Abstract
Objectives
The purpose of this review was to summarize recent developments regarding photodynamic therapy (PDT) in the field of dentistry.
Materials and methods
A review of pertinent literature was carried out in PubMED to determine the current position of PDT applications in dentistry. One hundred thirteen relevant articles were retrieved from PubMED by inserting the keywords “photodynamic therapy”, “dentistry”, “periodontology”, “oral surgery”, and “endodontics”. It is anticipated that this overview will create a specific picture in the practitioner’s mind regarding the current status and use of PDT.
Results
In spite of different results and suggestions brought about by different researchers, PDT can be considered as a promising and less invasive technique in dentistry.
Conclusion
PDT seems to be an effective tool in the treatment of localized and superficial infections. Within the limitations of the present review, it can be concluded that although PDT cannot replace antimicrobial therapy at its current stage, it may be used as an adjunctive tool for facilitating the treatment of oral infections.
Clinical relevance
Oral infections (such as mucosal and endodontic infections, periodontal diseases, caries, and peri-implantitis) are among the specific targets where PDT can be applied. Further long-term clinical studies are necessary in establishing a more specific place of the technique in the field of dentistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photodynamic therapy (also called PDT, photo radiation therapy, phototherapy, or photo chemotherapy) is a new treatment modality that has been developing rapidly within various medical specialties since the 1960s and has been defined as “the light induced inactivation of cells, microorganisms, or molecules.” The studies and clinical trials by Thomas Dougherty and his founding the International Photodynamic Association in 1986 helped the PDT approach to receive specific attention [1, 2]. Currently, a considerable number of researches and clinical investigations are being undertaken for the determination of optimal combinations of photosensitizers, light sources, and treatment parameters for a wide variety of different diseases.
Background and mechanism of PDT
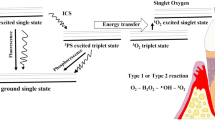
PDT was developed as a therapy for several diseases such as tumors, periodontitis, other oral lesions, and premalign diseases, involving the application and retention of an applied photosensitizing agent in target tissues [3]. Upon irradiation with light of an appropriate wavelength, the photosensitizer undergoes transition from low-energy-level “ground state” to a higher-energy “triplet state.” This triplet-state sensitizer can react with biomolecules to produce free radicals and radical ions or with molecular oxygen to produce singlet oxygen. These cytotoxic species can cause oxidation of cellular constituents such as plasma membranes and DNA, resulting in cell death. Clinically, this reaction is cytotoxic and vasculotoxic [4–7]. On the other hand, although DNA is one of the targets, it has been indicated that damage to DNA is not directly correlated with cell death, giving Deinococcus radiodurans as an example. This microorganism which possesses a very efficient DNA repair mechanism is readily killed by photodynamic processes [8]. Another type of damage caused by antimicrobial PDT is the damage caused to the cytoplasmic membrane of the bacteria by cytotoxic species generated by antimicrobial photodynamic therapy, leading to events such as inactivation of the membrane transport system, inhibition of plasma membrane enzyme activities, lipid peroxidation, and others [9].
Microorganisms such as bacteria, fungi, viruses, and protozoa can be killed by singlet oxygen species. Common herpes simplex infections can be successfully treated with antimicrobial photodynamic therapy [9].
Photosensitizers may be injected intravenously, ingested orally, or applied topically depending on the type of agent [10]. There are two mechanisms by which the triple-state photosensitizer can get into reaction with biomolecules. In type I mechanism, electron/hydrogen transfers directly from the photosensitizer producing ions or there is an electron/hydrogen removal from a substrate molecule to form free radicals. The free radicals get into a reaction with oxygen rapidly and result in producing highly reactive oxygen species [11]. In type II mechanism, an electronically excited and highly reactive state of oxygen is released, which is named singlet oxygen. Since type II reactions are mediated through singlet oxygen species, this is accepted as the major pathway in microbial cell destruction. With the action of both types of mechanisms, damage is created by oxygen tension as well as photosensitizer concentration [12, 13].
PDT has initially been introduced as a significant novel disinfection treatment modality in dentistry. Different definitions have been made for the inactivation of microorganisms by the PDT, such as antimicrobial photodynamic therapy, photodynamic antimicrobial chemotherapy (PACT), and photodynamic disinfection or lethal photosensitization [9, 14, 15].
This treatment model represents a highly promising alternative for the treatment of localized microbial infections, such as chronic ulcers and a variety of oral infections. PACT seems to be effective against antibiotic-sensitive and antibiotic-resistant microorganisms. In addition, repeated applications do not result in the selection of bacteria [14, 15].
Photosensitizers
For PDT to be successful in tumor therapy as well as for antimicrobial purposes, it is essential to select an appropriate and effective non-toxic photosensitizer capable of high absorption in the light length used [16]. Many natural and synthetic photoactive compounds have a photosensitizing effect. The characteristics of ideal photosensitizers are: (1) high absorption coefficient in the spectral region of the excitation light, (2) a triplet state of appropriate energy (ET/95 kJ mol−1) to allow for efficient energy transfer to ground-state oxygen, (3) high quantum yield of the triplet state (FT/0.4) and long triplet- state lifetimes (tT/1 ms) since the efficiency of the photosensitizer is dependent on the photophysical properties of its lowest excited triplet state, and (4) high photostability [17]. Several kinds of photosensitizers may be associated with laser, but each will have applicability dependent on the absorption of the light and wavelength. Most photosensitizers are activated by light between 630 and 700 nm, corresponding to a penetration depth of 0.5 cm (630 nm) to 1.5 cm (at ∼700 nm). The main photosensitizers found in the literature are: hematoporphyrin derivatives (620–650 nm), phenothiazine, like toluidine blue and methylene blue (620–700 nm), cyanine (600–805 nm), phytotherapic agents (550–700 nm), and hytalocyanines (660–700 nm) [16, 18, 19].
Some photosensitizers that are commercially available are Photofrin®, ALA, VisudyneTM (BPD; Verteporfin), and Foscan®. While all four are in use in Europe, the first three have been approved by the FDA. There are also photosensitizers commercially produced in kits. The Periowave product has been commercialized by Ondine Biopharma Corporation for the treatment of periodontitis. Furthermore, the Periowave product consists of a laser system with a custom-designed handpiece and patient treatment kits of methylene blue. There is another available kit in the market, including phenothiazine chloride for clinical photodynamic therapy in Austria, Germany, Switzerland, and the UK (Helbo®; Photodynamic Systems GmbH&Co. KG, Grieskirchen, Austria). There are also similar kits including toluidine blue O produced by other companies which include Dentofex Ltd., Dexcel Pharma Thechnologies Ltd., SciCan Medtech AG, and Cumdente GmbH [20].
Photofrin and hematoporphyrin derivates (620–650 nm) are the first-generation sensitizers whereas the 5-aminolevulin-ic acid (ALA; Levulan®, Dusa Pharmaceuticals, Inc., Wilmington, MA, USA; Metvix®, Galderma, F), benzoporphyrin derivative, lutetium texaphyrin, temoporfin (mTHPC; Foscan®, Biolitec Pharma, Edinburgh, Scotland), tinethyletiopurpurin, and talaporfin sodium are the second-generation sensitizers [12]. Foscan (temoporfin), the most potent second-generation photosensitizer, has been found to be 100 times more active than photofrin in animal studies [21]. Two ALA preparations, Metvix (PhotoCure ASA, Oslo, Norway) and Levulan Kerastic (Dusa Pharmaceuticals, Wilmington, MA, USA), have received approval from the European Agency for the Evaluation of Medicinal Products and by the FDA, respectively, for the treatment of nonhyperkeratotic actinic keratoses of the face and scalp. PDT has not yet been approved by FDA for dentistry use, and when clinical studies are conducted, the treatment procedure of the patients should be conducted according to FDA and local institutional review board approval [22].
