Abstract
Objective
The aim of the present in vitro study was to assess resistance to static fatigue of implants with different connections at various insertion levels.
Materials and methods
Sixty implants and abutments were used with the smallest diameter of each model. Four groups (n = 15) were created on the basis of the implant design and connection: cylindrical external hexagon Ø3.30 mm (group 1), cylindrical internal hexagon Ø3.30 mm (group 2), conical internal hexagon Ø3.50 mm (group 3), and conical Morse taper Ø3.50 mm (group 4). Three insertion levels in resin were tested, 0 mm at the platform level (l1), 3 mm (l2), and 5 mm (l3) above the platform of the resin. All groups were subjected to quasi-static loading at 30° to the implant axis in a universal machine.
Results
The mean fracture strengths for group 1 were 1,991 N (l1), 1,020 N (l2), and 767 N (l3); for group 2: 2,119 N (l1), 1,034 N (l2), and 903 N (l3); for group 3: 2,373 N (l1), 1,407 N (l2), and 929 N (l3); and for group 4: 1,710 N (l1), 1,680 N (l2), and 1,182 N (l3).
Conclusions
Resistance to loading decreases significantly with the loss of insertion, and the connection design between the implants and abutments can change the performance and resistance of the system.
Clinical relevance
When implants are used in areas where there is a possibility of bone loss, the selection of a connection type is an important consideration for the longevity of the system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The replacement of missing teeth with dental implants has become routine in dental practice. The success rate of this treatment is higher than 90 % and is consistently predictable [1]. On occasion, however, prosthetic implants will fail because of mechanical or biological causes [2].
Clinical observations have indicated that the primary causes of implant failure include incomplete osseointegration, complications from neighboring soft tissues and biomechanical problems. Still, the failure of dental implants related to defects or faults introduced during implant design or production [3] can be attributed to poor planning or the use of an improper design and/or dimensions [4] for a given region of the maxilla or mandible [3, 5]. A combination of these factors, along with an inadequate integration with the supporting structures, are considered as additional causes of failure [6–9] and may lead to overloading of the implant [10]. Occlusal conditions, such as parafunctional habits or excessive occlusal forces, have been identified as other potential causes of implant fracture [11]. Finally, the passive fit and seal between the implant and its abutment components are factors that further determine the success and longevity of the system.
Titanium has been used to manufacture implants owing to its physicochemical properties. However, its rigidity as compared with alveolar bone is the primary drawback of this implant material, as it removes stress from the bone and causes a loss of bone mass and ultimately resorption at the site. This phenomenon is known as stress shielding [12]. An important implication of this loss of bone is the risk of implant fracture. A small number of cases were reported to have implant fracture 6 to 7 years after placement [13]. These fractures were observed primarily at the implant neck. When this occurs, the remaining implant body is rigidly connected to the bone, and thus, removal of the fractured implant involves the removal of living bone, with subsequent pain experienced by the patient.
In an effort to reduce the frequency of this outcome, the mechanical causes of fracture are being scrutinized, with multiple studies examining mechanisms to retrieve fractured dental implants [14–18] and the causes of these fractures. Adequate crestal bone level is considered as an important clinical determinant for the success of implants [19]. The loss of peri-implant crestal bone will dramatically affect the biomechanical anchoring of the prosthetic restoration and possibly compromise proposed treatment [20] and may be attributed to several factors, such as excessive occlusal forces, trauma during the surgical procedure, inflammation/ infection, implant exposure during soft tissue healing, implant abutment gap present in the great majority of implant systems commercially available, early loading of a not biomechanically competent bone biomaterial interface, and implant bulk device design, particularly the crest module profile [21]. Furthermore, when vertical bone loss coincides with the internal chamber for fixation of the abutment screw in their apical limit, there is an increased risk of implant fracture [22].
