Abstract
Dental restorations are increasingly manufactured by CAD/CAM systems. Currently, there are two alternatives for digitizing dental implants: direct intra-oral data capturing or indirect from a master cast, both with transfer caps (scanbodies). The aim of this study was the evaluation of the fit of the scanbodies and their ability of reposition. At the site of the first molars and canines, implants were placed bilaterally in a polymer lower arch model (original model), and an impression was taken for fabricating a stone cast (stone model). Ten white-light scans were obtained from the original and the stone model with the scanbodies in place. The scanbodies were retrieved after each scan and re-attached to the same implant or lab analogue. The first scan of the series served as control in both groups. The subsequent nine scans and control were superimposed using inspection software to identify the discrepancies of the four scanbodies in both experimental groups. The systematic error of digitizing the models was 13 μm for the polymer and 5 μm for the stone model. The mean discrepancy of the scanbodies was 39 μm (±58 μm) on the original implants versus 11 μm (±17 μm) on the lab analogues. The difference in scanbody discrepancy between original implants and lab analogues was statistically significant (p < 0.05, Mann–Whitney U test). Scanbody discrepancy was higher on original implants than on lab analogues. Fit and reproducibility of the scanbodies on original implants should be improved to achieve higher accuracy of implant-supported CAD/CAM fabricated restorations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-precision transfer of the clinical situation into dental laboratory is one of the crucial factors for highly accurate prosthetics on natural teeth as well as dental implants. This transfer includes the implant position as well as the inclination [1, 2]. The absolute passive fit of the prosthodontics—the declared goal of any rehabilitation—is, however, ruled out by various sources of error [3]. For implant-supported restorations, discrepancies are particularly detrimental because of the rigid osseointegration of the implants. Those discrepancies may lead to both mechanical and biological complications. Stress-induced porcelain chippings, screw loosenings and fractures of the screw, abutment, or even the implant, were reported [4–9]. Biological complications as mucositis, periimplantitis [10–12], and implant loss caused by inappropriate loading were discussed, too [13].
The precision of intra-oral impressions is one of the most important factors to achieve a perfect fit [14]. Impression techniques and impression materials affect the precision of intra-oral data transfer [15, 16]. Conventional impressions are associated with transfer problems caused by shrinkage, variable layer thickness or separation of the impression material from the tray, and warping of the impression [17]. Additional problems are caused by the expansion of the dental stone during cast fabrication and dimension changes by casting of the framework and veneering in the ceramic furnace.
In order to minimize sources of error, CAD/CAM techniques (computer-aided design/computer-aided manufacturing) have been introduced. For CAD/CAM-assisted fabrication, digitization of the clinical situation means a prerequisite. Two alternatives of data capturing are available: (1) intra-oral scanning (direct data capturing) or (2) digitizing the casts made from conventional impressions by scanning in the dental lab (indirect data capturing) [18].
Dental implants, unlike natural abutments, with their inner configuration defy digital capturing. Therefore, implant manufacturers offer devices, so called scanbodies, for digitizing of implants. These are clip or screw retained on the implants intra-orally or on the lab analogues of the master cast during scanning. The fit of these scanbodies is decisive for a high-precision transfer of the implant position and inclination, which is important for the fabrication of prosthodontics.
This study evaluated the accuracy of these scanbodies both on implants and lab analogues and detected potential sources of error. The working hypothesis is that the reproducible fit of the scanbodies will be the same on original implants and lab analogues.
Material and methods
Two-piece implants (Screwline Promote ø4.3/13 mm; Camlog Biotechnologies, Wimsheim, Germany) were placed at the sites of the first molars and canines bilaterally in an edentulous mandibular model (B-3 NM J UK; Frasaco, Tettnang, Germany). Impression posts were screwed onto them and a polyether impression (Impregum; 3 M-ESPE, Seefeld, Germany) was made with an open-bite custom tray (Master Impression Tray, Water Pik, Ft. Collins, CO, USA). Impressions remained on the model for 8 min, counted from the start of mixing [19]. Four hours after taking the impression, the cast was fabricated (Rocky Mountain Sahara; Klasse IV Dental GmbH, Augsburg, Germany) [19].
Cover screws (Camlog) were seated on the original implants in the Frasaco model (original model), which was matted by applying contrast spray (Met-L-Check Cleaner; Met-L-Check, Santa Monica, CA, USA). The applied contrast spray was used because of its control and better measurability. In unpublished pilot studies, the authors determined that Met-L-Check Cleaner made uniform layers of approximately 10 μm, while other tested sprays produced layers of up to 100 μm.