5-Aminolevulinic acid can be applied intravenously, orally, or topically to allow greater tumor selectivity. 5-ALA is the sole photosensitizer to be applied topically. The remaining types can only be delivered intravenously. Topically applied ALA provides some advantages such as complete lack of systemic photosensitivity and lack of necessity of avoiding exposure to light after the treatment. A major disadvantage of topical application is the low treatment depth and penetration which is around 1–2 mm. Therefore, this approach is useful for the successful treatment of superficial tumors [23, 24]. Examples to these cases are premalignant and malignant lesions, leukoplakia and recalcitrant leukoplakia, mouth angles, buccal, labial, gingival, mandibular oral mucosa, dysplasia, squamous cell carcinoma, verrucous hyperplasia, and extraoral verrucous carcinoma [12].
The activating light is most often generated by lasers or in some cases by arc lamps or fluorescent light sources. Typically, the light must be of a specific wavelength as described by some; however, even broad-spectrum light can activate photosensitizers such as toluidine blue. Lasers are the most preferred sources of light used in PDT. The laser light used in PDT can be directed through a fiber optic to deliver the proper amount of light. Most of the light photons at wavelengths between 630 and 800 nanometers (nm) travel 23 cm through the surface tissue and muscle between input and exit at the photon detector [25]. As high-power lasers may induce trauma to surrounding tissues through thermal injury, low-power light with a photosensitizer is an attractive alternative therapy [12, 26]. Currently, diode lasers, which are much cheaper and more portable than metal vapor or tuned dye lasers, have become the preferred light source [23].
The choice of photosensitizers used in dentistry is strongly dependent on the light source used. Currently, light sources of a specific wavelength mostly applied in photodynamic therapy are helium–neon lasers (633 nm), gallium–aluminum–arsenide diode lasers ( 630–690, 830, or 906 nm), and argon lasers (488–514 nm). The wavelengths of these sources range from visible light to the blue of argon lasers or from the red of helium–neon and gallium–aluminum–aresenide lasers to the infrared area of some diode lasers. High-level-energy laser irradiation is not used to activate the photoactive dye because a relatively low-level exposure produces a high bactericidal effect [9].
Non-coherent light sources, such as tungsten filament, quartz halogen, xenon arc, and phosphor-coated sodium lamps, are used for the treatment of larger areas. Nonlaser sources such as light-emitting diodes (LED) are recently used in PDT because of their inexpensive, flexible, and lightweight properties [9].
Guidelines have also been developed by authors for achieving an efficient and practical use of PDT in non-melanoma skin cancers depending on the detailed evidence-based review of pertinent literature [27, 28].
Advantages of PDT
Selective uptake of photosensitizers to particular tissue layers, precise directing of laser light using optical fibers, lack of scarring, and highly selective tissue necrosis, which is achieved by localizing the drug to the proliferating tissue, are the potential advantages of PDT. It can be performed in out-patient or day-case settings and repeated doses can be given without the need for total dose limitations. Resistance to treatment does not develop with repeated treatment [12].
Limitations of PDT
PDT requires direction of the light to the appropriate site and tissue depth to be effective. Optimal light delivery with laser and collaboration and coordination between clinicians is complex and availability of the necessary light sources has been a problem. Currently, low-cost portable light sources are more readily available. PDT is an ablative procedure and does not yield material for histological diagnosis. Diagnosis should be made before treatment. Persistent skin photosensitivity, lasting for weeks with some photosensitizers, limits the use of PDT as a therapeutic regimen. PDT is also less effective in treating large tumors because the light cannot pass far into these pathologies [29–31]. PDT is a local treatment and generally cannot be used to treat cancer that has spread widely (metastasized) [31].
Side effects
The side effects of PDT depend on how the treatment is performed and it will vary between individuals. The side effects produced vary according to:
-
What part of the body is treated
-
The type of photosensitizing drug given
-
The time between administration of the drug and light application
-
The skin sensitivity to light following treatment
The major side effect of PDT is residual systemic photosensitization, which lasts for several days or weeks depending on the administered photosensitizer. When administered systemically, residual skin photosensitivity may ensue for a period. Daylight may be a means of activation of photosensitizer, resulting in first- or second-degree burns. The patients are therefore instructed to avoid exposure to bright light or sunlight until the drug is completely eliminated. Also, skin and eyes should be protected from intense exposure of light. Furthermore, though PDT treatment is not a painful procedure, pain may be experienced by patients several hours after PDT. Pain relief medications prior or after the laser treatment may be advocated. When used for the treatment of tumors, though damage to health tissues is minimal, burns, swelling, pain, and scarring in the nearby tissues are likely. Other side effects that are less frequent are coughing, trouble swallowing, stomach pain, painful breathing or shortness of breath, allergic reactions, change of liver parameters, etc. [12].
PDT has not been approved by the FDA for use in dentistry. In clinical trials, all patients are treated in accordance with the FDA and local institutional review board approval.
Applications of PDT in dentistry are growing rapidly such as the photodynamic diagnosis of malignant transformation of oral lesions [3] and the treatment of head and neck cancer, as well as bacterial and fungal infections.
Materials and methods
A review of pertinent literature was carried out in PubMED to determine the current position of PDT applications in dentistry. One hundred twenty-one relevant articles between 1981 and 2012 were retrieved from PubMED by inserting the keywords “photodynamic therapy”, “dentistry”, “periodontology”, “oral surgery”, and “endodontics”. It is anticipated that this overview will create a specific picture in the practitioner’s mind regarding the current status and use of PDT.
Results
Data obtained from this review can be summarized as presented in the following sections in terms of relevant topics.
Diagnosis and treatment of premalign and malign lesions of head and neck with PDT
Topical application of ALA is a relatively new diagnosing method of oral lesions. A pro-drug, 5-aminolevulinic acid (ALA), serves as a precursor of the photosensitizer, protoporphyrin IX (PpIX), in the heme biosynthetic pathway [30]. ALA-mediated photodynamic diagnosis, an intercellular accumulation of PpIX, increases tissue fluorescence. Subsequent illumination leads to fluorescence of the lesion caused by endogenous and ALA-induced PpIX. The difference in the fluorescence ratio between normal and premalignant/malignant tissue facilitates the distinction between malignant and nonmalignant lesions. ALA-based photodynamic diagnosis offers potential advantages such as non-invasive diagnosis, in situ monitoring, cost-effectiveness, and better tolerance than surgical biopsy for the patient. The use of ALA is restricted to superficial lesions (1–2 mm) due to the limited depth of topical ALA and the limited penetration of tissue at 635 nm [12].
Sharwani et al. [3] examined patients with clinically suspicious oral leukoplakia and showed that dysplastic lesions have significantly higher red fluorescence than benign oral lesions without changes in green autofluorescence.
PDT is a relatively minimally invasive treatment form of malign and premalignant lesions of head and neck including the oral cavity, the pharynx, the nasal cavity, and the larynx. These tumors are generally treated with conventional treatments, such as surgery, chemotherapy, and radiation, which may cause many side effects, including jaw pain, mouth sores, dysfunctional salivary glands, and difficulties in chewing, swallowing, and talking [32]. Selective tumor destruction and its minimal invasiveness are the main advantages of PDT over conventional treatments based on the preservation of healthy tissues.
