Abstract
Objectives
The objectives of the investigation were to describe changes in mandibular bone structure with aging and to compare the usefulness of cortical and trabecular bone for fracture prediction.
Materials and methods
From 1968 to 1993, 1,003 women were examined. With the help of panoramic radiographs, cortex thickness was measured and cortex was categorized as: normal, moderately, or severely eroded. The trabeculation was assessed as sparse, mixed, or dense.
Results
Visually, the mandibular compact and trabecular bone transformed gradually during the 24 years. The compact bone became more porous, the intertrabecular spaces increased, and the radiographic image of the trabeculae seemed less mineralized. Cortex thickness increased up to the age of 50 and decreased significantly thereafter. At all examinations, the sparse trabeculation group had more fractures (71–78 %) than the non-sparse group (27–31 %), whereas the severely eroded compact group showed more fractures than the less eroded groups only in 1992/1993, 24 years later. Sparse trabecular pattern was associated with future fractures both in perimenopausal and older women (relative risk (RR), 1.47–4.37) and cortical erosion in older women (RR, 1.35–1.55). RR for future fracture associated with a severely eroded cortex increased to 4.98 for cohort 1930 in 1992/1993. RR for future fracture associated with sparse trabeculation increased to 11.43 for cohort 1922 in 1992/1993.
Conclusion
Dental radiographs contain enough information to identify women most at risk of future fracture.
Clinical relevance
When observing sparse mandibular trabeculation, dentists can identify 40–69 % of women at risk for future fractures, depending on participant age at examination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone mineral density (BMD) only partly explains bone quality and strength [1]. As many as 73 % of all fractures occur in women with a negative test for osteoporosis [2]. Easily assessed clinical fracture risk factors are age, sex, prior fragility fractures, corticosteroid use, low body mass index, and smoking [3]. Several research groups have tried to predict osteoporosis or fractures by assessing mandibular bone mass and structure using dental radiographs. It is a reasonable approach because dental radiographs are taken anyway for other purposes.
Previously, mandibular sparse trabeculation, evaluated visually using intra-oral radiographs, was shown to be a better indicator of fracture risk than BMD [4]. Furthermore, in a longitudinal study, a sparse mandibular trabecular pattern was found to be a predictor of future fracture risk both in perimenopausal and in older women: the older the individual, the more effective [5]. No longitudinal study has reported age changes in the mandibular cortex. In the present study, radiographs from three examinations (from 1968/1969 to 1992/1993) were included in order to describe trabecular and compact bone aging and to compare the usefulness of trabecular and cortical bone for fracture prediction.
Materials and methods
Subjects
A total of 1,003 women, participating in the Prospective Population Study of Women in Gothenburg in the examinations 1968/1969, 1980/1981, and 1992/1993, were included in the present investigation (172 born in 1914, 447 born in 1922, and 384 born in 1930).
The non-participation analysis showed that the women who declined did not differ significantly in medical respect from the participants except for long-term survival which was lower in the initial refusers [6].
The Ethics Committee of the University of Gothenburg approved the study (T 453-04 and T 075-09), and participants gave their informed consent.
Fracture ascertainment
Information on fractures occurring before 1980 was collected from questionnaires. Since 1980, it has been possible to use medical registries to identify incident fractures and this was done every year after 1980. Thus, hospital-verified fracture follow-up was from 1980/1981 to 2006 (26 years), and total fracture follow-up (including data from questionnaires) was from 1946 to 2006 (60 years). Relative risk (RR) was calculated using only “future” fractures: dated fractures from examination dates and forward. Women with a fracture in childhood (<20 years old) were registered as not fractured. Women with one or more fractures were denoted “fractured” in all periods, regardless of the fracture number; 31 women had two fractures, 20 had three, and 12 women had more than three fractures; 55 % were forearm fractures, 17 % hip, 14 % spine, 14 % upper-arm, and 0.3 % pelvis fractures. No fractures of fingers, toes, or skull were recorded, and no attempt to separate fragility fractures from other fractures was made.