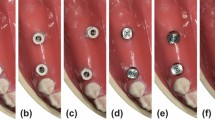
The cover screws were removed and scanbodies (Camlog) were seated on the implants clockwise with a defined torque of 5 Ncm (Torque Control; Camlog) (personal communication with Camlog Biotechnologies), (Fig. 1). In a pilot study, the cast model was scanned five times without removing the scanbodies to determine the systematic error. These five data sets were superimposed and evaluated (Comet INSPECT® plus 4.5; Steinbichler Optotechnik, Neubeuern, Germany). The inspection software calculated discrepancies of 6 μm between the data sets. This value represented the systematic error.
The original model was placed in a white-light scanner (Everest Scan Pro; KaVo, Biberach, Germany) for scanning the entire lower arch. When the first scan was completed, the object support was taken out of the scanner. The scanbodies were detached from the implants one by one and reattached on the same implant clockwise at a defined torque of 5 Ncm without removing the original model from the object support. Once all four scanbodies were relocated again, the object support was placed in the scanner and another scan was obtained for data acquisition. This was repeated nine times and the STL data (Standard Triangulation Language) of ten scans of the original model with the same scanbodies on the same implants were available. Those scanbodies were designed for a maximum of 20 uses (personal communication with Camlog Biotechnologies).
The same procedure was followed for the stone cast (cast model). As the dental stone used for the cast was specifically developed for the scanning technique, opaquing with scan powder was not required. Like the original model, the stone cast was left on the object support, while the scanbodies were released and relocated. Again, a series of ten scans were obtained, and data were stored in STL. The surface of each scanbody was defined by about 40.250 triangles.
Evaluating the positions of the scanbodies and their reproducible fit the STL data were imported and processed with an inspection software (Comet INSPECT® plus 4.5) for data comparison. The first scan model (control) was used as reference (Fig. 2) and compared with the remaining nine digital models. The original model remained the same, while the scanbodies were released and re-attached. Therefore, the scanbodies of the control were blanked out for superimposition with the nine digital models (target models 1–9) and only the model parts without the scanbodies were superimposed by the software. For optimal superimposing of the control and the target models (best fit), a three-run iterative approach (search radius 1, 0.5, and 0.1 mm and 5° search angle) was used. Additionally, the software (Comet INSPECT® plus 4.5) calculated the mean discrepancy of the control model and the target model for each superimposition. This represented the systematic error of the superimposition. The mean error of each superimposition was imported into a statistics program (SPSS 17.0, SPSS Inc, Chicago, IL, USA). The data of original models and lab casts were compared at a level of 5% (ANOVA). When the best fit was found and the value of the discrepancy was calculated and listed, the scanbodies were put back in the digitized control model, and the discrepancies of the four scanbodies in the nine target models were calculated. For this purpose, a plane was constructed through the upper third of the scanbodies on the control model (Fig. 3). In a distance of 0.6 mm, 21 measurement points were determined along the outline of each scanbody (Fig. 4), and the discrepancies between these particular 21 measurement points of the control and target were measured in this plane. The mean of these discrepancies were defined as scanbody discrepancy. This procedure was also used for the cast model with the lab analogues.
This provided nine comparisons of the four implant positions at the sites of the lower molars and canines for both the original model with the original implants and the cast model with the lab analogues. Twenty-one measuring points were defined for each scanbody, i.e., 84 measuring points for each model. Overall, the nine comparisons yielded 756 measuring points for both the original implants and the lab analogues (Fig. 5).
Data were imported into a statistics program (SPSS 17.0, SPSS Inc). For explorative data analysis, the Mann–Whitney U test and ANOVA test was used. The level of statistical significance was set at 5%.
Results
Superimposition of control and target models without scanbodies exhibited discrepancies of 13 μm (±3 μm) for the original model and 5 μm (±2 μm) for the lab casts, respectively. Table 1 shows the discrepancies between the particular superimpositions of the models. There were statistically significant discrepancies of the superimposition between original and cast models (p ≤ 0.001; ANOVA test).
The calculated mean discrepancies of the scanbodies were 39 μm (±58 μm) for the original model and 11 μm (±17 μm) for the stone model. Therefore, the scanbody discrepancies of the original implants differed significantly from that of the lab analogues (p ≤ 0.001; Mann–Whitney U test). The mean discrepancies of the scanbodies at the different implant sites (FDI 36, 33, 43, and 46) for the original implants and the lab analogues are shown in Fig. 6.