With the experience of 30 years by PDT, it can be concluded that this treatment regimen is appropriate for basically two groups of pathologies: these are early neoplasmic lesions (premalignant and in situ carcinoma) and advanced recurrences after previous surgery or radiotherapy, respectively. Outcomes of the treatment are less satisfactory in the treatment of advanced carcinomas with PDT. This is probably due to a limited ability to adequately deliver laser light to the tumor bed. However, the possibility of an effective treatment of early-stage tumors and premalignant lesions with the preservation of the surrounding normal structures is often a great benefit [33, 34].
Kubler et al. [35] evaluated the effectiveness of mTHPC-mediated PDT in 25 patients with primary squamous cell carcinomas of the lip. During 12 weeks of the evaluation period, complete response has been shown by 24 of the patients (96 %). Only one patient has shown a partial response and has been successfully retreated by mTHPC-mediated PDT, with a complete response at 7 months after retreatment. The functional results were excellent in all patients, without any signs of restricted mouth opening or impaired lip closure.
Copper et al. [36] performed a study to examine the long-term efficacy of mTHPC-mediated PDT in the treatment of 29 early-stage squamous cell carcinomas of the oral cavity and oropharynx. In 25 tumors (86 %), a complete remission of the primary tumor was obtained. Four lesions developed local recurrent disease after 1–6 months. All of these cases were salvaged by surgery and/or radiotherapy. None of the patients complained about impairment of mastication, swallowing, articulation, or speech after PDT.
Hopper et al. [37] demonstrated that tumor clearance was accompanied by excellent cosmetic and functional results, without impact on the patient’s performance status. Adverse events in the immediate post-treatment phase were limited to pain (82 %) and swelling (10 %) at the treatment site due to the tumor necrosis caused by PDT. The authors concluded that mTHPC-mediated PDT is a safe and effective method of dealing with early oral squamous cell carcinoma with a number advantages over conventional treatments in terms of improved organ function and cosmetic appearance.
In a prospective case series by Rigual et al. [38], PDT use was assessed for the treatment of laryngeal and oral cavity premalignant and malignant disease of the head and neck. Two cohorts of patients were included in this trial, one of which included patients having tumor grade T1 squamous cell carcinoma and the other containing dysplasia and carcinoma in situ. Among the patients, 12 had persistent and recurrent disease after previous surgery or radiotherapy and 14 had primary disease. The patients were followed up for a period ranging between 7 and 52 months (mean 15 months). Complete response was observed in 12 patients in the dysplasia group and 12 in the T1 group. Partial response was received from one T1 patient, whereas no response was observed in one patient following PDT. Recurrence was detected within 90 days in three patients with oral dysplasia and a second invasive primary cancer was observed in one T1 patient. Pain, edema, hoarseness, and phototoxicity were the other adverse effects observed.
Lin et al. [39] indicated that the laser light-mediated topical ALA-PDT is also very effective for oral verrucous hyperplasia (OVH) and oral erythroleukoplakia lesions (OEL). Therefore, they suggested that topical ALA-PDT using either LED or laser light may serve as the first-line treatment of choice for OVH and OEL lesions.
Carcinoma in situ, field characterization having large areas of superficial premalignant and malignant changes and multicentric malignancies, are among the pathologies that seem responsive to PDT. Conventional treatment regimes seem to be incapable of treating these tumors without morbidity. Relatively large affected areas can be treated with PDT by preserving tissue, and it is possible to repeat the treatment as often as required. By using more powerful second-generation photosensitizers and more penetrating laser light of 652 nm, PDT is expected to give even better outcomes in the treatment of early-stage head and neck carcinomas [10, 40]. Sieron et al. showed that PDT is a useful and effective method for the treatment of premalignant lesions of the oral cavity and the palliation of advanced lesions of the oropharynx and larynx [33].
The option of retreatment with PDT or conventional therapy remains in case a complete tumor response is not achieved after PDT. Equivalent or greater efficacy can be achieved with PDT in the treatment of premalignant and malignant lesions at the head and neck region when compared with conventional therapy, with the additional benefit of greatly reduced morbidity and disfigurement. However, the choice for an optimum therapy for head and neck cancer is a multidisciplinary decision. When deciding on treatment options for these patients, treatment-related morbidity and the quality of life as well as the risk of developing secondary primary tumors should be considered in addition to the probability of achieving tumor control.
Oral lesions
Oral lichen planus (OLP) is a chronic inflammatory disease exhibiting relapses and remissions. The disease has a cell-mediated immunological origin in which there is accumulation of T lymphocytes beneath the oral mucosa epithelium. Furthermore, the differentiation rate of the stratified squamous epithelium increases, which results in hyperkeratosis and erythema with or without ulceration [41]. There is currently no cure for OLP. Treatment is aimed primarily at reducing the length and severity of symptomatic outbreaks. Topical steroids are the first-choice agents for the treatment of symptomatic, active OLP [42]. Other topical agents that have been used in cases resistant to topical steroids include retinoids, azathioprine, cyclosporine, tacrolimus, and mycophenolate mofetil. One such promising modality is PDT, which may have immunomodulatory effects and may induce apoptosis in the hyperproliferating inflammatory cells which are present in psoriasis and lichen planus. This may reverse the hyperproliferation and inflammation of lichen planus. Aghahosseini et al. demonstrated that methylene blue (MB)-PDT seems to be an effective alternative treatment for the control of OLP. However, further studies are needed in order to show the efficacy of MB-PDT in the control of OLP for a longer period of time [43].
Candidiasis, caused by Candida species, the commonest being Candida albicans, is the most frequently encountered infection of the oral cavity [44]. Immunocompromised states, diabetes mellitus, dental prostheses, xerostomia, and prolonged use of antibiotics or immunosuppressive drugs are the predisposing factors for oral candidiasis to ensue [45]. In addition, biofilm formation on epithelial surfaces and prosthetic devices is critical in the development of denture-associated candidiasis, which is a frequent condition occurring in denture wearers [46–48]. Due to the risk of high frequency of Candida infections in immunocompromised patients, effective antifungal therapy is necessary. In the management of oral candidiasis, topical antifungal agents are often prescribed [49, 50]. However, these agents achieve only a transient response and relapses are frequent. Antimicrobial photodynamic therapy has been evaluated as a promising method of treatment of oral candidiasis to overcome the problems associated with antifungal resistance [51–54]. The mechanism of antimicrobial photodynamic therapy inactivation of fungi is completely different from that of antifungal agents. The reactive oxygen species promote perforation of the cell wall and membrane, thereby permitting the photosensitizer to translocate into the cell. Once inside the cell, oxidizing species generated by light excitation induce photodamage to internal cell organelles and cell death [55, 56].
Dovigo et al. [57], in an in vitro study, attempted to describe the association of Photogem® (Photogem, Moscow, Russia) with LED for the photoinactivation of fluconazole-resistant (FR) and American Type Culture Collection strains of C. albicans and Candida glabrata. They treated suspensions of each Candida strain with five Photogem® concentrations and exposed them to four LED light fluences (14, 24, 34, or 50 min of illumination). After incubation, colonies were counted. Single-species biofilms were generated on cellulose membrane filters, treated with 25.0 mg l−1 of Photogem® and illuminated at 37.5 J cm−2. The biofilms were then disrupted and the viable yeast cells present were determined. Planktonic suspensions of FR strains were determined to be effectively killed after PDT. It was observed that the fungicidal effect of PDT was strain dependent. Significant decreases in biofilm viability were observed for three strains of C. albicans and for two strains of C. glabrata. The authors concluded that although antimicrobial photodynamic therapy was effective against Candida species, fluconazole-resistant strains showed reduced sensitivity to PDT. Moreover, single-species biofilms were less susceptible to antimicrobial photodynamic therapy than their planktonic counterparts.
