Abstract
Objectives
This study was carried out to histologically assess the effect of bone grafting materials extracted from bovine origin on the bone healing process either alone or when mixed with autologous platelet-rich plasma which could be used in many procedures of oral and maxillofacial bone and implant reconstructive surgery.
Materials and methods
Sixteen rabbits were used; three intrabony defects in the femur bone of each rabbit were created, one left unfilled for normal healing process and served as control, the second filled with xenogenic graft (Gen-Ox-lyophilized bovine bone organic matrix), and the third filled with(Gen-Ox-lyophilized bovine bone organic matrix) mixed with autologous platelet-rich plasma . Histological examination of the sections was performed after staining with H&E and Van Geison stains. The histomorphometric analysis including counting of bone cells (osteoblasts, osteocytes, and osteoclasts) with performance of osteon diameter and lamellar thickness at the end of the fourth week postoperatively was obtained.
Results
It has been shown that with the use of autologous platelet-rich plasma in combination with the xenogenic bone graft prepared from bovine origin, new bone formation and neovascularization were enhanced significantly when compared with xenogenic graft alone.
Conclusion
The addition of PRP to xenogenic bone substitute in small bone defects of the rabbit femur showed a histomorphometric increase in bone formation (at the fourth week of healing).
Clinical relevance
Platelet concentrate might be used to accelerate the osseointegration of enosseous dental implants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although autogenous bone graft is considered the gold standard in functional rehabilitation and treatment of bone loss [1], autografting is limited by the amount of bone that can be retrieved, morbidity, and risk of infection [2, 3]. There is a continuing search for bone substitutes to avoid or minimize the need for autologous bone grafts. Biomaterials can be used for replacing autografts [3] and organic bone matrix; an osseoconductive biomaterial is used for these purposes [4]. In vivo, studies have demonstrated the feasibility of using xenogenic bone in orthognathic [2] and trauma surgeries [5]. Xenografts like Gen-Ox® have been widely used as bone graft materials due to abundant sources and accessible processing; however, the results remain controversial, with different outcomes according to the type of defect [6] and variable resorption rate [7]. The association of biomaterials with repair promoters like platelet-rich plasma (PRP) is promising [8]. Platelet-rich plasma has received significant attention because it is an autologous product with simple in-operatory preparation and purported wide-ranging therapeutic effects [9]. The use of PRP offers a potentially useful adjunct to autologous, allogenic, and xenogenic graft materials in oral and maxillofacial bone and implant reconstructive surgeries. PRP can be defined as a volume of autogenous plasma that has a platelet concentration above the baseline, and it is produced by centrifugation of the patient's own blood. So, it is the suspension of growth factors that has been demonstrated to induce healing and regeneration in soft as well as hard tissues [10]. Platelets release multiple wound healing growth factors and cytokines, including platelet-derived growth factor (PDGF), transforming growth factor B1 and B2 (TGF-B1 and B2), vascular endothelial growth factor (VEGF), platelet-derived endothelial growth factor, basic fibroblast growth factor, and platelet activating factor-4 [11, 12]. For several years, PRP has been thought to promote bone healing, but there are contradicting reports about its clinical efficacy. Several studies in humans show that PRP has a beneficial effect on bone healing [13–16]. Studies on animals reveal conflicting results; some were positive and others were negative [17–22]. The inconsistency of these results prompted this study on the effect of PRP on bone regeneration with a xenograft. This study examined histologically the influence of PRP when used as an adjunct to Gen-Ox bovine organic bone in the repair of small bone defects.
Materials and methods
Sixteen healthy male New Zealand rabbits aged 6–9 months and weighing between 2 and 2.5 kg were used as experimental animals. Approval was obtained from the animal care committee at the College of Medicine, University of Saladdin, Iraq. Platelet-rich plasma was prepared using a technique described by Shayestah et al. [23]. Briefly, 5 mL of autologous blood withdrawn from each rabbit was initially centrifuged at 1,200 rpm for 20 min to separate PRP and platelet-poor plasma (PPP) portions from the red blood cell fraction. The PRP and PPP portions were again centrifuged at 2,000 rpm for 15 min to separate the PRP from the PPP. The platelet count was carried out in the blood sample drawn, and the PRP sample of each rabbit was counted using a manual method under a microscope [24]. The platelet count in PRP was about 2–3-fold of that in peripheral blood. The PRP was activated before application with 10 % calcium chloride solution [10]. After activation, PRP turned into a gel-like substance with adhesive properties and mixed with organic bovine bone (Gen-Ox-organic®, Braumer SA, Brazil) in a ratio of 0.5 mL of PRP with 30 mg of organic bovine bone [23].