The hypothesis was that the type of implant and its connections would impact on the strength-to-fracture of the implant. The use of an implant more resistant to fracture would be particularly crucial in areas where bone loss is known to be more frequent. Thus, the aim of the present in vitro study was to assess resistance to static fatigue of implants with different connections at various insertion levels.
Materials and methods
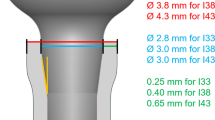
Sixty dental implants and 60 abutments were manufactured by the same company (Implacil De Bortoli, São Paulo, Brazil), with characteristics as described in Table 1. Four implant types were used in the final analysis: cylindrical external hexagon Ø3.30 mm (group 1), cylindrical internal hexagon Ø3.30 mm (group 2), conical internal hexagon Ø3.50 mm (group 3), and conical Morse taper Ø3.50 mm (group 4). Figure 1 illustrates the dimensions and appearance of the implants (13 mm in length).
Test implants were loaded with static compressive forces. The static fatigue strength of the dental implants was tested according to previous guidelines; these guidelines recommend selection of the smallest diameter of implant available for each model because this critically impacts on the efficacy of the implant and an implant angulation of 30° with respect to the applied load [22].
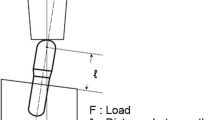
The implants were embedded in epoxy resin with the implant body placed at three different levels: (l1) surface level (level 1 = 0 mm), (l2) 3 mm above surface level (level 2 = 3 mm), and (l3) 5 mm above surface level (level 3 = 5 mm), simulating various marginal bone levels (Fig. 2). The epoxy resin had a Young's modulus of elasticity similar to cortical bone. For each level, five implant specimens per group were used (20 implants per level).
The abutments were set with the same final height and received a torque of 25 N, as recommended by the product manufacturer. A metal hemisphere was elaborated and cemented on the abutment (Fig. 3).
Scheme used in the compression test based on ISO 14801/2007 standards [22]. The distance between the red points (alpha) shows the extent of compression during the test
According to the study outline (Table 1), all groups were subjected to quasi-static loading until fracture using a properly calibrated universal testing machine (model AME-5kN, Técnica Industrial Oswaldo Filizola Ltda, Guarulhos, Brazil) with a test capacity of 5.0 kN. Tests were conducted at the Testing Laboratory of Biomechanics (Biotecnos, Santa Maria, Brazil). The test speed was set at 1 mm/min.
After the quasi-static loading test, all fractured samples were ultrasonically cleaned in 96 % isopropanol and observed under low power magnification. Digital photographs were taken using a Sony H9 digital camera (Tokyo, Japan), and the data were reported descriptively.
Statistical analyses were performed using a one-way analysis of variance (ANOVA) to determine the differences between the four groups.
Results
The fracture strength values of all groups recorded during quasi-static loading are shown in Figs. 4 and 5. All four implant types (groups 1–4) showed the greatest degree of resistance at level 1. Across the four groups, a 3-mm loss in insertion produced an average reduction in strength of 37.2 % and a 5-mm loss in insertion caused an average reduction in strength of 53.8 %. However, the most critical decreases in resistance were found between the groups.
Cylindrical external hex implants (group 1) showed a high level of resistance at level 1, with a mean of 1,991 N and presented a slight deformation of the hexagon upon crushing and fracture of the abutment. At level 2, a slight deformation to the hexagon was also observed; however, a partial tear (fissure) in the implant body at the origin of its insertion and a fracture of the abutment were also observed, with a 48.74 % reduction in average strength relative to that of level 1 (F = 1,020 N). At level 3, the change in the hexagon was similar to the previous levels and the partial tear to the body of the implant was slightly higher than at the previous level. The average resistance relative to level 1 was reduced by 61.45 % (F = 767 N).
