Abstract
Background
Interleukin 8 (IL-8) is a pro-angiogenic, pro-inflammatory mediator that belongs to the family of chemokines. Due to its pro-angiogenic characteristic, it may play a vital role in tumour angiogenesis and progression.
Objectives
This study was designed to estimate the levels of salivary IL-8 in oral precancer and oral squamous cell carcinoma (OSCC) patients and compare them with healthy controls. The aim was to evaluate its efficacy as a potential biomarker for these diseases.
Materials and methods
Each group comprised 25 individuals. The salivary IL-8 levels were determined by enzyme-linked immunosorbent assay.
Results
The levels of salivary IL-8 were found to be significantly elevated in patients with OSCC as compared to the precancer group (p < 0.0001) and healthy controls (p < 0.0001). However, the difference in salivary IL-8 concentrations among the precancer group and controls was statistically non-significant (p = 0.738).
Conclusions
Our results suggested that salivary IL-8 can be utilised as a potential biomarker for OSCC. Salivary IL-8 was found to be non-conclusive for oral premalignancy in this preliminary study. Hence, its possible role in transition from premalignancy to malignancy needs further research with larger sample sizes.
Clinical relevance
Saliva as a diagnostic biofluid offers a number of advantages over blood-based testing. The role of IL-8 in oral cancer if validated further by future research can provide an easy diagnostic test as well as a prognostic indicator for patients undergoing treatment. Therefore, if it’s role in tumourigenesis can be sufficiently assessed, it could open up new avenues to find out novel treatment modalities for oral cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) constitutes a major health problem worldwide, representing a leading cause of death. According to the World Health Organization, carcinoma of the oral cavity is the sixth commonest cancer in males and tenth commonest cancer in females in developing countries [1] and the most prevalent cancer related to the consumption of tobacco, alcohol and other carcinogenic products [2]. On the basis of the cancer registry data, it is estimated that annually 75,000–80,000 new oral cancer cases develop in India [3]. The survival index for OSCC continues to be small (50 %) as compared to the progress in the diagnosis and treatment of other malignant tumours. Hence, there is a need for improvement in the early detection of the oral carcinoma because in the initial stages, treatment is more effective and the morbidity is minimal [1].
Clinically, it is important to note that the therapeutic modality currently offered to patients is based on traditional stage predicting indices, based mostly on the tumour–node–metastasis (TNM) criteria and on histologic grading. Unfortunately, these predictors are subjective and relatively unreliable as often, two tumours with identical staging and grading behave in totally different fashions, and although one responds to therapy, the other is lethal [4, 5]. Circulatory tumour markers for OSCC were investigated in various studies and showed relatively moderate sensitivity and specificity values with relation to diagnosis, prognosis or treatment monitoring [6–11]. Accordingly, there has been an ever-growing effort dedicated to the basic research of oral cancer focusing on the identification of biological indicators for the diagnosis of its nature and aggressiveness.
Carcinogenesis involves multiple alterations of the genome progressively accumulated during a protracted period. In the course of this progression, changes are taking place at the cellular and tissue levels. The sum total of these physical and morphological alterations occurring in the oral mucosa are of diagnostic and prognostic relevance and are designated as precancerous changes [3].
Saliva is a mirror of the human health and a reservoir of analytes from systemic sources that reach the oral cavity through various pathways. Steroids, amines and peptides, such as melatonin, insulin, leptin and ghrelin, enter the saliva by passive diffusion. Immunoglobulins such as secretory IgA and enzymes such as amylase and lysozyme can be analysed in the saliva. In addition to these endogenous substances, various drugs can be measured in the saliva as an excellent option for therapeutic drug monitoring and in the assessment of drugs of abuse. Thus, the composition of saliva reflects the levels of hormonal, immunological, toxicological and infectious disease markers [12]. Consequently, this fluid provides a source for the monitoring of oral and also systemic health. The noninvasive nature of collection, the direct contact to the oral cavity, and the relationship between oral fluid and blood levels make saliva a useful and promising specimen to detect potential biomarkers for OSCC. As a biofluid, it provides a perfect medium to observe disease onset, progression, recurrence and treatment outcome through noninvasive means [13].