Surgical procedure
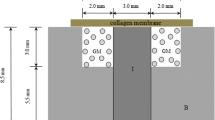
All surgical procedures were performed under systemic anesthesia (40 mg/kg ketamine and 5 mg/kg intramuscular xylazine). The bone surface of the femur was exposed by a latero-longitudinal incision. Three identical bone defects of 3 mm were created using a small round bur with a distance of approximately 4 mm between each hole [25] (Fig. 1). The created holes were dried of blood and were treated with one of the following two treatment modalities: (1) grafting with Gen-Ox-organic bovine bone alone or (2) grafting with Gen-Ox-organic bovine bone mixed with PRP. The last hole was left without grafting and served as control. All experimental areas were covered with the soft tissue flap with resorbable 4/0 suture (Vicryl, Johnson & Johnson, Somerville, NJ) and with 4/0 black silk suture for femoral skin. The rabbits recovered from anesthesia without complications. They were given pain medication (25 mg diclofenac sodium) during the first 3 days after surgery and an antibiotic (ampiclox 500 mg I.M.) for 1 week to prevent infection.
Experimental protocol
Four rabbits were sacrificed at the end of each healing period (i.e., at the end of the first, second, third, and fourth weeks postoperatively). The left femoral bone was resected without encroaching on the grafted area using a bone saw. The bone piece with the defect and the attached soft tissue were removed and immediately fixed in 10 % phosphate-buffered formaldehyde solution for 48 h. Thereafter, tissue blocks were decalcified with 50 % formic acid and 20 % sodium citrate for 4 weeks, dehydrated with graded alcohols, and embedded in paraffin. The histological sections of 4–6 μm obtained were stained with H&E and Van Gieson stains. Histological analysis of bone filling in the defective area, new bone formation, histological evaluation of collagen fibers, inflammatory cells, bone-forming cells, bone trabeculae, and neovascularization were assessed. Cell counting, diameter of the osteon, and lamellar thickness of the Haversian system were also assessed. For bone cell counting, osteoblasts, osteoclasts, and osteocytes were counted at the fourth week postoperatively (PO) for control and experimental groups according to the Qassim method [26]. Counting was performed in five randomly selected sites of each section at 40× magnification. The osteon diameter of 10 randomly selected sites was done using the measuring ruler of Visapan at 40×. The mean wall thickness was calculated as: ½(osteon diameter − Haversian canal diameter) [27]. The results obtained were submitted to ANOVA and Duncan t test using SPSS.18 (Chicago, IL). Differences were considered statistically significant if P < 0.05.
Results
During the experiment, all animals remained in good health and did not show any complications. No evidence of infection or fracture of the surgically treated site was observed.
Qualitative assessment
Light microscopic examination of the decalcified sections showed the following
One-week results
In group I (control group), the defective area showed newly formed granulation tissue with a large blood clot filling the defect site (Fig. 2a). In group II (Gen-Ox® only), dense granulation tissue was seen around multiple blood clots filling the defect (Fig. 2b). In group III (Gen-Ox®plus PRP), we observed a little new bone formation mainly from the edges of the defect with a central area of fibrovascular connective tissue (Fig. 2c).
Two-week results
In group I, a dense mixture of fibrocartilaginous callus and hyaline cartilage was seen with newly formed bone spicules starting from the edge of the defect (Fig. 3a). In group II, the newly formed bone spicules appeared more mature with more organized fibrocartilage callus formation (Fig. 3b). Group III showed well-vascularized bone marrow, and increased number and distribution of bone spicules were observed. (Fig. 3c).
Three-week results
Group 1 showed continuous healing through the formation of new bone trabeculae extending from the edge of the defect and diffused along the callus (Fig. 4a). Group II: more bone trabeculae with many primary osteon formations with active osteoblasts were seen (Fig. 4b). In group III were more bridging of new bone with increased surface area of bone trabeculae and prominent primary osteon formation (Fig. 4c).
Four-week results
Group I was characterized by newly formed osseous tissue with irregular distribution of osteoblasts and osteocytes and few number of osteoclasts. Primary osteons were formed but with few number (Fig. 5a).
In group II, More mature bony tissue was observed with little number of primary osteons and a significant increase in the number of osteoblasts (Fig. 5b). Group III revealed a well-formed osseous tissue with still irregular distribution of bone cells. The number of osteocyte was significantly increased indicating bone maturity, and the diameter of the osteon with lamellar thickness was significantly increased when compared with other groups (Fig. 5c and d).