The cylindrical internal hexagon implants (group 2) showed a high degree of resistance at level 1 (F = 2,119 N), with the greatest deformation observed when the hexagon was displaced by the applied load. At level 2, complete fracture of the implant body was observed at the level of insertion of the hexagon component, showing an average reduction of 51.19 % (F = 1,034 N). At level 3, the implants exhibited the same behavior as described for level 2, with complete fracture occurring at the head of the implant. The resistance average reduced by 57.36 % (F = 903 N) relative to that observed at level 1.
The highest level of resistance at level 1 was measured with the conical internal hexagon implants (group 3), with an average strength of 2,373 N. Deformation occurred along the hexagon with a fracture of the abutment. At level 2, there was a large deformation in the hexagon, a partial tear (fissure) of the implant body at the origin of its insertion, and a fracture of the abutment, showing an average 40.67 % reduction in resistance (F = 1,034 N). At level 3, there was a fracture in the abutment and changes to the hexagon. In addition, the body of the implants had a small crack at the level corresponding to the end of the internal chamber of the screw. An average reduction of 60.84 % (F = 929 N) was observed as compared with that at level 1.
The fourth group of conical Morse taper implants exhibited almost uniform resistance under all three conditions. At level 1, the implant bowed but did not fracture completely, deforming only in the cervical portion of the implant, with an average strength of 1,710 N. At level 2, there was also a slight deformation in the cervical portion of the implant and a fracture of the abutment; however, it did not show fissure or deformation in the body of the implant, with an average strength of 1,680 N. At level 3, no additional changes were observed in the cervical portion; however, a small fissure was observed in the body of the implants, with an average reduction of 30.87 % in strength (F = 1,182 N).
Significant differences between the four groups were observed using a one-way ANOVA test. In all cases, F-cal = 18.46106 was greater than F-crit = 4.25649, with significance set at p < 0.05. Within the groups 1, 2, and 3, variations in the level of insertion significantly affected the resistance of the implants, with a higher insertion causing a significant decrease in resistance between levels 1 and 2. The exception to this was observed for group 4, which showed no difference between levels 1 and 2 and the lowest reduction between the groups.
Discussion
Endosseous implants are widely used for prosthetic treatment in fully or partially edentulous patients. In general, these implants are considered to be consistent and predictable, with few failures [23]. In situations where implant fracture occurs, it is difficult to repair the implant because of technical and physiological complications. The possible causes of fracture can be classified into three broad groups: (1) failure of the implant design or the employed material, (2) an absence of passive adaptation of the prosthetic crown to the implant substructure, and (3) overload due to parafunctional habits. The type of treatment may also be influenced by the load and stress that is transmitted to the implant following reconstruction. The results of this study demonstrated that the insertion level of the implant can significantly influence the level of resistance offered by the implant, with a deeper insertion providing a larger resistance to external forces.
Some authors [11] have observed that bone loss occurs around the implant above its point of fracture, particularly when molar implant units are involved. Corono-apical resorption produces a high bending stress on the implant because of the loss of bony support. Bone resorption in response to peri-implantitis usually extends to the level of bone corresponding with the end of the abutment screw, where resistance to bending is diminished [24, 25]. This region is strongly related to the magnitude and direction of the stress that is transmitted to the implant. These forces are affected by the nature of the antagonist teeth, the bite force, the number of implants available to support the load, and the structure of the prosthesis with respect to the position of the implant [1]. Here, we examined the resistance to static fatigue of implants with different connections at various levels of integration and found significant differences between the connection types. To conduct this study according to ISO 14801:2007 [22], the smallest diameter implant of each model was used set at an inclination of 30°. The implant diameter relative to the dimension of the supporting bone is critical for successful treatment. The average maximum biting force exhibited by adults in the premolar and molar region is 789 N for man and 596 N for women [26]. In our study, fracture strength after static loading of the specimens was significantly higher for groups 1 and 2 at implant levels 0 and 3 mm. When the insertion was reduced to 5 mm above the surface level, the resistance of the models approximates the previously reported masticatory forces [27].