Zeina et al. have demonstrated that PDT with methylene blue under conditions that lead to effective killing of typical skin microbes, including C. albicans, causes neither cytotoxicity nor DNA damage to keratinocytes in vitro [58, 59].
Candida has been demonstrated to be susceptible to antimicrobial photodynamic therapy by using an agent (Photofrin) which is already used in clinics. This is an important step that shows the potential application of this novel treatment modality for fungal infections. Selectivity is an important factor in these treatments because healthy human cells are also affected and may be damaged by the use of these agents. In mucocutaneous candidiasis, topical application can be selected for the affected areas and light can be applied only to those regions, making these infections amenable to antimicrobial photodynamic therapy [60].
Herpes is a common infectious disease that is caused by human herpes viruses. Several treatments have been proposed, but none of them prevents reactivation of the virus. Treatment with low-level laser therapy has been considered as an option in the treatment of herpes labialis, decreasing the frequency of vesicle recurrence and providing comfort for patients. The lesions have healed rapidly and no significant acute side effects have been noted [61].
Photodynamic approach has also been used to kill microorganisms in root canals in vitro and in vivo [62]. These studies suggested the potential of antimicrobial photodynamic therapy adjunctive to standard endodontic antimicrobial treatment. Methylene blue, a well-established photosensitizer has been used in PDT for targeting endodontic bacteria. MB predominantly interacts with the anionic macromolecule lipopolysaccharide, resulting in the generation of MB dimers, which participate in the photosensitization process [62, 63].
Fonseca et al. [64] have investigated the effects of antimicrobial photodynamic therapy on endodontic pathogens by evaluating the decrease in numbers of Enterococcus faecalis colonies in the canals of extracted human teeth. After contaminating root canals with bacteria and incubation, teeth were divided into a control group and a test group. Half of the teeth did not undergo any intervention and served as the control, whereas in the test group the teeth received a solution of 0.0125 % toluidine blue for 5 min followed by irradiation using a 50-mW diode laser (Ga–Al–As) at a wavelength of 660 nm. Bacterial samples were taken before and after irradiation. The number of colony-forming units was counted and it was concluded that PDT was effective in E. faecalis-contaminated root canals.
In oral surgery, antimicrobial photodynamic therapy, with its use of non-toxic dye (photosensitizer) in combination with low-intensity laser light enabling singlet oxygen molecules to destroy bacteria, also represents a treatment alternative for alveolar osteitis and post-extraction pain. It has been stated that laser treatment is best combined with surgical opening of the implant site for cleaning and disinfecting the local defect. In this way, photodynamic therapy can be used successfully to decontaminate the implant surface [65].
Photodynamic antimicrobial chemotherapy
The science of PACT is still in its infancy but follows similar principles to that of PDT. Due to the problems of systemic light delivery, the use of PACT may also be limited to localized infection. What is important, both in PDT and PACT, is the ability to excite the photosensitizer at its target site with minimal photoeffect on the surrounding tissue [66]. The most common bacterial diseases causing human dental caries and periodontal diseases result from plaque biofilms on teeth and soft tissues of the mouth. Biofilm that forms on teeth contains many microbial species including aerobic and anaerobic Gram-positive and negative bacteria, fungi, mycoplasma, protozoa, and viruses. The effectiveness of PACT, both topical and systemic, tends to be minimized by the presence of this biofilm [67]. Dental plaque formation is one of the initial phases of tooth decay, which is a microbial disease that affects a tooth’s calcified tissues. Streptococcus mutans is one of the most important bacteria present in dental plaque. The elimination of pathogenic microorganisms on teeth is fundamental in the prevention and control of tooth decay [68]. The use of lasers or LEDs of different wavelengths, in association with various photosensitizing dyes, can play an important role as an alternative treatment to remove dental plaque [69–71]. Bevialacqua et al. demonstrated that PACT was efficient at killing microorganisms and preventing the formation of biofilms in a planktonic culture [69].
Antimicrobial photodynamic therapy and periodontology
Systemic use of antibiotics in conjunction with mechanical treatment is a commonly performed treatment modality in periodontology and is regarded as a reliable method in the treatment of periodontal diseases. On the other hand, it has been determined that bacteria in biofilms are protected within the plaque matrix, thus showing less susceptibility to antibiotics. Furthermore, frequent use of antibiotics poses the risk of bacterial resistance. Therefore, there has recently been an increase in attempts for the development of alternative antimicrobial concepts [72–75]. Recently, antimicrobial photodynamic therapy has been used for the treatment of localized microbial infections. Antimicrobial photodynamic therapy exerts a toxic effect over bacteria by free radical formation. It has been indicated by researchers that this is an effective means of bacterial elimination during periodontal treatment and shows promise as a new methodology that can be selected for the elimination of bacterial infection from periodontal pockets during the non-surgical treatment of periodontitis. It has been shown in an animal model that the progression of periodontal disease and destruction of periodontal tissues can be reduced significantly by the utilization of antimicrobial photodynamic therapy [67]. Furthermore, Sigusch et al. [76] reported a reduction in the markers of periodontal destruction in beagle dogs following treatment with antimicrobial photodynamic therapy. They tested the antimicrobial photodynamic therapy using two photosensitizers, chlorine e6 and BLC1010, on beagle dogs. The animals were infected with Porphyromonas gingivalis and Fusobacterium nucleatum in all subgingival areas. Microbiological monitoring before and after treatment was performed using polymerase chain reaction. Antimicrobial photodynamic therapy was conducted with a diode laser with a wavelength of 662 nm using a power of 0.5 W and the photosensitizers.
Several studies have shown that oral bacteria in planktonic cultures and in plaque scrapings are susceptible to antimicrobial photodynamic therapy [77–81]. Moreover, recent studies have reported that photodynamic therapy induced bacterial cell killing and reduced bacterial numbers by more than tenfold in S. mutans, Streptococcus sobrinus, and Streptococcus sanguinis biofilms when toluidine blue O or erythrosine was used as the photosensitizer [82–85]. Schneider et al. [86] assessed the effect of laser-induced antimicrobial photodynamic therapy on the viability of S. mutans cells employing an artificial biofilm model and concluded that laser irradiation is an essential part of antimicrobial photodynamic therapy to reduce bacteria within a layer of 10 μm.
However, other studies have demonstrated incomplete destruction of oral pathogens in plaque scrapings, monospecies biofilms, and multispecies biofilms derived from human saliva [87–90].
Yılmaz et al. [91] concluded that antimicrobial photodynamic therapy provided no additional microbiological and clinical benefits over conventional mechanical debridement. The reduced effectiveness of antimicrobial photodynamic therapy in their study may be a result of the indirect application of antimicrobial photodynamic therapy from the external surface of the gingiva.
Fontana et al. [77] investigated the photodynamic effects of methylene blue on human dental plaque microorganisms in the planktonic phase vs. the biofilm phase and found that oral bacteria in biofilms are affected less by antimicrobial photodynamic therapy than bacteria in the planktonic phase [77]. The mechanism responsible for the reduced susceptibility of biofilms to antimicrobial photodynamic therapy may also be related to the inactivation of methylene blue, the existence of biofilm bacteria in a slow-growing or starved state, and distinct and protected phenotypes expressed by biofilm species when they attach to the agar surface [92–94]. However, a recent in vivo study showed that scaling and root planing (SRP) combined with photodynamic therapy using methylene blue led to significant improvements of the investigated clinical parameters over the use of scaling and root planing alone [95].