The mandibular cortical index (MCI) and cortex thickness
The radiographic exposure factors were adjusted according to the size of the women. Larger women were exposed with higher kV and mA than smaller ones.
Longitudinal bone changes (24 years) were studied visually in 517 women examined three times. All 1,003 women had cortical evaluations; 517 women had radiographs from all three examinations, 282 had two, and 204 had one radiograph.
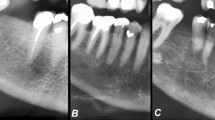
Cortical bone lying distal to the mental foramen was categorized into three groups (Fig. 1). Normal cortex (mandibular cortical index (MCI)-1) had a relatively even endosteal margin; moderately eroded cortex (MCI-2) had semilunar defects, severely eroded cortex (MCI-3) had heavy endosteal cortical residues, was clearly porous, or extremely thin (1–2 mm), which is an expansion of the original criterion [7, 8].
Reference images (panoramic radiographs) presenting dense trabeculation and a normal mandibular cortex with even and sharp endosteal margin (left), mixed trabeculation and a moderately eroded cortex with endosteal margin showing semilunar defects (middle), and sparse trabeculation and severely eroded cortex with cortical layer being clearly porous (right)
All cortex thickness measurements were performed by one observer (AH-N). Cortex was measured using the “natural size” of the digital panoramic radiograph and a specially developed transparent ruler that took the magnification of the panoramic radiograph into account. The thickness was measured slightly distal of the mental foramen because often a small area (2–4 mm) of cortex just below the foramen was clearly thicker than the rest of the distal cortex. When relative risk was calculated, the cortex thickness variable was transformed to a dichotomous variable: thickness < 3 mm~risk; thickness > 3 mm~no risk [9].
Ordinal classification of the radiographic mandibular alveolar trabecular pattern
The trabecular patterns were evaluated by one observer (GJ). Not all radiographs were evaluated: 78 radiographs showed too little alveolar bone, a consequence of tooth extraction and resorption of the alveolar trabecular bone at the extraction sites [10]. With the help of reference radiographs (Fig. 1) the alveolar trabeculation was classified as either sparse (value 1), mixed dense plus sparse (value 2), or dense (value 3) [11, 12]. Sparse trabeculation had large intertrabecular spaces in most of the alveolar processes, especially in the crestal, dentate, premolar area. Dense trabeculation had small intertrabecular spaces everywhere. Mixed dense plus sparse trabeculation was mostly dense crestally and sparse apically.
The bone surrounding teeth with marginal bone loss due to periodontitis, periapical sclerotic bone caused by periapical periodontitis, or bone under bridge pontics was unevaluated because of local alterations caused by these conditions.
Reliability
Three dentists evaluated thirty panoramics twice at four-week intervals. Their findings were evaluated using Kappa statistics. Intraobserver reliability for trabeculation was lower (0.65–0.92) than for cortex evaluation (0.80–0.92), and similar for interobserver reliability (0.72–0.84 versus 0.80–0.85).
Statistics
Differences between fracture rates were tested with ANOVA. RR was calculated both for the pooled cohorts and for the cohorts separately to study the association between “exposure” and “outcome”. “Exposure” variables were the three bone variables transformed to dichotomous variables: severely eroded cortex versus not severely eroded cortex; cortex thickness, <3 mm versus cortex thickness, >3 mm; sparse trabeculation versus non-sparse trabeculation. “Outcome” was incident fracture versus non-fracture. Only “future” fractures: dated fractures from examination dates and forward were used for RR calculations. Spearman’s regression analyses tested linear correlations between trabecular pattern and cortical erosion (both ordinal variables with three values). Epi-Info version 3.5 was used for all statistical analyses (Center for Disease Control, Atlanta, GA, USA).