Alterations in host immunity, inflammation, angiogenesis and metabolism have been noted as the prominent pathological features in patients with head and neck squamous cell carcinoma (HNSCC) [14–17]. The tumour-induced T lymphocyte, granulocyte and neoangiogenesis responses in the local tumour microenvironment have been associated with increased growth and metastasis and decreased survival. Although the origin of the signals and mechanisms underlying these responses are not well understood, the local and systemic nature of these responses suggest the hypothesis that cytokines with pro-inflammatory, pro-angiogenic and immunoregulatory activities are produced by squamous cell carcinomas and could contribute to its pathogenesis[18]. Accordingly, a number of molecules (Table 1) have been studied in the saliva to evaluate their role as possible biomarkers for OSCC and oral precancer [18–26].
Interleukin-8 (IL-8) is a prototypical member of the CXC chemokine family, the chemokines in which the first two amino terminal cysteine residues are separated by an intervening amino acid. The amino terminus of the majority of the CXC chemokines contains three amino acid residues, glutamic acid–leucine–arginine, the ELR motif. Members that contain the ELR motif (ELR+) are potent promoters of angiogenesis, and those that lack the ELR motif (ELR−) are potent inhibitors of angiogenesis [27]. IL-8 is an ELR+ pluripotent cytokine whose effect on tumour cells involves several pathways. IL-8 is a well-known neutrophil chemoattractant that is also responsible for neutrophil adhesion and transendothelial migration. It is intimately involved in new vessel formation required for tumour growth [28]. As a known angiogenic factor, IL-8 could play a pivotal role in the angiogenesis seen in OSCC.
This study was carried out with the perspective to determine the extent to which this pro-inflammatory, pro-angiogenic cytokine can be detected in the local tumour environment, its relevance as a diagnostic indicator and a possible role in the malignant transformation of precancerous lesions/conditions.
Patients and methods
Patients and study design
A total of 75 subjects, between the age group of 16 and 75 years, were enrolled in this preliminary study, from the outpatient department of Government Dental College and Hospital, Nagpur, India. The study population consisted of three groups: group I—OSCC patients (n = 25); group II—oral precancer patients (n = 25) with oral submucous fibrosis (OSF, n = 13) and oral leukoplakia (n = 12); and group III—age- and sex-matched healthy individuals enrolled as controls (n = 25). Oral examination of all subjects was performed thoroughly in good light. OSCC patients were grouped clinically using the AJCC tumour staging system with TNM parameters [29]. The leukoplakia lesions were classified by the LSCP system (L—extent of leukoplakia, S—site of leukoplakia, C—clinical aspect, P—histopathological feature) as described by Schepman et al. [30]. Written informed consent was obtained from all the subjects. The institutional ethics committee approved the protocol for the study.
Individuals with history of any other systemic disorder; individuals suffering from acute inflammatory conditions of the oral cavity (e.g. dental abscess, pericoronitis); patients receiving chemotherapy/radiotherapy, individuals taking drugs that induce hyposalivation (e.g. anticholinergics, antihistaminics, antihypertensives and beta adrenergic blockers); and individuals using secretogogues were excluded from the study.
Saliva collection
Whole unstimulated saliva was collected from all the subjects. The subjects were refrained from eating, drinking, using chewing gum, mints, etc., for at least 1.5 h prior to the evaluation. Samples were obtained by requesting the subjects to swallow first, tilt their head forwards and expectorate the saliva into 2-ml plastic vials for 5 min [31]. The samples were centrifuged at 6,000 rpm for 20 min to remove squamous cells and debris. The clear supernatant were drawn off and stored in aliquots at −70 °C until the biochemical analysis.
Assessment of salivary interleukin 8
The estimation of salivary IL-8 was carried out by enzyme-linked immunosorbent assay (ELISA) using a commercial ELISA kit specific for human IL-8 (quantikine human IL8/CXCL8 immunoassay kit, R&D Systems, Minneapolis, MN, USA). The assay employed the quantitative sandwich enzyme immunoassay technique. The procedure was performed as per the manufacturer’s instruction manual. The results of the colorimetric reaction were read as the value of the optical density (absorbance) directly on the automatic microplate reader at 450 nm. The results were expressed in picograms per millilitre.
Statistical analysis
Statistical analysis of the data was done using the statistical package for social sciences (SPSS, version 11.0). The concentrations of salivary IL-8 amongst the various groups were compared using analysis of variance, Scheffe’s analysis and two-tailed independent samples t test. Values lower than 0.05 (p < 0.05) were considered statistically significant. The results were expressed as the mean ± standard deviation.