In a recent split-mouth clinical study, it was demonstrated that non-surgical periodontal treatment performed on patients with aggressive periodontitis by applying antimicrobial photodynamic therapy alone showed similar clinical improvements in comparison to scaling and root planing [96]. It has been demonstrated that scaling and root planing combined with photodisinfection or the application of antimicrobial photodynamic therapy alone leads to reduction of pocket depths and results in clinical attachment gain in the non-surgical treatment of periodontitis [97]. Braun et al. [98], in a study assessing the effect of adjunctive antimicrobial photodynamic therapy in chronic periodontitis, concluded in favor of the use of this treatment modality and suggested that clinical outcomes of conventional subgingival debridement can be improved by adjunctive aPDT. De Oliveira et al. [99] treated ten patients with aggressive periodontitis in a split-mouth design study with either PDT with laser source scaling and root planing. They determined that both methods showed similar clinical results in a 3-month follow-up period. The authors, in a similar study design, evaluated the results in a biochemical perspective and indicated that SRP and PDT had similar effects on crevicular TNF-α and RANKL levels in patients with aggressive periodontitis [100].
Recently, residual periodontal pockets have received particular attention from some authors in terms of their response to PDT. Campos et al. [101], who evaluated the effects of PDT in addition to SRP at baseline and 3 months post-therapies, demonstrated additional clinical benefits for residual pockets in single-rooted teeth and suggested that this treatment modality may be an alternative therapeutic strategy in supportive periodontal maintenance. Giannopoulou et al. [102], on the other hand, observed no significant differences for any biochemical parameters when they compared the local biologic effects of PDT, diode soft laser therapy, and conventional deep SRP in residual pockets.
All these aforementioned studies reveal that there is some controversy between the results of different studies. Most probably, this must be related with the designs of the investigations. More structured and better designed studies are mandatory to reach firmer conclusions.
Peri-implantitis and antimicrobial photodynamic therapy
Peri-implantitis is considered to be a multifactorial process involving bacterial contamination of the implant surface. It is a local and relatively superficial infection caused by well-known specific microflora colonization on the implant surface [103–105]. It is unknown to what extent bacterial and non-bacterial residues have to be removed from an implant surface to obtain a predictable, stable clinical result after treatment. Decontamination by mechanical, chemical, and physical methods has been used so far. Surgical intervention has also been considered as an option [106, 107]. Cleaning rough implant surfaces is very difficult since bacteria are protected in microirregularities or undercuts of the surface [103, 108].
A new technique for cleaning of infected implant surfaces in vivo is antimicrobial photodynamic therapy. Experimental examinations revealed that light from a helium/neon laser or a gallium–arsenide laser, in combination with appropriate photosensitizers, can achieve a significant reduction in the viability of both aerobics and anaerobics in a solution of subgingival plaque from patients with chronic periodontitis [109, 110].
Shibli et al. [70] investigated the effects of photodynamic therapy on peri-implantitis and reported that PDT was able to reduce bacterial counts. Prevotella sp., Fusobacterium sp., and Streptococcus beta heamolyticus were not 100 % destroyed in all samples; however, a significant reduction resulted.
Dörtbudak et al. [111] reported that treatment of peri-implantitis with the application of the photosensitizer toluidine blue alone (i.e., without light sensitization) resulted in significant reductions of Prevotella intermedia and Aggregatibacter actinomycetemcomitans compared to baseline values. The bacterial counts of P. gingivalis also decreased in comparison with the initial value, but the change was not statistically significant [111]. On the other hand, the lethal photosensitization of the toluidine blue with a diode laser of a wavelength of 690 nm resulted in significantly higher reductions of P. intermedia, A. actinomycetemcomitans, and P. gingivalis compared to both baseline. PDT resulted in a significant bacterial reduction, although complete elimination of bacteria was not achieved. The authors concluded in favor of the combined application of TBO and laser depending on the significant reduction of the initial values in all three groups of bacteria.
Hayek et al. [112] indicated that antimicrobial photodynamic therapy is an effective non-invasive method for treating peri-implantitis compared to conventional therapy with elevated mucoperiosteal mucosa flaps for scaling the implant surface. The use of azulene delivered in a paste as photosensitizer seemed to be effective against peri-implantitis pathogenic microorganisms and did not stain the implant surface and/or surrounding tissues.
The possible advantages of PDT over conventional antibiotic therapy in peri-implantitis include topical treatment where only the affected sites requiring antimicrobial treatment receive the dye and illumination and, unlike antibiotics, there is no disruption of the microflora in the unaffected sites. Also, there is no evidence of resistance development in the target bacteria after PDT [113, 114].
Although the application of antimicrobial photodynamic therapy in the treatment of periodontitis and peri-implantitis is an interesting therapeutic approach, current reports have not shown significant superior effects of antimicrobial photodynamic therapy compared with conventional mechanical therapy. Therefore, the potential effects of antimicrobial photodynamic therapy should be studied more extensively to establish the optimal conditions during clinical application. However, antimicrobial photodynamic therapy holds promise as a novel non-invasive treatment method that might be beneficial when applied alone or in conjunction with conventional mechanical periodontal and peri-implantitis therapy.
Many factors may interfere with the effectiveness of laser irradiation, including the capacity for light absorption by the photosensitized microorganism, wavelength of the laser, physiological state of the bacteria, emission from the laser, time of laser exposure, pH of the medium, staining of the area to be irradiated, water content, thermal conductivity, and the organic matrix [15]. New types of light delivery devices and new photosensitizing drugs will expand the usefulness of PDT in the future.
Endodontics
Disinfection of the root canal space and elimination of microoorganisms to induce periapical repair is one of the fundamental goals of endodontic treatment. Recently, new systems and substances have been proposed to improve root canal disinfection either by replacing conventional chemomechanical procedures or by supplementing their effects [115]. Fimple et al. [116] investigated the photodynamic effects of methylene blue on Actinomyces israelii, F. nucleatum, P. gingivalis and P. intermedia in experimentally infected root canals of extracted teeth and found up to 80 % reduction of colony-forming unit counts when root canal systems were incubated with methylene blue (25 μg/mL) for 10 min followed by exposure to red light at 665 nm with an energy fluence of 30 J/cm. The authors suggested PDT to be an effective adjunct to standard antimicrobial endodontic treatment when PDT parameters were optimized.
Xu et al. [117], in an in vitro study, assessed the synergistic effect of methylene blue and red light on human gingival fibroblasts and osteoblasts. They sensitized both cell types with 50 μg/mL MB followed by exposure to red light at 665 nm for 5 min with an irradiance of 10, 20, and 40 mW/cm 2. The results showed that light at 20 and 40 mW/cm2 with MB had modest effects at 24 h on osteoblasts, whereas sodium hypochlorite completely eliminated cells. The authors interpreted the results as the presence of a therapeutic safe window by which PDT can inactivate cells without affecting host cell viability.