Quantitative assessment
Cell counting was performed at the end of the fourth week PO for all tested groups. The number of osteoblasts in Gen-Ox-treated groups was significantly higher than other groups (P < 0.05); however, the number of osteocytes was significantly higher in the Gen-Ox + PRP group than other groups (P < 0.05). No significant difference in number of osteoclasts was observed among groups. Regarding osteon diameter and lamellar thickness, a significant increase was observed in the Gen-Ox + PRP group than other groups (Table 1, Fig. 6).
Discussion
One of the dilemmas in oral and maxillofacial surgery is the question of how to treat extensive bone injuries accompanied with tissue loss. Large bone defects cannot heal spontaneously, preventing the natural repair of the damaged bone. Therefore, the use of graft material became a must. Although autogenous grafts are commonly used in such cases, the need for additional intervention increases the duration of surgery and the risk of infection, pain, and discomfort at the donor site. During the last decade, several bone grafting materials produced from bovine bone, with physicochemical characteristics similar to those of human bone, have been developed for use in oral and orthopedic surgeries as an alternative to autologous grafts [28]. The performance of organic bone replacement is not very clear, but some studies in orthognathic and trauma surgeries [2, 8] demonstrated good results. Bovine organic bone was rapidly absorbed, and the histological analysis demonstrated that osteoblastic activity is significantly increased; this indicates a marked osteoinductive ability of the material. The newly formed bone seen in this study is not different from that seen by others [29]. Therefore, an attempt was made to accelerate the process of bone healing through mixing bovine organic matrix with PRP [14].
PRP is a potent mitogenic and chemotactic factor for both fibroblasts and osteoblasts. It is a strategic vehicle for growth factors that influence bone regeneration [10]. Platelets are a natural source of growth factors that play an important role in the wound healing process [12]. In in vivo studies, PDGFs have shown to stimulate bone formation and consistently enhance wound fill [30, 31]. Increasing the concentration of platelets in bone defect may lead to improved bone formation. However, the association of PRP to biomaterials remains controversial [32]. Some in vivo studies demonstrated the effectiveness of PRP associated with bone substitutes for treating periodontal defects or for sinus floor augmentation [33]. Hanna [34] found that periodontal infrabony defects treated with bovine-derived xenograft and PRP have significant reduction in pocket depth and increased clinical attachment levels when compared with defects treated only with bovine-derived xenografts. In the present investigation, both lamellar thickness and osteon diameter were significantly enhanced in bony defects treated with bovine organic matrix mixed with PRP. This finding is in accordance with Fuerst and colleagues [35] who documented that dental implants placed in conjunction with PRP achieve accelerated bone-to-implant contact during the early stage of implant healing. Other studies [21, 22] found no effect of PRP in new bone formation in the PRP-treated bone graft. Although the potential clinical applications for PRP are numerous and have shown promising benefits, a number of studies question the efficacy of this growth factor product. Raghoebar [36], for example, found no beneficial effect on wound healing or bone remodeling when PRP was added to subantral augmentation grafts. Sanchez [37] found that the addition of PRP to xenografts in the treatment of peri-implant defects demonstrated low regeneration potential. One explanation for the discrepancies in many recent PRP studies is that all PRP preparation systems are not created equal. The variation in the concentration of the platelets may play an important role in the conflicting results reported in various animal experimental studies using PRP. In the present study, the platelet count used was about three times the usual baseline platelet count. The high concentration of PRPs used in this study might lead to the expectation that when a small amount of bone graft is mixed with a large volume of PRP, the bone cells either residing in the adjacent tissue or transferred in the graft would be exposed to various growth factors present in PRP for activation and hence new bone formation. The histological evaluation of Gen-Ox-lyophilized bovine bone organic matrix was assessed in this study at different intervals. It reveals that the material has good osteogenic property; it induces mesenchymal cell recruitment, differentiation of chondroblasts, cartilage formation, vascular ingrowth, and eventually, bone formation. This biomaterial is a biocompatible, osteoconductive grafting material with no sign of foreign body reaction and/or severe inflammation. Adding PRP to this biomaterial did not affect its biocompatibility. The same findings were seen in other studies [38–41]. The trabecular bone maturation (from woven bone to lamellar bone) was seen in all tested groups; however, the amount of lamellar bone was significantly greater in the Gen-Ox + PRP group than the other; this could be attributed to the combined effect of bone morphogenic proteins present in Gen-Ox bovine organic bone matrix and the growth factors release from the platelet concentrate that stimulate bone formation such as PDGF, IGF, and VEGF [11, 12], since PDGF induces the proliferation of mesenchymal cells, angiogenesis and macrophage recruitment which are crucial for bone regeneration [42]. Marx et al. [14] observed the same finding when PRP was used in combination with autogenous bone graft, and they suggested that such combination could increase the rate of osteogenesis and qualitatively enhance bone formation. Furthermore, Kim et al. [43] reported that PRP in combination with bovine cancellous bone allografts increased bone formation in calvarial defects in rabbits. More basic researches into the optimal concentration of PRP and the optimal bone defect size are necessary to capitalize on the ability of platelet growth factors to enhance bone formation in a graft.