Results
The clinical data and salivary IL-8 levels for groups I (OSCC) and II (precancer) are presented in Tables 2 and 3, respectively. The mean age for the OSCC group was 53.2 ± 11.42 years; for the precancer group, it was 32.16 ± 12.97 years, and for the control group was 45.12 ± 14.65 years. There was a male predilection in both the study groups, the male-to-female ratios being 1.78:1 and 3.17:1 for OSCC and precancer groups, respectively.
Salivary IL-8 levels in oral cancer
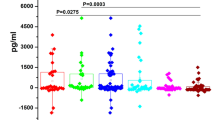
The mean salivary IL-8 concentration for the OSCC group was 1,718.610 ± 668.294 pg/ml, whereas it was 299.513 ± 158.165 and 210.096 ± 142.302 pg/ml for precancer and control subjects, respectively (Fig. 1). The difference between OSCC and precancer was found to be statistically significant (p < 0.0001). Similarly the difference between OSCC and controls was also statistically significant (p < 0.0001).
The salivary IL-8 levels were also compared as per the TNM stage and histopathologic variants (Fig. 2). The mean salivary IL-8 concentrations for patients with stage II (n = 2), stage III (n = 8) and stage IV (n = 15) cancer were 1,694.905 ± 60.677, 1,633.325 ± 359.773 and 1,767.256 ± 832.992 pg/ml, respectively. Though the levels were highest for stage IV disease, the difference between the stages was statistically non-significant (p = 0.907). The mean salivary IL-8 concentration for moderately differentiated squamous cell carcinoma (MDSCC) patients (n = 7) was 1,991.916 ± 57.268 pg/ml, whereas for well-differentiated squamous cell carcinoma (WDSCC) patients, (n = 18), it was 1,612.324 ± 659.795 pg/ml. Though the mean levels were higher for MDSCC patients as compared with WDSCC patients, this difference did not reach the level of statistical significance (p = 0.209).
Salivary IL-8 in oral precancer
The mean salivary IL-8 concentration for the precancer group was found to be 299.513, with a standard deviation of ±158.165. Though the levels where higher compared with the controls (210.096 ± 142.302 pg/ml), this difference was found to be statistically non-significant (p = 0.738). Within the precancer group, the mean salivary IL-8 concentration for OSF was 325.535 ± 70.152 pg/ml, whereas it was 271.32 ± 146.046 pg/ml for leukoplakia. The mean level was thus marginally higher for OSF compared with leukoplakia, but this difference was statistically non-significant ( p = 0.403).
Amongst the leukoplakia lesions, the mean level for stage 2 lesions (n = 6) was 323.43 ± 157.587 pg/ml, whereas for stage 1 lesions (n = 6), it was 219.212 ± 124.795 pg/ml. This difference was found to be statistically non-significant (p = 0.233). The levels were also compared amongst the males and females for each group, and the difference amongst the genders was found to be statistically non-significant (group I: p = 0.401; group II: p = 0.138; group III: p = 0.530).
Discussion
The connection between chronic inflammation and cancerogenesis is well known, and increasing evidence indicates that chemokines play multiple roles in the development and progression of human cancer [32–34]. Indeed, IL-8 has been identified as a mediator of proliferation in a variety of tumour types, including colorectal cancer [35], melanoma [36], gliomas [37] and ovarian cancer [38].
Saliva has been used for diagnostics over more than 2,000 years. Doctors of ancient cultures considered saliva as a part of the circulation, and changes in saliva were believed to be indicative of certain aspects of patients’ health. For example, the viscosity and odour, as well as an individual’s gustatory sensation of his own saliva, have been used to identify disease states [13]. The ability to use saliva to monitor a patient’s health and disease states is a highly desirable goal for health promotion and health care research. It is possible to measure the concentration of drugs, hormones, antibodies and other molecules in saliva. Drug monitoring includes therapeutic drugs like theophylline, lithium, methadone and cyclosporine as well as abusive drugs such as alcohol, cocaine, marijuana, opiates and methamphetamines [39]. Virtually all natural steroid hormones of significance in routine endocrinology—estrogens, testosterone, DHEA, progesterone, cortisol and melatonin—can be monitored in saliva. For example, salivary estriol testing is approved by the FDA to predict preterm birth [40]. In addition, the onset and severity of infectious diseases can be determined by monitoring the presence of antibodies to viruses (HIV, hepatitis A, B, and C, and measles) [41, 42].
