontic treatment. Considering the duration of orthodontic treatment, more permanent nonadhesive or antimicrobial coatings that kill bacteria upon contact are preferable. However, neither low surface free energy polytetrafluoroethylene coatings on brackets [52] nor polymer brush-coatings [53] to discourage bacterial adhesion or photocatalytic TiO2 on wires [54] to discourage bacterial growth have yet found their way toward clinical application. Alternative directions include modified composites with antimicrobial surface properties that kill bacteria upon adhesion [55]. Recently, polymerization of antimicrobial cross-linked quaternary ammonium polyethylenimine nanoparticles into composite matrix has been demonstrated to significantly prevent oral biofilm formation in vivo and exhibit a potent broad spectrum antibacterial activity against salivary bacteria [56]. Contact-killing coatings may have greater potential for the future than antimicrobial release coatings as their efficacy is not hampered over time by a reduced release rate of antimicrobials from a reservoir with a limited volume.
Conclusions
The number of patients at risk of biofilm-related complications, including white spot lesions, caries, and gingivitis, has increased tremendously over the past two decades as a result of the success of orthodontic intervention to restore functional and facial esthetics and now encompasses sizeable juvenile and adult populations. Based on this study, a conservative estimate of 60 % of all orthodontic patients acquires one or more biofilm-related complications as a result of orthodontic treatment. Fixed braces and other orthodontic appliances hamper the maintenance of oral hygiene and provide numerous additional surfaces in the oral cavity to which bacteria can adhere and form a biofilm. With the growing demand for orthodontic treatment and a high occurrence of oral biofilm-related complications requiring professional care, orthodontic treatment is at risk of becoming a public health threat requiring improved preventive measures, including information for patients, effective personal oral care products like powered toothbrushes demonstrating noncontact removal of biofilms, pastes, and rinses, and the development of antimicrobial materials, preferentially contact-killing rather than materials relying on limited release of antimicrobials over time. Only through concerted action, we will be able to prevent biofilm-related complications during orthodontic treatment from overshadowing its obvious advantages.
References
American Association of Orthodontists (2012) AAO patient census surveys 1989-2010. Bull Am Assoc Orthod
U.S. Government Printing Office. Washington, DC (2011) America’s children: key national indicators of well-being. Federal Interagency Forum on Child and Family Statistics
Christopherson EA, Briskie D, Inglehart MR (2009) Objective, subjective, and self-assessment of preadolescent orthodontic treatment need? A function of age, gender, and ethnic/racial background? J Public Health Dent 69:9–17
Van der Weijden GA, Echeverria JJ, Sanz M, Lindhe J (2008) Mechanical supragingival plaque control. In: Lindhe J, Lang NP, Karring T (eds) Clinical Periodontology and Implant Dentistry, 5th edn. Blackwell Munskgaard, Copenhagen, pp 705–733
Marsh PD (2010) Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am 54:441–454
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Filoche S, Wong L, Sissons CH (2010) Oral biofilms: emerging concepts in microbial ecology. J Dent Res 89:8–18
Subramani K, Jung RE, Molenberg A, Hammerle CH (2009) Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants 24:616–626
Al Mulla AH, Kharsa SA, Kjellberg H, Birkhed D (2009) Caries risk profiles in orthodontic patients at follow-up using Cariogram. Angle Orthod 79:323–330
Liu Y, Zhang Y, Wang L, Guo Y, Xiao S (2013) Prevalence of Porphyromonas gingivalis four rag locus genotypes in patients of orthodontic gingivitis and periodontitis. PLoS One 8:e61028
Øgaard B (2008) White spot lesions during orthodontic treatment: mechanisms and fluoride preventive aspects. Semin Orthod 14:183–193
Demling A, Heuer W, Elter C, Heidenblut T, Bach F, Schwestka-Polly R, Stiesch-Scholz M (2009) Analysis of supra- and subgingival long-term biofilm formation on orthodontic bands. Eur J Orthod 31:202–206
Sukontapatipark W, El‐Agroudi MA, Selliseth NJ, Thunold K, Selvig KA (2001) Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod 23:475–484
Mei L, Busscher HJ, Van der Mei HC, Chen Y, De Vries J, Ren Y (2009) Oral bacterial adhesion forces to biomaterial surfaces constituting the bracket-adhesive-enamel junction in orthodontic treatment. Eur J Oral Sci 117:419–426
Ahn SJ, Lee SJ, Lim BS, Nahm DS (2007) Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic brackets. Am J Orthod Dentofac Orthop 132:815–821