Results
Longitudinal bone changes
The mandibular alveolar trabeculation and compact bone changed visibly during 24 years. In most cases, the intertrabecular spaces increased, the trabeculae seemed less mineralized, and the compact bone became thinner and more porous. Table 1 shows that the “Fracture group” in 1992/1993 contained a higher percentage of women (64 %) with a severely eroded cortex (MCI-3) and a less percentage number (5 %) with a normal cortex (MCI-1) whereas the distribution was opposite 24 years earlier in 1968/1969 in the same women (54 % for MCI-1 and 3 % for MCI-3, respectively). Table 2 shows that the sparse group increased with age (21 to 46 %, pooled group) but the change between trabecular groups was not as large as the change between cortical groups. Mean cortex thickness increased slightly from ages 38 to 50 (3.3 to 3.4 mm in cohort 1930); from 50 to 78 years the thickness decreased significantly (3.4 to 2.3 mm, Table 3).
The MCI and cortex thickness
The group with severely eroded cortex increased from 0.5 % in the youngest group (38 year olds) to 75.4 % in oldest cohort group (78 year olds) (Table 3). The distribution of women in the cortex classes changed markedly. In 1968/1969 most fractures were found in the group with normal eroded cortex (54 %), in 1980/1981 in moderately eroded (62 %), and in 1992/1993 in the severely eroded cortex group (64 %) (Table 1).
RR calculations (pooled cohorts) showed statistically significant increased risk for fracture in women with severely eroded cortex in 1980/1981 (RR = 1.35; 95 % CI, 1.01–1.80) and in 1992/1993 (RR = 1.55; 95 % CI, 1.20–2.02) (Table 1). When the three cohorts were analyzed separately, RR was only significantly for those born 1930 in the last study in 1992/1993 (RR = 4.98; 95 % CI, 2.27–10.93) (Table 3).
No significant differences were found for cortex thickness between fractured and non-fractured groups for any cohort or for all women pooled together.
Ordinal classification of the radiographic mandibular alveolar trabecular pattern
RR calculations showed statistically significant increased risk for fracture in women with sparse trabeculation for the pooled cohorts (RR, 1.47–4.37) (Table 2) and also for the cohorts studied separately (RR, 1.60–11.43) (Table 3). Cohort born 1922 was the largest group and had 48.3 % of all dated fractures. Approximately 80 % of women with sparse trabeculation (cohort 1922 and 1914) had a fracture at least once during their lifetime (Table 3). In cohort born 1930 as many as 54–61 % of those with sparse trabeculation experienced a fracture (Table 3). Cohort born 1914 had less sparse trabeculation at the age of 54 than the other cohorts in similar age group but with aging this difference disappeared (Table 3). The sparse fracture ratio, meaning fractures in women with sparse trabeculation relative to the total number of fractures in all women, also increased as subjects grew older (40–69 %, Table 2). Fracture rate for women with sparse trabeculation was significantly higher (71.3–78.0 %) than for the non-sparse groups (27.0–30.7 %).
Correlations between trabecular pattern and cortical index
At all examinations dates, significant correlations were found between trabecular pattern and cortical index (r = 0.26–0.37; p < 0.00001). The older the subjects the better was the correlation between trabecular and cortical bone.
Discussion
The fracture rate was significantly higher in the group with sparse trabeculation in the mandible 1968/1969 (78 %, Table 2) and in the group with severely eroded mandibular inferior compact bone from 1992/1993 (64 %, Table 1), than in the groups with denser trabeculation and less eroded compact bone. The percentage of women with severely eroded compact basal bone increased with age from 3 % in 1968/1969 to 54 % in 1992/1993 and those with sparse trabeculation increased from 21 to 46 % (Table 2). Also, the ratio of fractures in the sparse group of women relative to the total fracture number experienced of all women, increased, parallel to the increased number of women in the sparse trabeculation group (40–69 %).
Trabecular pattern index performed better as a fracture risk predictor than compact bone index which is not surprising since bone changes are seen earlier in trabecular bone than in compact bone. Similarly, it seems natural that in 1968/1969 most future fractures were found in the group with normal erosion whereby there is less erosion at a younger age. In 1992/1993, the process of erosion had had 24 years to work so there were fewer subjects with normal erosion. In 1980/1981, only 12 % of the 709 women (85 women) had severely eroded compact bone but 61 % of them (52 women) had suffered a fracture. Twelve years later, 54 % of the 556 examined women (300) had severely eroded compact bone and 55 % had suffered a fracture (165 women). The high percentage of fractured women in 1980/1981 was probably caused by the low number of subjects with severely eroded cortex. RR in women with sparse trabecular pattern was increased as early as 1968/1969 whereas RR in women with severely eroded compact bone was so from 1980/1981.
