Abstract
Enamel matrix derivative (EMD) used to promote periodontal regeneration has been shown to exert anti-inflammatory effects. This in vitro study was performed to investigate if the anti-inflammatory actions of EMD are modulated by the local cellular environment, such as inflammation or occlusal, i.e., biomechanical, loading. Human periodontal ligament cells were seeded on BioFlex plates and incubated with EMD under normal, inflammatory, and biomechanical loading conditions for 1 and 6 days. In order to mimic inflammatory and biomechanical loading conditions in vitro, cells were stimulated with interleukin (IL)-1β and exposed to dynamic tensile strain, respectively. The gene expression of IL-1β, IL-1 receptor antagonist (IL-1RN), IL-6, IL-8, IL-10, and cyclooxygenase (COX)-2 was analyzed by real-time RT-PCR and the IL-6 protein synthesis by enzyme-linked immunoassay. For statistical analysis, Student's t test, ANOVA, and post-hoc comparison tests were applied (p < 0.05). EMD downregulated significantly the expression of IL-1β and COX-2 at 1 day and of IL-6, IL-8, and COX-2 at 6 days in normal condition. In an inflammatory environment, the anti-inflammatory actions of EMD were significantly enhanced at 6 days. In the presence of low biomechanical loading, EMD caused a downregulation of IL-1β and IL-8, whereas high biomechanical loading significantly abrogated the anti-inflammatory effects of EMD at both days. Neither IL-1RN nor IL-10 was upregulated by EMD. These data suggest that high occlusal forces may abrogate anti-inflammatory effects of EMD and should, therefore, be avoided immediately after the application of EMD to achieve best healing results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis represents a multifactorial inflammatory disease and is characterized by the destruction of periodontal tissues. Periodontopathogenic microorganisms, their components, and products from the subgingival biofilm trigger the production of inflammatory molecules, such as interleukin (IL)-1β, IL-6, IL-8, and cyclooxygenase (COX)-2, by infiltrating immunoinflammatory and resident cells of the periodontium and, thereby, initiate and perpetuate soft tissue degradation and bone resorption [1–4]. Although anti-inflammatory cytokines, such as IL-1 receptor antagonist (IL-1RA encoded by IL-1RN) and IL-10, are also produced by periodontal and inflammatory cells, the balance is shifted toward inflammation [5, 6].
The main goal of periodontal therapy is to arrest the inflammatory and tissue-destructive processes by removing the subgingival biofilm and creating a local environment and microflora which is compatible with periodontal health. However, conventional periodontal treatment, which comprises nonsurgical or surgical debridement, sometimes applied in combination with antibiotics, achieves periodontal healing mainly by repair but not by reconstitution of the initial form, structure, and architecture, i.e., regeneration, of the lost periodontal tissues [7, 8]. Several bioactive molecules have been suggested to promote periodontal regeneration, enamel matrix derivative (EMD) being one of them. EMD has been shown in preclinical and clinical studies to support the formation of periodontal ligament (PDL), cementum, and bone [9–14].
Interestingly, in addition to its role in periodontal regeneration, EMD seems to reduce the incidence of postoperative pain/swelling and to accelerate wound healing, suggesting an anti-inflammatory role for EMD in periodontal healing [12, 15–18]. Healing is mainly characterized by inflammation, cell migration, and proliferation, followed then by matrix deposition and, finally, tissue remodeling [19, 20]. Parkar and Tonetti have revealed that EMD downregulates the expression of genes involved in the early inflammatory phase of periodontal wound healing, while simultaneously upregulating genes encoding growth and repair-promoting molecules [21]. These findings are in accordance with observations by Myhre and co-workers, who found that EMD limits the release of proinflammatory cytokines from stimulated blood cells, suggesting that EMD has anti-inflammatory properties [22]. Similarly, EMD has been shown to exhibit anti-inflammatory properties in rat monocytes, which were exposed to bacterial lipopolysaccharide [23]. All these studies suggest that EMD may support periodontal healing by downregulation of proinflammatory molecules and, thereby, shortening the inflammatory stage of healing.
The cell culture conditions chosen in most in vitro studies on EMD undoubtedly allowed to assess the full potential of EMD and helped unravel the mechanism whereby EMD directs healing under optimal conditions. Clinically, the beneficial effects of EMD may be jeopardized by the local cellular environment, such as inflammation because of a residual periodontal infection, or biomechanical loading. The periodontium comprises load-bearing tissues, and teeth affected by periodontitis are frequently subject to comparatively high occlusal, i.e., biomechanical, forces during mastication and functional dental habits. However, the role of biomechanical signals in the response of periodontal cells to bioactive molecules has yet to be elucidated. A better understanding of the interactions between regenerative molecules, biomechanical forces, and inflammatory mediators may improve the outcome of regenerative treatment approaches with bioactive molecules in periodontally diseased patients. The aim of this in vitro study was therefore to investigate whether the anti-inflammatory effects of EMD are modulated by biomechanical and inflammatory signals.