The fracture load values found for the titanium implants in this study are lower than those reported by Strub and Gerds [28] for implant metal abutment combinations after chewing simulation. This difference may be explained by differences in methodology. For example, in this study, the static load measurements were stopped after a deflection of 4 mm, while Strub and Gerds [28] continued until they observed a deviation from the linear slope in the load displacement graph. The fatigue test established by ISO 14801:2007 [22] is extremely important in the evaluation of dental implants. These guidelines serve to analyze the samples mechanically with the intention of mimicking clinical behavior. Our tests using static implant fatigue for different products at various levels of insertion demonstrated that implant strength is maximized when fully embedded. A 3-mm loss in insertion has an average reduction in strength of 37.2 %, whereas a 5-mm loss in insertion has an average reduction in strength of 53.8 %. Morse taper implants showed the lowest loss in strength of all groups because of the type of abutment used in this system. These results demonstrate that the type of implant and abutment can change the performance and resistance of the system, and suggest that in areas where there is a possibility of bone loss, the selection of a connection type is an important consideration to the longevity of the implant system in dental repair. While other meaningful results have been reported in chewing simulation or fatigue loading studies of implant abutment systems [29–31], clinical trials are necessary to validate the results of these investigations as well as the present in vitro study.
Conclusions
Within the limitations of this in vitro study, we conclude that:
-
1.
The level of cervical insertion of implants affects the resistance to external forces during the application of non-axial strength.
-
2.
Implants integrate firmly into bone and fracturing generally takes place at the neck portion of the implant.
-
3.
Connection design between implants and abutments can change the performance and resistance of the system.
References
Albrektsson T, Zarb GA, Worthington P, Eriksson AR (1986) The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants 1(1):11–25
Manda MG, Psyllaki PP, Tsipas DN, Koidis PT (2009) Observations on an in vivo failure of a titanium dental implant/abutment screw system: a case report. J Biomed Mater Res B Appl Biomater 89B(1):264–273
Balshi TJ (1996) An analysis and management of fractured implants: a clinical report. Int J Oral Maxillofac Implants 11(5):660–666
Siddiqui AA, Sosovicka M, Goetz M (2006) Use of mini implants for replacement and immediate loading of 2 single-tooth restorations: a clinical case report. J Oral Implantol 32(2):82–86
Piattelli A, Scarano A, Paolantonio M (1998) Clinical and histologic features of a nonaxial load on the osseointegration of a posterior mandibular implant: report of a case. Int J Oral Maxillofac Implants 13(2):273–275
Abu-Hammad O, Khraisat A, Dar-Odeh N, Jagger DC, Hammerle CH (2007) The staggered installation of dental implants and its effect on bone stresses. Clin Implant Dent Relat Res 9(3):121–127
Quirynen M, Naert I, van Steenberghe D (1992) Fixture design and overload influence marginal bone loss and fixture success in the Brånemark system. Clin Oral Implant Res 3(3):104–111
Stegaroiu R, Sato T, Kusakari H, Miyakawa O (1998) Influence of restoration type on stress distribution in bone around implants: a three-dimensional finite element analysis. Int J Oral Maxillofac Implants 13(1):82–90
Sütpideler M, Eckert SE, Zobitz M, An KN (2004) Finite element analysis of effect of prosthesis height, angle of force application, and implant offset on supporting bone. Int J Oral Maxillofac Implants 19(6):819–825
Isidor F (1999) Occlusal loading in implant dentistry. In: Lang NP, Karring T, Lindhe J (eds) Proceedings of the 3rd European workshop on periodontology: implant dentistry. Quintessence, London, pp 358–375