Van der Veen MH, Attin R, Schwestka-Polly R, Wiechmann D (2010) Caries outcomes after orthodontic treatment with fixed appliances: do lingual brackets make a difference? Eur J Oral Sci 118:298–303
Van Gastel J, Quirynen M, Teughels W, Coucke W, Carels C (2007) Influence of bracket design on microbial and periodontal parameters in vivo. J Clin Periodontol 34:423–431
Pandis N, Vlachopoulos K, Polychronopoulou A, Madianos P, Eliades T (2008) Periodontal condition of the mandibular anterior dentition in patients with conventional and self-ligating brackets. Orthod Craniof Res 11:211–215
Pellegrini P, Sauerwein R, Finlayson T, McLeod J, Covell DA Jr, Maier T, Machida CA (2009) Editor’s summary, Q & A, reviewer’s critique: plaque retention by self-ligating vs elastomeric orthodontic brackets: quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am J Orthod Dentofac Orthop 135:426–427
Batoni G, Pardini M, Giannotti A, Ota F, Rita Giuca M, Gabriele M, Campa M, Senesi S (2001) Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur J Oral Sci 109:388–392
Levin L, Samorodnitzky-Naveh GR, Machtei EE (2008) The association of orthodontic treatment and fixed retainers with gingival health. J Periodontol 79:2087–2092
Jongsma MA, Pelser FD, Van der Mei HC, Atema-Smit J, Van de Belt-Gritter B, Busscher HJ, Ren Y (2013) Biofilm formation on stainless steel and gold wires for bonded retainers in vitro and in vivo and their susceptibility to oral antimicrobials. Clin Oral Investig 17:1209–1218
Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds EC (2010) New approaches to enhanced remineralization of tooth enamel. J Dent Res 89:1187–1197
Murphy TC, Willmot DR, Rodd HD (2007) Management of postorthodontic demineralized white lesions with microabrasion: a quantitative assessment. Am J Orthod Dentofac Orthop 131:27–33
Miyawaki S, Koyama I, Inoue M, Mishima K, Sugahara T, Takano-Yamamoto T (2003) Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofac Orthop 124:373–378
Kravitz ND, Kusnoto B (2007) Risks and complications of orthodontic miniscrews. Am J Orthod Dentofac Orthop 131:S43–S51
Erverdi N, Biren S, Kadir T, Acar A (2000) Investigation of bacteremia following orthodontic debanding. Angle Orthod 70:11–14
Gürel HG, Basciftci FA, Arslan U (2009) Transient bacteremia after removal of a bonded maxillary expansion appliance. Am J Orthod Dentofac Orthop 135:190–193
Beyth N, Bahir R, Matalon S, Domb AJ, Weiss EI (2008) Streptococcus mutans biofilm changes surface-topography of resin composites. Dent Mater 24:732–736
Busscher HJ, Rinastiti M, Siswomihardjo W, Van der Mei HC (2010) Biofilm formation on dental restorative and implant materials. J Dent Res 89:657–665
Eliades T, Bourauel C (2005) Intraoral aging of orthodontic materials: the picture we miss and its clinical relevance. Am J Orthod Dentofac Orthop 127:403–412
Ogaard B (1989) Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofac Orthop 96:423–427
Enaia M, Bock N, Ruf S (2011) White-spot lesions during multibracket appliance treatment: a challenge for clinical excellence. Am J Orthod Dentofac Orthop 140:e17–e24
Hadler-Olsen S, Sandvik K, El-Agroudi MA, Ogaard B (2012) The incidence of caries and white spot lesions in orthodontically treated adolescents with a comprehensive caries prophylactic regimen—a prospective study. Eur J Orthod 34:633–639
Bollen A, Cunha-Cruz J, Bakko DW, Huang GJ, Hujoel PP (2008) The effects of orthodontic therapy on periodontal health: a systematic review of controlled evidence. J Am Dent Assoc 139:413–422
Renkema AA, Dusseldorp JK, Middel B, Ren Y (2010) Enlargement of the gingiva during treatment with fixed orthodontic appliances. Ned Tijdschr Tandheelkd 117:507–512
Tariefbeschikking Tandheelkundige zorg. Nederlandse Zorg Autoriteit. http://www.nza.nl/98174/139255/654366/TB-CU-7042-02.pdf
Rafe Z, Vardimon A, Ashkenazi M (2006) Comparative study of 3 types of toothbrushes in patients with fixed orthodontic appliances. Am J Orthod Dentofac Orthop 130:92–95
Schätzle M, Sener B, Schmidlin PR, Imfeld T, Attin T (2010) In vitro tooth cleaning efficacy of electric toothbrushes around brackets. Eur J Orthod 32:481–489
Schmidt JC, Zaugg C, Weiger R, Walter C (2013) Brushing without brushing?—a review of the efficacy of powered toothbrushes in noncontact biofilm removal. Clin Oral Investig 17:687–709
Busscher HJ, Jager D, Finger G, Schaefer N, Van der Mei HC (2010) Energy transfer, volumetric expansion, and removal of oral biofilms by non-contact brushing. Eur J Oral Sci 118:177–182
Sari E, Birinci I (2007) Microbiological evaluation of 0.2 % chlorhexidine gluconate mouth rinse in orthodontic patients. Angle Orthod 77:881–884