Materials and methods
Cell culture
PDL cells were harvested from six donors, who had to undergo tooth extraction for orthodontic reasons. Approval of the ethics committee of the University of Bonn and informed parental consent were obtained. Cells were dissected from the mid-third portion of the roots of the periodontally healthy and caries-free teeth grown in Dulbecco's minimal essential medium (Invitrogen®, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (FBS, Invitrogen®), 100 units penicillin, and 100 μg/ml streptomycin (Biochrom®, Berlin, Germany) at 37°C in a humidified atmosphere of 5% CO2; seeded (50,000 cells/well) on BioFlex® collagen-coated culture plates (Flexcell International, Hillsborough, USA); and grown to 80% confluence. One day before experiments, the FBS concentration was reduced to 1%, and the medium was changed every other day. Cells were used between passages 3 and 5.
In vitro inflammation and biomechanical loading
PDL cells were stimulated with EMD (0.1 mg/ml; Straumann AG, Basel, Switzerland) dissolved in 0.1% acetic acid (Fisher Scientific, Schwerte, Germany) in PBS (PAA Laboratories, Cölbe, Germany) under normal, inflammatory, or biomechanical loading conditions. Cells in the absence of EMD under these conditions served as control. In order to mimic inflammation in vitro, cells were stimulated with IL-1β (1 ng/ml; Calbiochem, San Diego, USA), the levels of which are raised in the gingival crevicular fluid and gingival tissues at inflamed periodontal sites [24–27]. Biomechanical loading conditions were created by exposing PDL cells to equibiaxial cyclic tensile strain (CTS) of low (CTSL, 3%) and high (CTSH, 20%) magnitudes at a rate of 0.05 Hz by using a strain device, which was developed at the University of Bonn. This system had already been used for the application of static strain in previous studies [28, 29]. Briefly, the BioFlex® culture plates were positioned in such a way that posts were centered directly beneath the flexible-bottom wells of the plates. By cyclic upward and downward movement of a moving table, which was located directly above the culture plate, the flexible membrane of each well was pulled over the posts, which caused the cells grown on the flexible membrane to be dynamically stretched. In order to unravel the intracellular signaling induced by EMD, some cells were preincubated with a specific nuclear factor-κB (NF-κB) inhibitor [pyrrolidine dithiocarbamate (PDTC), 10 μM; Calbiochem] or a SMAD1/5/8 inhibitor (dorsomorphin, 5 μM; Calbiochem) 1 h prior to the experiments.
Real-time RT-PCR
In order to analyze the effect of EMD at transcriptional level, RNA was extracted with a Qiagen® RNA extraction kit (Qiagen®, Hilden, Germany) and reverse transcribed with iScript™ Select cDNA Synthesis Kit (Bio-Rad, Munich, Germany) at 42°C for 90 min followed by 85°C for 5 min. The expression of IL-1β, IL-1RN, IL-6, IL-8, IL-10, COX-2, and GAPDH was analyzed by real-time RT-PCR using the iCycler iQ detection system (Bio-Rad), SYBR Green (Qiagen®) and specific primers (QuantiTect Primer Assay, Qiagen®). One microliter of cDNA was amplified as a template in a 25-μl reaction mixture containing 12.5-μl 2× QuantiFast SYBR Green PCR Master Mix (Qiagen®), 2.5 μl of primers, and deionized water. The mixture was heated initially at 95°C for 5 min and then followed by 40 cycles with denaturation at 95°C for 10 s and combined annealing/extension at 60°C for 30 s. For data analysis, the comparative threshold cycle method was applied [30].
ELISA
Concentration of IL-6 in cell supernatants was measured by a commercially available enzyme-linked immunoassay (ELISA) kit (RayBiotech, Norcross, USA) according to the manufacturer's instructions and a microtiterplate reader (PowerWave X, BioTek Instruments, USA) at 450 nm. Data were normalized by cell number.
Data analysis
The statistical analysis was performed by using SPSS 17.0 (SPSS Inc., USA). Mean values and standard errors of the mean were calculated (n = 6). For statistical analysis, Student's t test as well as ANOVA with post-hoc Dunnett's and Tukey's tests were applied (p < 0.05). Experiments were repeated at least twice.