Green NT, Machtei EE, Horwitz J, Peled M (2002) Fracture of dental implants: literature review and report of a case. Implant Dent 11(2):137–143
Gefen A (2002) Computational simulations of stress shielding and bone resorption around existing and computer-designed orthopaedic screws. Med Biol Eng Comput 40:311–322
Takeshita F, Kuroki H, Yamasaki A, Suetsugu T (1995) Histopatologic observation of seven removed endosseus dental implants. Int J Oral Maxillofac Implants 10:367–372
Siegmund C, Scbimming R, Swaid S (2000) Implant failure caused by screw head fractures–a new type of complication in a reconstruction plate: a case report. J Oral Maxillofac Surg 58(8):909–910
Piattelli A, Scarano A, Piattelli M, Vaia E, Matarasso S (1998) Hollow implants retrieved for fracture: a light and scanning electron microscope analysis of 4 cases. J Periodontol 69(2):185–189
Morgan MJ, James DF, Pilliar RM (1993) Fractures of the fixture component of an osseointegrated implant. Int J Oral Maxillofac Implants 8(4):409–414
Piattelli A, Piattelli M, Scarano A, Montesani L (1998) Light and scanning electron microscopic report of four fractured implants. Int J Oral Maxillofac Implants 13(4):409–414
Yokoyama K, Ichikawa T, Murakami H, Miyamoto Y, Asaoka K (2002) Fracture mechanisms of retrieved titanium screw thread in dental implant. Biomaterials 23(12):2459–2465
Zarb GA, Albrektsson T (1998) Consensus report: towards optimized treatment outcomes for dental implants. J Prosthet Dent 80(6):641
Leonard G, Coelho PG, Polyzois I, Stassen L, Claffey N (2009) A study of the bone healing kinetics of plateau versus screw root design titanium dental implants. Clinical Oral Implants Res 20(3):232–239
Marincola M, Coelho PG, Morgan V, Cicconetti A (2010) The importance of crestal bone preservation in the use of short implants. J Adv Dent Res 1(1):15–18
International Organization for Standardization (2007) ISO 14801: dentistry-implants-dynamic fatigue test for endosseous dental implants. The Organization, Geneva
Pylant T, Triplett RG, Key MC, Brunsvold MA (1992) A retrospective evalutation of endosseus titanium implants in the partially edentulous patient. Int J Oral Maxillofac Implants 7(2):195–202
Rangert B, Krogh PH, Langer B, Van Roekel N (1995) Bending overload and implant fracture: a retrospective clinical analysis. Int J Oral Maxillofac Implants 10(3):326–334
Sánchez-Pérez A, Moya-Villaescusa MJ, Jornet-García A, Gomez S (2010) Etiology, risk factors and management of implant fractures. Med Oral Patol Oral Circ Bucal 15(3):e504–e508
Piattelli A, Corigliano M, Scarano A (1996) Microscopical observations of the osseous responses in early loaded human titanium implants: a report of two cases. Biomaterials 17(13):1333–1337
Bidez MW, Misch CE (1999) Clinical biomechanical in dentistry. In: Misch CE (ed) Contemporary implant dentistry, 2nd edn. CV Mosby, St Louis, pp 303–316
Strub JR, Gerds T (2003) Fracture strength and failure mode of five different single-tooth implant-abutment combinations. Int J Prosthodont 16:167–171
Cibirka RM, Nelson SK, Lang BR, Rueggeberg FA (2001) Examination of the implant-abutment interface after fatigue testing. J Prosthet Dent 85:268–275
Gratton DG, Aquilino SA, Stanford CM (2001) Micromotion and dynamic fatigue properties of the dental implant-abutment interface. J Prosthet Dent 85:47–52
Khraisat A, Hashimoto A, Nomura S, Miyakawa O (2004) Effect of lateral cyclic loading on abutment screw loosening of an external hexagon implant system. J Prosthet Dent 91:326–334
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gehrke, S.A., Souza dos Santos Vianna, M. & Dedavid, B.A. Influence of bone insertion level of the implant on the fracture strength of different connection designs: an in vitro study. Clin Oral Invest 18, 715–720 (2014). https://doi.org/10.1007/s00784-013-1039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-013-1039-7