Several salivary biomarkers that provide diagnostic information about cancers in the oral cavity or in the head and neck region have been identified (Table 1). Saliva counts of three oral bacteria species were found to be diagnostic indicators of OSCC [43]. Genetic alterations in the tumour tissue have been traced in the cellular component of oral fluid [44]. These can be epigenetic changes of methylation, loss of heterozygosity or point mutations of genomic or mitochondrial DNA. Also, elevated mitochondrial DNA was associated with HNSCC and with advanced-stage disease [45]
A major drawback to using saliva as a diagnostic fluid has been the notion that informative analytes generally are present in lower amounts in saliva than in serum [46]. With new and highly sensitive techniques, however, the lower level of analytes in saliva is no longer a limitation. Compelling reasons exist to use saliva as a diagnostic fluid. It meets the demands for inexpensive, noninvasive and easy-to-use diagnostic methods. As a clinical tool, saliva has many advantages over serum, including ease of collection, storing and shipping, and it can be obtained at low cost in sufficient quantities for analysis. For patients, the noninvasive collection techniques dramatically reduce anxiety and discomfort and simplify the procurement of repeated samples for monitoring over time. Saliva also is easier to handle for diagnostic procedures because it does not clot, thus lessening the manipulations required [47].
Previous studies have been carried out to detect the role of IL-8 in OSCC tissue samples, cell culture homogenates and tissue lysates using different methods [48, 49]. Their results suggested that IL-8 is overexpressed in HNSCC, consistent with a functional role in tumorigenesis. However, there is limited literature reporting the levels of IL-8 in the saliva of patients suffering from OSCC. The role of IL-8 in precancer has been relatively unexplored. With this backdrop, this study is unique as it was designed to evaluate salivary IL-8 levels in a diverse group of patients including OSCC, OSF and oral leukoplakia. The ultimate aim was to evaluate the efficacy of salivary IL-8 levels as a potential biomarker for oral cancer and precancer. To the best of our knowledge, this was the first such study carried out in the Indian population.
There was a statistically significant difference in salivary IL-8 levels for OSCC patients compared with both precancer patients and controls (p < 0.0001). This finding was in accordance with the study carried out by Rhodus et al. [18] and St. John et al. [50]. The findings of the present study were not in accordance with the studies carried out by Katakura et al. [51] and SahebJamee et al. [52]. These conflicting results may be attributable to the differences in ELISA technique, the population being studied and the fact that these studies involved a smaller sample size compared with the present study. The association of IL-8 levels with the TNM stage and histopathologic variation is similar to that reported by Chen et al. [49].
Altered levels of various cytokines have been reported in patients with oral premalignant lesions, such as oral lichen planus, OSF and oral leukoplakia [53–55]. The role of salivary IL-8 in OSF has not been investigated previously, and there has been only one published report, by Rhodus et al. [18], regarding its role in leukoplakia. Though the levels where higher compared with the controls, this difference was found to be statistically non-significant (p = 0.738). This finding was not in accordance with the findings of Rhodus et al. [18]. A possible explanation for this difference amongst the two studies could be the histopathologic nature of the precancerous tissue. All the preneoplastic lesions studied by Rhodus et al. showed evidence of moderate or severe dysplasia. In the present study, none of the OSF samples showed any evidence of dysplasia, whereas only five cases of leukoplakia out of 12 showed evidence of mild dysplasia on histopathologic examination (Table 2).
The present study showed that the pro-inflammatory, pro-angiogenic cytokine IL-8 was significantly elevated in the whole saliva of subjects with OSCC compared with the oral precancer group and controls, suggesting that salivary IL-8 can be utilised as a potential biomarker for OSCC. It can also be suggested that there could be a possible role played by IL-8 in the pathogenesis of OSCC. This aspect merits further research in the future to find novel therapeutic modalities for OSCC. Though the salivary IL-8 levels were higher in the precancer group as compared with the controls, they cannot be considered as predictive values for precancer. Hence, from the findings of this preliminary study, it can be concluded that IL-8 was not a useful as a biomarker for the identification of oral premalignant lesions. A previous study on IL-8 using salivary transcriptome analysis with a sample size of 300 has shown 91 % sensitivity as a biomarker for OSCC [47]. Similar sample sizes with a broader representation of disease sites and stage, as well as prospective studies of treated patient populations, will be needed to confirm the results of this study with respect to direct protein analysis using ELISA as done in this study. Therefore, also the use of a larger sample size would define better the role of IL-8 in oral premaligancy.
Abbreviations
- IL-8:
-
Interleukin 8
- OSCC:
-
Oral squamous cell carcinoma
- HNSCC:
-
Head and neck squamous cell carcinoma.