The cohort differences may be explained by the age differences, differences in life conditions during childhood, and/or a random selection effect. The women born in 1914 had less sparse trabeculation in 1968/1969 but the sparse group increased later in size. It is possible that this cohort difference is biased because of the small size of the longitudinal group (the healthiest had survived and attended the examination) but the loading of the skeleton had perhaps been largest in the oldest cohort.
Cortical thickness increased slightly until age of 50 years (0.1 mm) and decreased significantly thereafter (−0.6 mm in the 1930 cohort), parallel to change in menopausal status. After menopause, deficiency in estrogen induces increases bone turnover, leading to skeletal bone loss [13]. No cortical thickness difference was found between fractured and non-fractured groups in contrast to another study where traumatic fractures were separated from osteoporotic fractures [14]. Thus, the cortical thickness seems less relevant for fractures than the MCI index.
Visually, it seemed that the “intertrabecular spaces” increased during the 24 years, the trabeculae seemed less mineralized, and the compact bone became thinner and more porous. However, some of the older participants had a surprisingly dense trabeculation with distinct well-mineralized trabeculae and thick compact bone. Other women with dense trabeculation maintained their trabeculation pattern with small intertrabecular spaces but the mineralisation of the trabeculae visibly decreased. A thick compact bone 1968/1969 could transform to moderately eroded in 1980/1981 and severely eroded compact bone in 1992/1993.
Our results are supported by studies showing more pronounced age changes in alveolar bone than in basal bone [15]. Reduction in the complexity of the trabecular pattern was found in osteoporotic individuals [16, 17]. Increase in distance between adjacent trabeculae accounted for more than twice the age-related bone loss compared with the decrease in trabecular width [16]. Furthermore, advanced image analyses have shown that changes in radiographic trabecular structure is predictive of hip fracture in elderly women [18], partially independent of BMD [19].
The vertebral radiographic trabecular pattern is significantly related to compressive strength and ash density [20] and our results indicated that even the mandibular trabecular pattern is associated with skeletal bone strength. The mandibular central trabeculae are mostly thin whereas the trabeculae are thicker in the transitional layers between cortex and trabecular bone. Therefore, the radiographic trabecular pattern depicts mostly these layers. However, even the trabeculae situated in the middle of the bone and the cortical bone contribute, to some extent, to the radiographic image of the alveolar trabecular bone [21]. Although not all changes occurring in mandibular trabeculae may be visually evident on the radiographic image, it seems that sufficient information can be obtained from panoramic radiographs to identify most of the subjects with the highest fracture risk (sparse trabeculation), and those with the lowest risk (dense trabeculation).
Cortical erosion as bone fracture predictor has been tested previously. One study [14] has reported that subjects with a self-reported history of osteoporotic fractures tend to have increased resorption of the mandibular lower cortex. Another study [22] found that cortical measurements on panoramic radiographs were not significantly associated with the occurrence of fractures.
The main limitation of this study is the lack of DXA measurements and other measurements, such as bone texture measurements on digitized radiographs [4, 23, 24]. However, longitudinal data of large groups, with a long follow-up period and the large number of fractures perceived in that period, may be the optimal material for studying relations between radiographic appearance and future fractures. Probably such a material conveys more important information about fracture risk than any bone mineral density measurement.
No step wedge was included in the panoramic radiographs but the trabeculation index is rather robust and independent on differences in the radiographic processing if these differences are not extreme. Only high class radiographs were included. Normally, a reference object is necessary for measuring cortical thickness with a fair reliability. However, all panoramic radiographs had a magnification factor of 1.3 and our Department of Oral and Maxillofacial Radiology has developed a transparent ruler which can be used for different magnifications of the radiographs including this one. Panoramic radiographs are less sharp than intraoral radiographs and practice is needed before the trabecular assessment can be performed [25]. No periapical radiographs were available and the advanced objective methods developed for evaluation of the interdental trabecular pattern [18, 23, 26] are not easily adapted to panoramics. Due to cortex erosion we found it difficult to measure accurately the thickness of the compact bone of some old participants. An automated measurement of cortical thickness has been developed [27], and compared with the FRAX index but not with sustained fractures [28].