Discussion
Scanbodies on lab analogues exhibited higher reproducibility of fit compared to original implants, rejecting the working hypothesis. Comparing the systematic error of the pilot study (6 μm), which was done only on the stone model, with the calculated systematic error of the inspection software (5 μm for the stone model), the results were almost equal. Therefore, the calculated systematic error of the original model (13 μm) appears correct. Del Corso reported of a systematic error between 14 and 21 μm, simulating an intra-oral data capturing in an in vitro simulation [20]. Apparently, the scans with the white-light scanner, described above, were quite exact. KaVo states a systematic error for the Everest Scan Pro scanning a complete jaw of 8–20 μm (personal communication). Also Mehl described the systematic error of extra-oral optical measurement systems for scanning stone casts of 20 μm or less [21].
The statistically significant superimposition discrepancy of 8 μm between the original model and the stone model without scanbodies suggests that the scans of the original model are less precise. This might be due to the scan powder which was applied on the original model. However, scan powder is required for scanning reflecting surfaces like polymer models or most intra-oral scanning systems for opaquing the intra-oral surfaces [22].
The applied contrast spray was used because of its uniform layers of approximately 10 μm. Other tested sprays produced layers of up to 100 μm. This may be a reason, why the calculated systematic error of the digitizing of the original polymer model was less than the data reported by Del Corso and Mehl [20, 21]. However, this contrast spray is not allowed for intra-oral use, and scanners not requiring scan powder for digitizing objects would be desirable.
For superimposition of the control and the target models, the scanbodies and parts of the alveolar crest of the control were blanked out. The “sublingual” and retromolar area showed the highest data quality and seemed to be most suitable for precise superimposition. Some parts of the alveolar crest showed bad edges and were not included in the superimposing process. The flat “sublingual” area gave an exact field for the space coordinates X and Y, the retromolar area for the space coordinates Z. Hence, the superimposition algorithm can be calculated precisely by the inspection software, shown by the low systematic error.
Comparing the mean discrepancies of the scanbody misfit on the original model/implant (39 μm) and on the stone model/lab analogue (11 μm), there is a statistically significant difference. Minus the systematic error of the digitizing of the models, the misfit on the original implant would be 26 μm (39 − 13 μm) versus 6 μm (11 − 5 μm) for the lab analogue. The accuracy of the interfaces of the original implants and the lab analogues should be identical (personal communication Camlog Biotechnologies). However, there is a production tolerance of about 15 μm. If the inner diameter of the lab analogues was on the lower limit and the original implants on the upper limit of manufacturing tolerance, a discrepancy of about 25 μm could be possible.
Ma described tolerances between the implant and the impression copings, abutment replicas, and abutments from 22 to 100 μm [23]. These discrepancies are not only machining tolerances but also a function of the design of the positional index [24]. The positional index depends between internal and external implant–abutment connections [25]. Semper described rotational discrepancies of the abutment having three different geometric patterns of dental implants from 3.7° to 1.4° [26]. Based on these data, this study was conducted with an implant that showed an internal connection with less rotational discrepancy of 1.4°.
If multiple implants are parallel-inserted, there will be no horizontal shift in the transfer; if the implants are angulated, the rotational misfit leads to a horizontal discrepancy. An angulation of 20° and a rotational freedom of 1.5° can result in a horizontal misfit up to 127 μm [27].
Also the value of the discrepancy is dependent to the vertical extension of the scanbodies and the constructed plane for the measurement. The higher the plane is constructed in relation to the implant shoulder, the higher the measured discrepancy relating to the discrepancy in the internal connection. The plane for the measurements was determined in the upper third of the scanbodies. This vertical dimension showed approximately the length of a natural tooth, for determination the discrepancy in the occlusal plane.
Currently, it seems to be more precise to do a conventional impression and white-light scanning of stone casts for an accurate fit of implant-supported prosthodontics according to the results of this study. This is also stated by Luthardt [28]. However, it has to be considered that indirect data capturing consists of more steps in the working sequence. This includes the possibility of more discrepancies in the individual process.
Conclusions
Within the limitations of this study, the results showed that
-
1.
the systematic error by scanning the stone models was less in contrast to the polymer models; therefore, white-light scanning of stone casts seems to produces more accurate data than original (polymer) in vitro models matted with scan powder.
-
2.
the ability of repositioning of the scanbody is better on lab analogues than on original implants.
-
3.
the companies have to improve the reproducible fit of the scanbodies in the original implants by reducing the production tolerance.