- OSF:
-
Oral submucous fibrosis
- MDSCC:
-
Moderately differentiated squamous cell carcinoma
- WDSCC:
-
Well-differentiated squamous cell carcinoma
References
Mehrotra R, Yadav S (2006) Oral squamous cell carcinoma: etiology, pathogenesis and prognostic value of genomic alterations. Indian J Cancer 43:60–66
Khanna SS, Karjodkar FR (2006) Circulating immune complexes and trace elements (copper, iron and selenium) as markers in oral precancer and cancer: a randomised, controlled clinical trial. Head Face Med 2:33
Shafer WG, Levy BM, Hine MK, Rajendran R, Sivapathasundharam B (2006) Shafer’s textbook of oral pathology. Elsevier, New Delhi
Oliveira LR, Ribeiro-Silva A (2011) Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 40:298–307
Chang YC, Nieh S, Chen S-F, Jao S-W, Lin Y-L, Fu E (2010) Invasive pattern grading score designed as an independent prognostic indicator in oral squamous cell carcinoma. Histopathology 57:295–303
Nagler RM, Barak M, Ben-Aryeh H, Peled M, Filatov M, Laufer D (1999) Early diagnostic and treatment monitoring role of Cyfra 21-1 and TPS in oral squamous cell carcinoma. Cancer 35:1018–1025
Bhatavdekar JM, Patel DD, Vora HH, Balar DB (1993) Circulating markers and growth factors as prognosticators in men with advanced tongue cancer. Tumour Biol 14:55–58
Bhatavdekar JM, Patel DD, Vora HH, Balar DB (1993) Circulating prolactin and TPS in monitoring the clinical course of male patients with metastatic tongue cancer: a preliminary study. Anticancer Res 13:237–240
Krimmel M, Hoffmann J, Krimmel C, Cornelius CP, Schwenzer N (1998) Relevance of SCC-Ag, CEA, CA 19.9, and CA125 for diagnosis and follow-up in oral cancer. J Craniomaxillofac Surg 26:243–248
Kuo WR, Lee KW, Ho KY, Tsai SM, Chiang FY, Juan KH (1999) Tissue polypeptide antigen, carcinoembryonic antigen, carbohydrate antigen, and CA125 levels as tumor markers in squamous cell carcinoma of the head and neck. Kaohsiung J Med Sci 15:152–158
Hoffmann J, Munz A, Krimmel M, Alfter G (1998) Intraoperative and postoperative kinetics of serum tumor markers in patients with oral carcinoma. J Oral Maxillofac Surg 56:1390–1393
Groschl M, Kohler H, Topf HG, Rupprecht T, Rauh M (2008) Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J Pharm Biomed Anal 47:478–486
Zimmermann BG, Wong DT (2008) Salivary mRNA targets for cancer diagnostics. Oral Oncol 44:425–429
Young MRI, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL et al (1997) Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte–macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer 74:69–74
Gleich LL, Biddinger PW, Duperier FD, Gluckman JL (1997) Tumor angiogenesis as a prognostic indicator in T2–T4 oral cavity squamous cell carcinoma: a clinical–pathologic correlation. Head Neck 19:276–280
Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR (1995) Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte–macrophage colony-stimulating factor. Clin Cancer Res 1:95–103
Smith CW, Chen Z, Dong G, Loukinova E, Pegram MY, Figueroa LN, Waes CV (1998) The host environment promotes the development of primary and metastatic squamous cell carcinomas that constitutively express proinflammatory cytokines IL-1 α, IL-6, GM-CSF, and KC. Clin Exp Metastasis 16:655–664
Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F (2005) NF-kappa B dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev 29:42–45
Liao PH, Chang YC, Huang MF, Tai KW, Chou MY (2000) Mutation of p53 gene codon 63 in saliva as a molecular marker for oral squamous cell carcinomas. Oral Oncol 36:272–276
Zhong LP, Chen GF, Xu ZF, Zhang X, Ping FY, Zhao SF (2005) Detection of telomerase activity in saliva from oral squamous cell carcinoma patient. Int J Oral Maxillofac Surg 34:566–570
Balicki R, Grabowskaa SZ, Citkob A (2005) Salivary epidermal growth factor in oral cavity cancer. Oral Oncol 41:48–55
Nagler R, Bahar G, Shpitzer T, Feinmesser R (2006) Concomitant analysis of salivary tumor markers—a new diagnostic tool for oral cancer. Clin Cancer Res 12:3979–3984
Pickering V, Jordan RCK, Schmidt BL (2007) Elevated salivary endothelin levels in oral cancer patients—a pilot study. Oral Oncol 43:37–41
Rhodus NL, Cheng B, Myers S, Miller L, Ho V, Ondrey F (2005) The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog 44:77–82
Chaiyarit P, Ultrawichian A, Leelayuwat C, Vatanasapt P, Chcnchareonsook N, Samson MH, Giraud AS (2011) Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin Oral Investig. doi:10.1007/s00784-011-0667-z