We studied alveolar bone where age changes are different than in basal bone due to different development patterns and loading [29]. In the excluded, resorbed, edentulous mandibles, we observed that the radiographic basal bone was mostly dense and the cortex rather thick; the trabeculae are probably reinforced as a result of a biologic compensatory reaction to avoid jaw fracture. These observations are supported by other investigators [6, 29, 30].
The advantage of this study is the prospective design, the relatively large, representative study population, and a high participation rate. Fractures were carefully checked from questionnaires and hospitals. Previously, we found that the mandibular trabecular pattern was a better correlate of fracture than BMD identifying 48 % of all previous fractures compared with the 19 % identified using BMD [4]. Sparse trabeculation was found as early as in 38-year-old women (21 %). In this age group, exercise and diet may improve BMD without medication [23]. An early identification of pre-menopausal women at high risk for fracture is particularly interesting for the dental profession because of the risk of mandibular bone necrosis combined with bisphoshonate medication for osteoporosis [31].
Conclusion and clinical relevance
The mandibular trabecular pattern seems to be a highly significant predictor of future fracture risk both in perimenopausal and older women whereas cortical erosion, but not thickness, performed well for older women. Sparse trabeculation indicated that 71–78 % of these women would suffer a bone fracture. By analyzing dental radiographs, dentists can identify people who are at greater risk of fractures long before the first fracture occurs.
References
Genant HK, Jiang Y (2006) Advanced imaging assessment of bone quality. Ann N Y Acad Sci 1068:410–428
Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA (2006) The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int 17:1404–1409
White SC, Atchison KA, Gornbein JA, Nattiv A, Paganini-Hill A, Service SK (2006) Risk factors for fractures in older men and women: the Leisure World Cohort Study. Gend Med 3:110–123
Jonasson G, Alstad T, Vahedi F, Bosaeus I, Lissner L, Hakeberg M (2009) Trabecular pattern in the mandible as bone fracture predictor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:e42–e51
Jonasson G, Sundh V, Ahlqwist M, Hakeberg M, Björkelund C, Lissner L (2011) A prospective study of mandibular trabecular bone to predict fracture risk: a low-cost screening tool in the dental clinic. Bone 49:873–879
Bengtsson C, Ahlqwist M, Andersson K, Björkelund C, Lissner L, Söderström M (1997) The Prospective Population Study of Women in Gothenburg, Sweden, 1968–69 to 1992–93. A 24-year follow-up study with special reference to participation, representativeness, and mortality. Scand J Prim Health Care 15:214–219
Klemetti E, Kolmakov S, Kröger H (1994) Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res 102:68–72
Taguchi A, Asano A, Ohtsuka M, Nakamoto T, Suei Y, Tsuda M et al (2008) Observer performance in diagnosing osteoporosis by dental panoramic radiographs: results from the osteoporosis screening project in dentistry (OSPD). Bone 43:209–213
Devlin H, Karayianni K, Mitsea A, Jacobs R, Lindh C, van der Stelt P, Marjanovic E, Adams J, Pavitt S, Horner K (2007) Diagnosing osteoporosis by using dental panoramic radiographs: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:821–828
Ulm C, Solar P, Blahout R, Matejka M, Gruber H (1992) Reduction of the compact and cancellous bone substances of the edentulous mandible caused by resorption. Oral Surg Oral Med Oral Pathol 74:131–136
Lindh C, Petersson A, Rohlin M (1996) Assessment of the trabecular pattern before endosseous implant treatment: diagnostic outcome of periapical radiography in the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82:335–343
Jonasson G, Bankvall G, Kiliaridis S (2001) Estimation of skeletal bone mineral density by means of the trabecular pattern of the alveolar bone, its interdental thickness, and the bone mass of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:346–352