Wiegand A, Bichsel D, Magalhães AC, Becker K, Attin T (2009) Effect of sodium, amine and stannous fluoride at the same concentration and different pH on in vitro erosion. J Dent 37:591–595
Noel L, Rebellato J, Sheats RD (2003) The effect of argon laser irradiation on demineralization resistance of human enamel adjacent to orthodontic brackets: an in vitro study. Angle Orthod 73:249–258
Otten MP, Busscher HJ, Van der Mei HC, Abbas F, Van Hoogmoed CG (2010) Retention of antimicrobial activity in plaque and saliva following mouthrinse use in vivo. Caries Res 44:459–464
Chin MYH, Sandham A, Rumachik EN, Ruben JL, Huysmans MDNJM (2009) Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofac Orthop 136:547–553
Lee S, Kim H, Kong Y, Kim H, Lee S, Chang Y (2005) Fluoride coatings on orthodontic wire for controlled release of fluorine ion. J Biomed Mater Res B Appl Biomater 75B:200–204
Banks P, Chadwick S, Asher-McDade C, Wright J (2000) Fluoride-releasing elastomerics—a prospective controlled clinical trial. Eur J Orthod 22:401–407
Benson PE, Douglas CWI, Martin MV (2004) Fluoridated elastomers: effect on the microbiology of plaque. Am J Orthod Dentofac Orthop 126:325–330
Sehgal V, Shetty VS, Mogra S, Bhat G, Eipe M, Jacob S, Prabu L (2007) Evaluation of antimicrobial and physical properties of orthodontic composite resin modified by addition of antimicrobial agents—an in vitro study. Am J Orthod Dentofac Orthop 131:525–529
Mei L, Ren Y, Loontjens TJ, Van der Mei HC, Busscher HJ (2012) Contact-killing of adhering streptococci by a quaternary ammonium compound incorporated in an acrylic resin. Int J Artif Organs 35:854–863
Demling A, Elter C, Heidenblut T, Bach F, Hahn A, Schwestka-Polly R, Stiesch M, Heuer W (2010) Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur J Orthod 32:414–418
Roosjen A, De Vries J, Van der Mei HC, Norde W, Busscher HJ (2005) Stability and effectiveness against bacterial adhesion of poly (ethylene oxide) coatings in biological fluids. J Biomed Mater Res B Appl Biomater 73B:347–354
Chun MJ, Shim E, Kho EH, Park KJ, Jung J, Kim JM, Kim B, Lee KH, Cho DL, Bai DH, Lee SI, Hwang HS, Ohk SH (2007) Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod 77:483–488
Tiller JC, Liao C, Lewis K, Klibanov AM (2001) Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci 98:5981–5985
Beyth N, Yudovin-Farber I, Perez-Davidi M, Domb AJ, Weiss EI (2010) Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc Natl Acad Sci 107:22038–22043
Gorelick L, Geiger AM, Gwinnett AJ (1982) Incidence of white spot formation after bonding and banding. Am J Orthod 81:93–98
Mizrahi E (1982) Enamel demineralization following orthodontic treatment. Am J Orthod 82:62–67
Årtun J, Brobakken BO (1986) Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod 8:229–234
Geiger AM, Gorelick L, Gwinnett AJ, Griswold PG (1988) The effect of a fluoride program on white spot formation during orthodontic treatment. Am J Orthod Dentofac Orthop 93:29–37
Boersma JG, Van der Veen MH, Lagerweij MD, Bokhout B, Prahl-Andersen B (2005) Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res 39:41–47
Lovrov S, Hertrich K, Hirschfelder U (2007) Enamel demineralization during fixed orthodontic treatment—incidence and correlation to various oral-hygiene parameters. J Orofac Orthop 68:353–363
Chapman JA, Roberts WE, Eckert GJ, Kula KS, González-Cabezas C (2010) Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am J Orthod Dentofac Orthop 138:188–194
Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ (2011) Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod 81:206–210
Lucchese A, Gherlone E (2012) Prevalence of white spot lesions before and during orthodontic treatment with fixed appliances. Eur J Orthod. doi:10.1093/ejo/cjs070
Julien KC, Buschang PH, Campbell PM (2013) Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthod 83:641–647
Acknowledgments
This study has been supported by the Departments of Orthodontics and BioMedical Engineering, University Medical Centre Groningen, University of Groningen, the Netherlands.
Conflict of Interest
H.J. Busscher is also director of a consulting company, SASA BV (GN Schutterlaan 4, 9797 PC Thesinge, The Netherlands). The authors declare no potential conflicts of interest with respect to authorship and/or publication of this article. Opinions and assertions contained herein are those of the authors and are not construed as necessarily representing views of the funding organizations or their respective employers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, Y., Jongsma, M.A., Mei, L. et al. Orthodontic treatment with fixed appliances and biofilm formation—a potential public health threat?. Clin Oral Invest 18, 1711–1718 (2014). https://doi.org/10.1007/s00784-014-1240-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1240-3