Treatment of teeth with periapical lesions has always been a challenge for the practitioner and attempts have been made so far in order to eliminate irritating agents from the root canal system to provide healing in the periradicular tissues. This usually necessitates multiple appointments for confirming a thorough eradication of microorganisms within the root canal system. Recently, Silva et al. [118], conducted an in vivo study where they evaluated the response of apical and periapical tissues of dogs' teeth with apical periodontitis after one-session endodontic treatment with and without antimicrobial photodynamic therapy. The authors concluded that photodynamic therapy may serve as a promising adjunct to intracanal cleaning and shaping specifically for teeth with periapical lesions undergoing one-session endodontic treatment. Another study on the effects of diode laser in combination with photodynamic therapy is one by Nagoyashi et al. [119], who suggested that utilization of a diode laser in combination with a photosensitizer may be useful for clinical treatment of periapical lesions.
There have also been some attempts to eliminate E. faecalis, one of the major etiological factors of persistent endodontic infections. The study by Pagonis et al. [120] showed that the utilization of poly(lactic-co-glycolic acid) nanoparticles loaded with the photosensitizer MB and encapsulated with photoactive drugs may be a promising adjunct in antimicrobial endodontic treatment. The authors determined that the nanoparticle concentration was higher mainly on the cell walls of microorganisms at the 2.5-, 5-, and 10-min time periods. The synergism of light and MB-loaded nanoparticles resulted in approximately 2 and 1 log10 reduction of colony-forming units in planktonic phase and root canals, respectively. Light-activated disinfection targeting E. faecalis in a planktonic suspension and mono-species biofilms and on P. aeruginosa in a planktonic suspension and mono-species biofilms was tested by Upadya and Kishen [121]. The authors concluded that modifications in photosensitizer formulations enhanced the efficacy of light-activated disinfection on biofilms positively. Further studies favored the use of PDT for the elimination of biofilms and residual and drug-resistant microorganisms [122–125]. Since tooth staining and discoloration has been indicated as one of the major concerns of PDT, there have been some attempts to overcome this disadvantage by evaluating the efficacy of some chemical compounds. It has been concluded that 2.5 % NaOCl, associated or not with Endo-PTC cream (a cream consisting of 10 % urea peroxide, 15 %, Tween 80 (detergent), and 75 % carbowax (vehicle) used as a lubricant during cleaning and shaping of the rootcanals), was effective in avoiding tooth staining caused by MB during PDT [126]. As observed from the aforementioned data, there is yet limited information pertaining to the use of antimicrobial photodynamic therapy in endodontic treatment. However, this treatment option seems to be a promising adjunctive supplement, specifically in persistent cases where E. faecalis plays a major role. Further trials are necessary to make more reliable conclusions regarding the use of PDT in endodontics.
Concluding remarks
The advantages of photodynamic therapy compared with surgery or radiotherapy are reduced long-term morbidity and the fact that photodynamic therapy does not compromise future treatment options for recurrent, residual, or another primary disease. Based upon the present analysis of pertinent literature, where tumors are concerned, PDT appears to be a promising technique for early neoplasmic lesions (premalignant and in situ carcinoma) and advanced recurrences after previous surgery or radiotherapy. Also, superficial infections as well as bacterial and fungal infections seem to be areas which hold promise to incorporate photodynamic therapy as a treatment option more frequently in the future. Further evidence-based accumulation of data is definitely required to make a definite statement.
References
Dougherty TJ, Gomer CJ, Henderson BW et al (1998) Photodynamic therapy. J Natl Cancer Ins 90:889–905
Dougherty TJ (2002) An update on photodynamic therapy applications. J Clin Laser Med Surg 20:3–7
Sharwani A, Jerjes W, Salih V et al (2006) Fluorescence spectroscopy combined with 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in detecting oral premalignancy. J Photochem Photobiol B 83:27–33
Babilas P, Schreml S, Landthaler M, Szeimies RM (2010) Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed 26:118–132
Sharman WM, Allen CM, van Lier JE (1999) Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today 4:507–517
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42:13–28
Maisch T, Szeimies RM, Jori G, Abels C (2004) Antibacterial photodynamic therapy in dermatology. Photochem Photobiol Sci 3:907–917
Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G (2006) Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med 38:468–81
Takasaki AA, Aoki A, Mizutani K et al (2009) Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000 2000(51):109–140
Hematoporphyrin GJL (1991) Photodynamic therapy: is there truly a future in head and neck oncology? Reflections on a 5-year experience. Laryngoscope 101:36–42
Foote CS (1991) Definition of type I and type II photosensitized oxidation. Photochem Photobiol 54:659
Konopka K, Goslinski T (2007) Photodynamic therapy in dentistry. J Dent Res 86:694–707
Thomas B, Saatian S, Saeidi R et al. Photodynamic therapy: is it more effective than the current standard of care? An evidence-based study of the literature. http://www.utoronto.ca/dentistry/newsresources/evidence_based/PhotodynamicTherapy.pdf
Rossoni RD, Junqueira JC, Santos EL, Costa AC, Jorge AO (2010) Comparison of the efficacy of Rose Bengal and erythrosin in photodynamic therapy against Enterobacteriaceae. Lasers Med Sci 25:581–586
Wainwright M, Crossley KB (2004) Photosensitizing agents circumventing resistance and breaking down biofilms: a review. Int Biodeterior Biodegrad 53:119–126
Meisel P, Kocher T (2005) Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B 79:159–170
DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and itsapplications. Coord Chem Rev 233–234:351–371
Pinheiro SL, Schenka AA, Neto AA, de Souza CP, Rodriguez HM, Ribeiro MC (2009) Photodynamic therapy in endodontic treatment of deciduous teeth. Lasers Med Sci 24:521–526
Sigusch BW, Pfitzner A, Albrecht V, Glockmann E (2005) Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol 76:1100–1105
Soukos NS (2000) Goodson JM (2011) Photodynamic therapy in the control of oral biofilms. Periodontol 2000 55:143–166
Allison RR, Downie GH, Cuenca R, Hu XH, Childs C, Sibata CH (2004) Photosensitizers in clinical PDT. Photodiagn Photodyn Ther 1:27–42
Konopka K, Goslinski T (2008) Prospects for photodynamic therapy in dentistry. Biophotonics Int 15:32–35
Jerjes W, Upile T, Betz CS et al (2007) The application of photodynamic therapy in the head and neck. Dent Update 34:478–80, 483-4, 486
Hopper C (2000) Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol 1:212–219
Klot LO, Pellieux C, Briviba K, Pierlot C, Aubry JM, Sies H (1999) Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur J Biochem 260:917–922
Kübler AC (2005) Photodynamic therapy. Med Laser Appl 20:37–45
Christensen E, Warloe T, Kroon S, Funk J, Helsing P, Soler AM, Stang HJ, Vatne O, Mørk C; Norwegian Photodynamic Therapy (PDT) Group (2010) Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J Eur Acad Dermatol Venereol 24(5):505–12