Riggs BL, Khosla S, Atkinson EJ, Dunstan CR, Melton LJ 3rd (2003) Evidence that type I osteoporosis results from enhanced responsiveness of bone to estrogen deficiency. Osteoporos Int 14:728–733
Bollen AM, Taguchi A, Hujoel PP, Hollender LG (2000) Case–control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90:518–524
Atkinson PJ, Woodhead C (1968) Changes in human mandibular structure with age. Arch Oral Biol 13:1453–1463
Weinstein RS, Hutson MS (1987) Decreased trabecular width and increased trabecular spacing contribute to bone loss with aging. Bone 8:137–142
White SC, Rudolph DJ (1999). Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 88:628–35. |
White SC, Atchison KA, Gornbein JA, Nattiv A, Paganini-Hill A, Service SK, Yoon DC (2005) Change in mandibular trabecular pattern and hip fracture rate in elderly women. Dentomaxillofac Radiol 34:168–174
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD (2007) Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433
Korstjens CM, Mosekilde L, Spruijt RJ, Geraets WG, van der Stelt PF (1996) Relations between radiographic trabecular pattern and biomechanical characteristics of human vertebrae. Acta Radiol 37:618–624
Jett S, Shrout MK, Mailhot JH, Potter BJ, Borke JL (2004) An evaluation of the origin of trabecular bone patterns using visual and digital image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:598–604
Okabe S, Morimoto Y, Ansai T, Yoshioka I, Tanaka T, Taguchi A et al (2008) Assessment of the relationship between the mandibular cortex on panoramic radiographs and the risk of bone fracture and vascular disease in 80-years-olds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:433–442
Jonasson G, Jonasson L, Kiliaridis S (2006) Changes in the radiographic characteristics of the mandibular alveolar process in dentate women with varying bone mineral density: a five-year prospective study. Bone 38:714–721
Jonasson G, Jonasson L, Kiliaridis S (2007) Skeletal bone mineral density in relation to thickness, bone mass, and structure of the mandibular alveolar process in dentate men and women. Eur J Oral Sci 115:117–123
Pham D, Jonasson G, Kiliaridis S (2010) Assessment of trabecular pattern on periapical and panoramic radiographs: a pilot study. Acta Odontol Scand 68:91–97
Southard TE, Southard K, Lee A (2001) Alveolar process fractal dimension and postcranial bone density. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:486–491
Devlin H, Allen PD, Graham J, Jacobs R, Karayianni C, Lindh C et al (2007) Automated osteoporosis risk assessment by dentists: a new pathway to diagnosis. Bone 40:835–842
Horner K, Allen P, Graham J, Jacobs R, Boonen S, Pavitt S et al (2010) The relationship between the OSTEODENT index and hip fracture risk assessment using FRAX. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110:243–249
Kingsmill VJ, Boyde A (1998) Variation in the apparent density of human mandibular bone with age and dental status. J Anat 192:233–244
Ulm CW, Kneissel M, Hahn M, Solar P, Matejka M, Donath K (1997) Characteristics of the cancellous bone of edentulous mandibles. Clin Oral Implants Res 8:125–130
Filleul O, Crompot E, Saussez S (2010) Bisphosphonate-induced osteonecrosis of the jaw: a review of 2.400 patient cases. J Cancer Res Clin Oncol 136:1117–1124
Acknowledgements
This study was funded by research grants from the Health and Medical Care Committee of the Regional Executive Board, Region Västra Götaland, the Research and Development Councils of Southern Älvsborg and Göteborg and Southern Bohuslän Counties, the Swedish Council for Working Life and Social Research (FAS Center EpiLife), and the Swedish Research Council. No commercial funding was received and no conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jonasson, G., Sundh, V., Hakeberg, M. et al. Mandibular bone changes in 24 years and skeletal fracture prediction. Clin Oral Invest 17, 565–572 (2013). https://doi.org/10.1007/s00784-012-0745-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0745-x