Rai B, Kaur J, Jacobs R, Anand SC (2011) Adenosine deaminase in saliva as a diagnostic marker of squamous cell carcinoma. Clin Oral Investig 15:347–349
Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM (2000) CXC chemokines in angiogenesis. J Leukoc Biol 68:1–6
Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL (1997) Co-expression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg 174:507–512
Greenberg MS, Glick M, Ship JA (2008) Burkets oral medicine. BC Decker, Ontario
Greenberg MS, Glick M (2003) Burket’s oral medicine diagnosis and treatment. BC Decker, Ontario
Navazesh M, Christensen CM (1982) A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res 61:1158–1162
Balkwill F (2004) Cancer and the chemokine network. Nat Rev Cancer 4:540–545
Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA (2006) Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer 42:768–778
Yeudall WA, Miyazaki H (2007) Chemokines and squamous cancer of the head and neck: targets for therapeutic intervention. Expert Rev Anticancer Ther 7:351–360
Bailey C, Negus R, Morris A, Ziprin P, Goldin R, Allavena P et al (2007) Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis 24:121–130
Varney ML, Johansson SL, Singh RK (2006) Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol 125:209–216
Brat DJ, Bellail AC, Van Meir EG (2005) The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol 7:122–133
Son DS, Parl AK, Rice VM, Khabele D (2007) Keratinocyte chemoattractant (kc)/human growth-regulated oncogene (gro) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther 6:1302–1312
Mandel ID (1993) Salivary diagnosis: more than a lick and a promise. J Am Dent Assoc 124:85–87
Ramsey PS, Andrews WW (2003) Biochemical predictors of preterm labor: fetal fibronectin and salivary estriol. Clin Perinatol 30:701–733
Hodinka R, Nagashunmugam T, Malamud D (1998) Detection of human immunodeficiency virus antibodies in oral fluids. Clin Diagn Lab Immunol 5:419–426
Mortimer PP, Parry JV (1994) Detection of antibody to HIV in saliva: a brief review. Clin Diagn Virol 2:231–243
Mager DL (2005) The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med 3:27
Rosas SL, Koch W, da Costa Carvalho MG (2001) Promoter hypermethylation patterns of p16, O6-methylguanine-DNAmethyl-transferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res 61:939–942
Jiang WW, Masayesva B, Zahurak M et al (2005) Increased mitochondrial DNA content in saliva associated with head and neck cancer. Clin Cancer Res 11:2486–2491
Miller SM (1994) Saliva testing: a nontraditional diagnostic tool. Clin Lab Sci 7:39–44
Wong DT (2006) Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc 137:313–321
Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL (1995) Interleukin-8 expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 121:202–209
Chen Z, Malhotra PS, Thomas GS, Ondrey FG, Duffey DC, Smith CW et al (1999) Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res 5:1369–1379
St John MAR, Li Y, Zhou X, Denny P, Ho CM, Montemagno C et al (2004) Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 130:929–935
Katakura A, Kamiyama I, Takano N, Shibahara T, Muramatsu T, Ishihara K et al (2007) Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull Tokyo Dent Coll 48:199–203
SahebJamee M, Eslami M, Moghadam FA, Sarafnejad A (2008) Salivary concentration of TNFα, IL1α, IL6, and IL8 in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 13:292–295
Khan A, Farah CS, Savage NW, Walsh LJ, Harbrow DJ, Sugerman PB (2003) Th1 cytokines in oral lichen planus. J Oral Pathol Med 32:77–83
Haque MF, Meghji S, Khitab S, Harris M (2000) Oral submucous fibrosis patients have altered levels of cytokine production. J Oral Pathol Med 20:123–128
Brailo V, Boras VV, Arambasin AC, Alajbeg IZ, Milenovic A, Lukac J (2006) The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol 42:370–373
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Silky Rajesh Punyani and Ramhari Shankarrao Sathawane contributed equally to this work.
Rights and permissions
About this article
Cite this article
Punyani, S.R., Sathawane, R.S. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin Oral Invest 17, 517–524 (2013). https://doi.org/10.1007/s00784-012-0723-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0723-3