References
Assuncao WG, Filho HG, Zaniquelli O (2004) Evaluation of transfer impressions for osseointegrated implants at various angulations. Implant Dent 13:358–366
Carr AB (1991) Comparison of impression techniques for a five-implant mandibular model. Int J Oral Maxillofac Implants 6:448–455
Kan JY, Rungcharassaeng K, Bohsali K, Goodacre CJ, Lang BR (1999) Clinical methods for evaluating implant framework fit. J Prosthet Dent 81:7–13
Balshi TJ (1996) An analysis and management of fractured implants: a clinical report. Int J Oral Maxillofac Implants 11:660–666
Burguete RL, Johns RB, King T, Patterson EA (1994) Tightening characteristics for screwed joints in osseointegrated dental implants. J Prosthet Dent 71:592–599
Eckert SE, Meraw SJ, Cal E, Ow RK (2000) Analysis of incidence and associated factors with fractured implants: a retrospective study. Int J Oral Maxillofac Implants 15:662–667
Jemt T, Rubenstein JE, Carlsson L, Lang BR (1996) Measuring fit at the implant prosthodontic interface. J Prosthet Dent 75:314–325
Sahin S, Cehreli MC (2001) The significance of passive framework fit in implant prosthodontics: current status. Implant Dent 10:85–92
Wee AG, Aquilino SA, Schneider RL (1999) Strategies to achieve fit in implant prosthodontics: a review of the literature. Int J Prosthodont 12:167–178
Augthun M, Conrads G (1997) Microbial findings of deep peri-implant bone defects. Int J Oral Maxillofac Implants 12:106–112
Leonhardt A, Renvert S, Dahlen G (1999) Microbial findings at failing implants. Clin Oral Implants Res 10:339–345
Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C (1992) Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res 3:9–16
Binon PP, McHugh MJ (1996) The effect of eliminating implant/abutment rotational misfit on screw joint stability. Int J Prosthodont 9:511–519
Karl M, Winter W, Taylor TD, Heckmann SM (2004) In vitro study on passive fit in implant-supported 5-unit fixed partial dentures. Int J Oral Maxillofac Implants 19:30–37
Al-Bakri IA, Hussey D, Al-Omari WM (2007) The dimensional accuracy of four impression techniques with the use of addition silicone impression materials. J Clin Dent 18:29–33
Lee H, So JS, Hochstedler JL, Ercoli C (2008) The accuracy of implant impressions: a systematic review. J Prosthet Dent 100:285–291
Christensen GJ (2008) Will digital impressions eliminate the current problems with conventional impressions? J Am Dent Assoc 139:761–763
Beuer F, Schweiger J, Edelhoff D (2008) Digital dentistry: an overview of recent developments for CAD/CAM generated restorations. Br Dent J 204:505–511
Luthardt RG, Walter MH, Quaas S, Koch R, Rudolph H (2010) Comparison of the three-dimensional correctness of impression techniques: a randomized controlled trial. Quintessence Int 41:845–853
Del Corso M, Aba G, Vazquez L, Dargaud J, Dohan Ehrenfest DM (2009) Optical three-dimensional scanning acquisition of the position of osseointegrated implants: an in vitro study to determine method accuracy and operational feasibility. Clin Implant Dent Relat Res 11:214–221
Mehl A, Ender A, Mormann W, Attin T (2009) Accuracy testing of a new intraoral 3D camera. Int J Comput Dent 12:11–28
Masek R (2005) Margin isolation for optical impressions and adhesion. Int J Comput Dent 8:69–76
Ma T, Nicholls JI, Rubenstein JE (1997) Tolerance measurements of various implant components. Int J Oral Maxillofac Implants 12:371–375
Semper W, Kraft S, Kruger T, Nelson K (2009) Theoretical considerations: implant positional index design. J Dent Res 88:725–730
Binon PP (2000) Implants and components: entering the new millennium. Int J Oral Maxillofac Implants 15:76–94
Semper W, Kraft S, Kruger T, Nelson K (2009) Theoretical optimum of implant positional index design. J Dent Res 88:731–735
Semper W, Kraft S, Mehrhof J, Nelson K (2010) Impact of abutment rotation and angulation on marginal fit: theoretical considerations. Int J Oral Maxillofac Implants 25:752–758
Luthardt RG, Loos R, Quaas S (2005) Accuracy of intraoral data acquisition in comparison to the conventional impression. Int J Comput Dent 8:283–294
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stimmelmayr, M., Güth, JF., Erdelt, K. et al. Digital evaluation of the reproducibility of implant scanbody fit—an in vitro study. Clin Oral Invest 16, 851–856 (2012). https://doi.org/10.1007/s00784-011-0564-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-011-0564-5