Braathen LR, Szeimies RM, Basset-Seguin N, Bissonnette R, Foley P, Pariser D, Roelandts R, Wennberg AM, Morton CA (2005) International Society for Photodynamic Therapy in Dermatology (2007) Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Am Acad Dermatol 56(1):125–43
Wilson M (1993) Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease: a review. J App Bacteriol 75:299–306
Vrouenraets MB, Visser GWM, Snow GB, van Dongen GAMS (2003) Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res 23:505–522
Capella MAM, Capella LS (2003) A light in multidrug resistance: photodynamic treatment of multidrug-resistant tumors. J Biomed Sci 10:361–366
Silverman S (1999) Oral cancer: complications of therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:122–126
Sieron A, Namyslowski G, Misiolek M, Adamek M, Kawczyk-Krupka A (2001) Photodynamic therapy of premalignant lesions and local recurrence of laryngeal and hypopharyngeal cancers. Eur Arch Otorhinolaryngol 258:349–352
Nauta JM, von Leengoed HL, Star WM, Roodenburg JL, Witjes MJ, Verney A (1996) Photodynamic therapy of oral cancer. A review of basic mechanisms and clinical applications. Eur J Oral Sci 104:69–81
Kubler AC, de Carpentier J, Hopper C, Leonard AG, Putnam G (2001) Treatment of squamous cell carcinoma of the lip using Foscan-mediated photodynamic therapy. Int J Oral Maxillofac Surg 30:504–509
Copper MP, Tan IB, Oppelaar H, Ruevekamp MC, Stewart FA (2003) Meta-tetra (hydroxyphenyl)chlorin photodynamic therapy in early-stage squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 129:709–711
Hopper C, Kubler A, Lewis H, Tan IB, Putnam G (2004) mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer 111:138–146
Rigual NR, Thankappan K, Cooper M et al (2009) Photodynamic therapy for head and neck dysplasia and cancer. Arch Otolaryngol Head Neck Surg 135:784–788
Lin HP, Chen HM, Yu CH, Yang H, Wang YP, Chiang CP (2010) Topical photodynamic therapy is very effective for oral verrucous hyperplasia and oral erythroleukoplakia. J Oral Pathol Med 39:624–630
Biel MA (1995) Photodynamic therapy of head and neck cancers. Semin Surg Oncol 11:355–359
Dissemond J (2004) Oral lichen planus: an overview. J Dermatolog Treat 15:136–140
Cerero R, Garcia-Pola MJ (2004) Management of oral lichen planus. Med Oral 9:124
Aghahosseini F, Arbabi-Kalati F, Fashtami LA, Fateh M, Djavid GE (2006) Treatment of oral lichen planus with photodynamic therapy mediated methylene blue: a case report. Med Oral Patol Oral Cir Bucal 11:126–129
Golecka M, Oldakowska-Jedynak U, Mierzwinska-Nastalska E, Adamczyk-Sosinska E (2006) Candida-associated denture stomatitis in patients after immunosuppression therapy. Transplant Proc 38:155–156
Jin Y, Samaranayake LP, Samaranayake Y, Yip HK (2004) Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol 49:789–798
Shulman JD, Rivera-Hidalgo F, Beach MM (2005) Risk factors associated with denture stomatitis in the United States. J Oral Pathol Med 34:340–346
Kulak Y, Arikan A, Delibalta N (1994) Comparison of three different treatment methods for generalized denture stomatitis. J Prosthet Dent 72:283–288
Samaranayake LP, MacFarlane TW (1981) A retrospective study of patients with recurrent chronic atrophic candidosis. Oral Surg Oral Med Oral Pathol 52:150–3
Banting DW, Greenhorn PA, McMinn JG (1995) Effectiveness of a topical antifungal regimen for the treatment of oral candidiasis in older, chronically ill, institutionalized adults. J Can Dent Assoc 61(199–200):203–205
Lombardi T, Budtz-Jorgensen E (1993) Treatment of denture-induced stomatitis: a review. Eur J Prosthodont Restor Dent 2:17–22
Bliss JM, Bigelow CE, Foster TH, Haidaris CG (2004) Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother 48:2000–2006
Chabrier-Rosello Y, Foster TH, Pérez-Nazario N, Mitra S, Haidaris CG (2005) Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob Agents Chemother 49:4288–4295
Donnelly RF, McCarron PA, Tunney MM, David Woolfson A (2007) Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue OJ. Photochem Photobiol B 86:59–69
Teichert MC, Jones JW, Usacheva MN, Biel MA (2002) Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:155–160
Donnelly RF, McCarron PA, Tunney MM (2008) Antifungal photodynamic therapy. Microbiol Res 163:1–12
Bertoloni G, Reddi E, Gatta M, Burlini C, Jori G (1989) Factors influencing the haematoporphyrin-sensitized photoinactivation of Candida albicans. J Gen Microbiol 135:957–966
Dovigo LN, Pavarina AC, de Oliveira Mima EG, Giampaolo ET, Vergani CE, Bagnato VS (2011) Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses 54:123–30
Zeina B, Greenman J, Corry D, Purcell WM (2003) Antimicrobial photodynamic therapy: assessment of genotoxic effects on keratinocytes in vitro. Br J Dermatol 148:229–232
Zeina B, Greenman J, Corry D, Purcell WM (2002) Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br J Dermatol 146:568–573
Bliss JM, Bigelow CE, Foster TH, Haidaris CG (2004) Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother 48:2000–2006
Marotti J, Aranha AC, Eduardo Cde P, Ribeiro MS (2009) Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomed Laser Surg 27:357–363
Fimple JL, Fontana CR, Foschi F et al (2008) Photodynamic treatment of endodontic polymicrobial infection in vitro. J Endod 34:728–734
Usacheva MN, Teichert MC, Biel MA (2003) The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg Med 33:311–319
Fonseca MB, Júnior PO, Pallota RC (2008) Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomed Laser Surg 26:209–213
Neugebauer J (2005) Using photodynamic therapy to treat peri-implantitis. Interview Dent Implantol Update 16:9–16
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42:13–28
Komerik N, Nakanishi H, Robert MAJ, Henderson B, Wilson M (2003) In vivo killing of Porphyromonas gingivalis by toluidine blue photosensitization in an animal model. Antimicrobial Agents Chemother 47:932–940
Paulino TP, Ribeiro KF, Thedei G Jr, Tedesco AC, Ciancaglini P (2005) Use of hand held photopolymerizer to photoinactivate Streptococcus mutans. Arch Oral Biol 50:353–359
Bevilacqua IM, Nicolau RA, Khouri S et al (2007) The impact of photodynamic therapy on the viability of Streptococcus mutans in a planktonic culture. Photomed Laser Surg 25:513–518
Shibli JA, Martins MC, Theodoro LH, Lotufo RFM, Garcia VG, Marcantonio E Jr (2003) Lethal photosensitization in microbiological treatment of ligature-induced peri-implantitis: a preliminary study in dogs. J Oral Sci 45:17–23
Wilson M (1994) Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J 44:181–189
Socransky SS (2000) Haffajee AD, (2002) Dental biofilms: difficult therapeutic targets. Periodontol 2000 28:12–55
Haffajee AD (2006) Systemic antibiotics: to use or not to use in the treatment of periodontal infections. That is the question J Clin Periodontol 33:359–361
Preshaw PM (2004) Antibiotics in the treatment of periodontitis. Dent Update 31:448–450, 453-454, 456
Levy SB (2002) The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J Antimicrob Chemother 49:25–30
Sigusch BW, Pfitzner A, Albrecht V, Glockmann E (2005) Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol 76:1100–1105
Fontana CR, Abernethy AD, Som S et al (2009) The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res 44:751–759
Wilson M, Dobson J, Sarkar S (1993) Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 8:182–187
Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T (1998) Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother 42:2595–2601
Williams JA, Pearson GJ, Colles MJ, Wilson M (2003) The effect of variable energy input from a novel light source on the photoactivated bactericidal action of toluidine blue O on Streptococcus mutans. Caries Res 37:190–193