Conclusion
On the basis of the observations presented in this study, it can be concluded that when PRP is used as an adjunct to Gen-Ox-lyophilized bovine organic matrix in the repair of small bone defects, faster bone healing would take place. Additional randomized, controlled clinical studies evaluating PRP long-term effects are certainly warranted and may someday put this question to rest.
References
Maus U, Andereya S, Gravius S, Siebert GH, Ohnsorge JA, Niedhart C (2008) Lack of effect on bone healing of injectable BMP-2 augmented hyaluronic acid. Arch Orthop Trauma Surg 128:1461–1466
Lye KW, Deatherage JR, Waite PD (2008) The use of demineralized bone matrix for grafting during Lefort I and chin osteotomies: techniques and complications. J Oral Maxillofac Surg 66:1580–1585
Nacamuli RP, Longaker MT (2005) Bone induction in craniofacial defects. Orthod Craniofac Res 8:259–266
Eppley BL, Pietrzak WS, Blanton MW (2005) Allograft and alloplastic bone substitutes: a review of science and technology for the craniofacial surgeon. J Craniofac Surg 16:981–989
Bostrom MP, Seigerman DA (2005) The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study. HSSJ 1:9–18
Torricelli P, Fini M, Giavaresi G, Rimondini L, Giardino R (2002) Characterization of bone defect repair in young and aged rat femur induced by xenogenic demineralized bone matrix. J Periodontol 73:1003–1009
Norton MR, Odell EW, Thompson ID, Cook RJ (2003) Efficacy of bovine bone mineral for alveolar study. Clin Oral Implants Res 14:775–783
Kim SG, Kim WK, Park JC, Kim HJ (2002) A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg 60:1018–1025
Thaller SR, Hoyt J, Dart A, Borjeson K, Tesluk H (1994) Repair of experimental calvarial defects with Bio-oss particles and collagen sponges in a rabbit model. J Craniofacial Surg 5:242–246
Tözum TF, Demirlap B (2003) Platelet rich plasma. A promising innovation in dentistry. J Can Dent Assoc 69:664
Lynch SE, Genco RJ, Marx RE (1999) Tissue engineering, application in maxillofacial surgery and periodontics, 1st edn. Quintessence Pub Co., Chicago
Weibrich G, Kleis WK, Hafner G, Hitzler WE, Wagner W (2003) Comparison of platelet leukocyte, and growth factor levels in point-of-care platelet-enriched plasma prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res 14:357–362
Galindo-Moreno P, Avila G, Fernandez-Barbero JE, Aguilar M, SanChez-Fernandez E, Cutando A, Wang HL (2007) Evaluation of sinus floor elevation using composite bone graft mixture. Clin Oral Implants Res 18:376–382
Marx RE, Carlson ER, EichstaedtRM SSR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
Simon EN, Merkx MAW, Shubi FM, Kalyanyama BM, Stoelinga PJW (2006) Reconstruction of the mandible after ablative surgery for the treatment of aggressive, benign odontogenic tumors in Tanzania: a preliminary study. Int J Oral Maxillofac Surg 35:421–426
Thorn JJ, Sørensen H, Weis-Fogh U, Andersen M (2004) Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg 33:95–100
Fennis JPM, Stoelinga PJW, Jansen JA (2004) Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet-rich plasma in goats. Int J Oral Maxillofac Surg 33:48–55
Kim SG, Chung CH, Kim YK, Park JC, Lim SC (2002) Use of particulate dentin-plaster of Paris combination with/without platelet-rich plasma in the treatment of bone defects around implants. Int J Oral Maxillofac Implants 17:86–94
Rocha FS, Ramos LMA, Batista JD, Barbosa DZ, Decbicibi P (2011) Organic bovine graft associated with PRP in rabbit calvaria. Int Arch Otorhinolaryngol 15:208–213
Schlegel KA, Donath K, Rupprecht S, Flak S, Zimmermann R, Felszeghy E, Wiiltfang J (2004) De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 25:5387–5393
Schlegel KA, Kloss FR, Kessler P, Schultze-Mosgau S, Nkenke E, Wiltfang J (2003) Bone conditioning to enhance implant osseointegration: an experimental study in pigs. Int J Oral Maxillofac Implants 18:505–511
You TM, Choi BH, Li J, Jung JH, Lee HJ, Lee SH, Jeong SM (2007) The effect of platelet-rich plasma on bone healing around implants placed in bone defects treated with Bio-Oss: a pilot study in the dog tibia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:e8–e12
Shayesteh YS, Khirsand A, Dehghan M, Ardestani MA (2005) Evaluation of platelet-rich plasma in combination with deproteinized bovine bone mineral in rabbit cranium. J Dent 2:127–134
Coles EF (1980) Veterinary clinical pathology, 3rd edn. Saunders, Philadelphia, pp 144–161
Guzzardella GA, Torricelli P, Nicoli-Aldini N, Giardino R (2003) Osseointegration of endosseous ceramic implant after post operative lower power laser stimulation: an in vivo comparative study. Clin Oral Implants Res 14:226–232
Qassim AH (2004) Histological and histochemical studies of human fetal liver during different stages of intrauterine life. M.Sc Thesis, College of Medicine, University of Mosul, Iraq
Marins LV, Cestari TM, Sottovia AD, Granjerio JM, Taga R (2004) Radiographic and histological study of perennial bone defect repair in rat calvaria after treatment with blocks of porous bovine organic graft material. J Appl Oral Sciences 12:62–69
Vajda EG, Kneissel M, Muggenburg B, Miller SC (1999) Increased intracortical bone remodeling during lactation in Beagle dogs. Biol Reprod 61:1439–1444
Munhoz EA, Bodanezi A, Cestari TM, Taga R, de Carvalho PSP, Junior OF (2011) Long-term rabbits bone response to titanium implants in the presence of inorganic bovine-derived graft. J Biomat Appl Feb 22 (in press)
Grageda E (2004) Platelet-rich plasma and bone graft materials: a review and a standerdized research protocol. Implant Dent 75:1668–1677
Nash T, Howlett C, Martin C, Steele J, Johnson KA, Hicklin DJ (1994) Effect of platelet-derived growth factor on tibial osteotomes in rabbit. Bone 15:203–208
Plachokova AS, van den Dolder J, Stoelinga PJ, Jansen JA (2007) Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res 18:244–251
Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, Saito Y, Wolff LF, Yoshiex H (2005) Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of infra bony periodontal defects in humans. A comparative controlled clinical study. Periodontol 76:890–898
Hanna R, Trejo P, Weltman R (2004) Treatment of intrabony defects with bovine-derived xenograft alone and in combination with platelet-rich plasma: a randomized clinical trial. J Periodontol 75:1668–1677
Furest G, Reinhard G, Tangl S, Sanroman F, Watzek G (2005) Enhanced bone to implant contact by platlet released growth factors in mandibular cortical bone. A histomorphometric study in minipig. Int J Oral maxillofac Implant 18:685–690
Raghoebar G, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A (2005) Does platelet rich plasma promote remodelling of autologous bone graftes used for augmentation of the maxillary sinus floor? Clin Oral Implant Res 16:349–356
Sanchez A, Sheridan P, Eckert S, Al W (2005) Regenerative potential of platelet rich plasma added to xenogenic bone grafts in peri-implant defects: a histomorphometric analysis in dogs. J Periodontol 76:1637–1644
Beglundh T, Lindhe J (1997) Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res 8:117–124
Donos N, Bosshardt D, Lang N, Graziani F, Tonetti M, Karring T, Kostopoulos L (2005) Bone formation by enamel matrix proteins and xenografts: an experimanetal study in the rat ramus. Clin Oral Implants Res 16:140–146
Slotte C, Lundgren D (1999) Augmentation of calvarial tissue using non-permeable silicon domes and bovine bone mineral. An experimental study in the rat. Clin Oral Implants Res 10:468–476
Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, Landesberg R (2005) Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg 63:521–528
Wiltfang J, Kloss FR, Kessler P, Nkenke E, Schultze-Mosgan S, Zimmermann R, Schlegel KA (2004) Effect of platelet rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical size defect (an animal experiment). Clin Oral Implants Res 15:187–193
Kim ES, Park EJ, Choung PH (2001) Platelet concentrate and its effect on bone formation in calvarial defect: an experimental study in rabbits. J Prosthet Dent 86:428–433
Conflict of interest
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurikchy, M.Q., Al-Rawi, N.H., Ayoub, R.S. et al. Histological evaluation of bone healing using organic bovine bone in combination with platelet-rich plasma (an experimental study on rabbits). Clin Oral Invest 17, 897–904 (2013). https://doi.org/10.1007/s00784-012-0751-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0751-z