Sarkar S, Wilson M (1993) Lethal photosensitization of bacteria in subgingival plaque samples from patients with chronic periodontitis. J Periodont Res 28:204–210
Metcalf D, Robinson C, Devine D, Wood S (2006) Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother 58:190–192
Wood S, Metcalf D, Devine D, Robinson C (2006) Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 57:680–684
Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, Hofling JF, Goncalves RB (2006) Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci 114:64–69
Zanin IC, Goncalves RB, Junior AB, Hope CK, Pratten J (2005) Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother 56:324–330
Schneider M, Kirfel G, Berthold M, Frentzen M, Krause F, Braun A (2012) The impact of antimicrobial photodynamic therapy in an artificial biofilm model. Lasers Med Sci 27:615–20
Soukos NS, Socransky SS, Mulholland SE, Lee S, Doukas AG (2000) Photomechanical drug delivery into bacterial biofilms. Pharm Res 17:405–409
Soukos NS, Mulholland SE, Socransky SS, Doukas AG (2003) Photodestruction of human dental plaque bacteria: enhancement of the photodynamic effect by photomechanical waves in an oral biofilm model. Lasers Surg Med 33:161–168
Qin Y, Luan X, Bi L et al (2008) Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers Med Sci 23:49–54
Ogura M, Abernethy AD, Blissett RD et al (2007) Photomechanical wave-assisted molecular delivery in oral biofilms. World J Microbiol Biotechnol 23:1637–1646
Yilmaz S, Kuru B, Kuru L, Noyan U, Argun D, Kadir T (2002) Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med 30:60–66
Foley I, Gilbert P (1996) Antibiotic resistance of biofilms. Biofouling 10:331–346
Brown SM, Allison DG, Gilbert P (1988) Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother 22:777–783
Whiteley M, Gita Bangera M, Bumgarner RE et al (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864
Andersen R, Loebel N, Hammond D, Wilson M (2007) Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent 18:34–38
Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M (1997) Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem Photobiol 65:1026–1031
Anderson GG, O’Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol 322:85–105
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy periodontal treatment: a randomized clinical trial. J Clin Periodontol 35:877–84
de Oliveira RR, Schwartz-Filho HO, Novaes AB Jr, Taba M Jr (2007) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol 78:965–973
de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, Scombatti de Souza SL, Ribeiro FJ (2009) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol 80:98–105
Campos GN, Pimentel SP, Ribeiro FV, Casarin RC, Cirano FR, Saraceni CH, Casati MZ (2012) The adjunctive effect of photodynamic therapy for residual pockets in single-rooted teeth: a randomized controlled clinical trial. Lasers Med Sci, in press
Giannopoulou C, Cappuyns I, Cancela J, Cionca N, Mombelli A (2012) Effect of photodynamic therapy, diode laser, and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J Periodontol 83(8):1018–27
Mombelli A (2000) Lang NP (1998) The diagnosis and treatment of peri-implantitis. Periodontol 2000 17:63–76
Ericsson I, Person LG, Berglundh T, Edlund T, Lindhe J (1996) The effect of antimicrobial therapy on peri-implantitis lesions. An experimental study in the dog. Clin Oral Implant Res 7:320–328
Machado MAN, Stefani CM, Sallum EA, Sallum AW, Tramontina VA, Nociti FH Jr (1999) Treatment of ligature-induced peri-implantitis defects by regenerative procedures: a clinical study in dogs. J Oral Sci 41:181–185
Mombelli A (2002) Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000 2000(28):177–189
Spiekermann H (1995) Implantology. Color atlas of dental medicine. Chicago, Quintessence, pp 317–322
Esposito M, Hirsch J, Lekholm U, Thomsen P (1999) Differential diagnosis and treatment strategies for biologic complications and failing oral implants: a review of the literature. Int J Oral Maxillofac Implants 14:473–490
Wilson M, Burns T, Pratten J, Pearson GJ (1995) Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J Appl Bacteriol 78:569–574
Haas R, Dörtbudak O, Mensdorff-Pouilly N, Mailath G (1997) Elimination of bacteria on different implant surfaces through photosensitization and soft laser. Clinical Oral Implant Research 8:249–254
Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G (2001) Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res 12:104–108
Hayek RR, Araújo NS, Gioso MA et al (2005) Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol 76:1275–1281
Kömerik N, MacRobert AJ (2006) Photodynamic therapy as an alternative antimicrobial modality for oral infections. J Environ Pathol Toxicol Oncol 25:487–504
Wilson BC (2002) Photodynamic therapy for cancer: principles. Can J Gastroenterol 16:393–396
Siqueira JF Jr, Rôças IN (2011) Optimising single-visit disinfection with supplementary approaches: a quest for predictability. Aust Endod J 37:92–8. doi:10.1111/j.1747-4477.2011.00334.x
Fimple JL, Fontana CR, Foschi F, Ruggiero K, Song X, Pagonis TC, Tanner AC, Kent R, Doukas AG, Stashenko PP, Soukos NS (2008) Photodynamic treatment of endodontic polymicrobial infection in vitro. J Endod 34:728–734
Xu Y, Young MJ, Battaglino RA, Morse LR, Fontana CR, Pagonis TC, Kent R, Soukos NS (2009) Endodontic antimicrobial photodynamic therapy: safety assessment in mammalian cell cultures. J Endod 35:1567–1572
Silva LA, Novaes AB Jr, de Oliveira RR, Nelson-Filho P, Santamaria M Jr, Silva RA (2012) Antimicrobial photodynamic therapy for the treatment of teeth with apical periodontitis: a histopathological evaluation. J Endod 38(3):360–6
Nagayoshi M, Nishihara T, Nakashima K, Iwaki S, Chen KK, Terashita M, Kitamura C (2011) Bactericidal effects of diode laser irradiation on Enterococcus faecalis using periapical lesion defect model. ISRN Dent.:870364.
Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, Foschi F, Dunham J, Skobe Z, Yamazaki H, Kent R, Tanner AC, Amiji MM, Soukos NS (2010) Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod 36:322–328
Upadya MH, Kishen A (2010) Influence of bacterial growth modes on the susceptibility to light-activated disinfection. Int Endod J 43:978–987. doi:10.1111/j.1365-2591.2010.01717.x
Garcez AS, Nuñez SC, Hamblim MR, Suzuki H, Ribeiro MS (2010) Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: a preliminary report. J Endod 36:1463–1466
Kishen A, Upadya M, Tegos GP, Hamblin MR (2010) Efflux pump inhibitor potentiates antimicrobial photodynamic inactivation of Enterococcus faecalis biofilm. Photochem Photobiol 201:1343–1349. doi:10.1111/j.1751-1097.2010.00792.x
Ng R, Singh F, Papamanou DA, Song X, Patel C, Holewa C, Patel N, Klepac-Ceraj V, Fontana CR, Kent R, Pagonis TC, Stashenko PP (2011) Soukos NS (2011) Endodontic photodynamic therapy ex vivo. J Endod 37:217–222
Nunes MR, Mello I, Franco GC, de Medeiros JM, Dos Santos SS, Habitante SM, Lage-Marques JL, Raldi DP (2011) Effectiveness of photodynamic therapy against Enterococcus faecalis, with and without the use of an intracanal optical fiber: an in vitro study. Photomed Laser Surg 29:803–8. doi:10.1089/pho.2011.2995
Carvalho Edos S, Mello I, Albergaria SJ, Habitante SM, Lage-Marques JL, Raldi DP (2011) Effect of chemical substances in removing methylene blue after photodynamic therapy in root canal treatment. Photomed Laser Surg 29:559–63, Epub 2011 Jun 1
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gursoy, H., Ozcakir-Tomruk, C., Tanalp, J. et al. Photodynamic therapy in dentistry: a literature review. Clin Oral Invest 17, 1113–1125 (2013). https://doi.org/10.1007/s00784-012-0845-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0845-7