Results
Effects of EMD on inflammatory mediators in normal condition
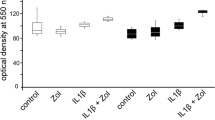
At 1 day, EMD downregulated significantly the mRNA expression of IL-1β and COX-2 as compared to control. Although EMD also reduced the IL-8 mRNA expression, the inhibitory effect was not significant. IL-6 was the only inflammatory mediator, the gene expression of which was significantly increased in EMD-treated cells as compared to control (Fig. 1a). At 6 days, EMD had no effect on the IL-1β mRNA expression and caused a significant IL-6, IL-8, and COX-2 mRNA downregulation (Fig. 1b). The regulatory effects of EMD on IL-6 were also analyzed and confirmed at protein level. While IL-6 protein levels in supernatants were significantly increased by EMD at 1 day, significant lower levels of IL-6 protein were measured in EMD-treated cells as compared to control at 6 days (Fig. 1c). Furthermore, incubation of cells with PDTC, a specific inhibitor of NF-κB signaling, partially abrogated the stimulatory effect of EMD on the IL-6 mRNA expression (Fig. 1d). By contrast, dorsomorphin, which inhibits SMAD signaling, did not interfere with the stimulation of IL-6 gene expression by EMD (data not shown).
Effect of EMD on IL-1β, IL-6, IL-8, and COX-2 under normal and inflammatory conditions. Regulation of IL-1β, IL-6, IL-8, and COX-2 mRNA expression by EMD under normal condition at 1 day (a) and 6 days (b). Asterisk, significantly different from control. IL-6 protein concentration in supernatants of cells cultured in the presence and absence of EMD under normal condition for 1 and 6 days (c). Asterisk, significant difference between groups. Effect of PDTC, a specific inhibitor of NF-κB signaling, on the EMD-induced upregulation of IL-6 under normal condition at 1 day (d). Asterisk, significant difference between groups. Actions of EMD on IL-1β, IL-6, IL-8, and COX-2 mRNA expression in the presence of IL-1β, i.e., under simulated inflammatory condition at 1 days (e) and 6 days (f). Asterisk, significantly different from control
Actions of EMD on inflammatory mediators in the presence of IL-1β
Next, we wondered whether the gene expressions of IL-1β, IL-6, IL-8, and COX-2 are regulated by EMD in an inflammatory environment. As shown in Fig. 1e, EMD caused a significant decrease of mRNA levels for IL-1β and COX-2 but not IL-6 and IL-8 under inflammatory condition at 1 day. By contrast, the mRNA expression of all molecules was significantly reduced when cells were exposed to EMD for 6 days in this condition (Fig. 1f).
Regulation of inflammatory mediators by EMD in low biomechanical loading
Since PDL represents a load-bearing tissue, we also studied if low biomechanical strain would alter the response of cells to EMD with regard to the expression of proinflammatory molecules. At 1 day, the gene expression of IL-1β and IL-8 in cells exposed to low biomechanical forces was downregulated by EMD but a significant reduction was only observed for IL-8 (Fig. 2a). Furthermore, EMD induced an IL-6 mRNA upregulation and did not exert a regulatory effect on COX-2 gene expression (Fig. 2a). Similarly, EMD raised the IL-6 mRNA level at 6 days (Fig. 2b). Moreover, mRNA levels of IL-1β and IL-8 were again decreased and of COX-2 not altered in EMD-treated cells at this time point (Fig. 2b).
Effect of EMD on IL-1β, IL-6, IL-8, and COX-2 under low and high biomechanical loading conditions. Regulation of IL-1β, IL-6, IL-8, and COX-2 mRNA expression by EMD under low biomechanical loading at 1 day (a) and 6 days (b) and under high biomechanical loading at 1 day (c) and 6 days (d). Asterisk, significantly different from control. IL-6 protein concentration in supernatants of cells cultured in the presence and absence of EMD and/or biomechanical loading (3% or 20%) for 1 days (e) and 2 days (f). Asterisk, significant difference between groups
Regulation of inflammatory mediators by EMD in high biomechanical loading
In cells exposed to high biomechanical strain, EMD did not induce a significant mRNA downregulation of any inflammatory mediator at 1 day. By contrast, the IL-6 mRNA expression was significantly increased by EMD (Fig. 2c). At 6 days, the gene expression of IL-6 and COX-2 was significantly upregulated by EMD, whereas no significant effects of EMD on the IL-1β and IL-8 mRNA expression were observed (Fig. 2d). EMD also caused a significant increase in protein levels for IL-6 at 1 and 2 days, as revealed by ELISA (Fig. 2e, f). The highest IL-6 protein synthesis was observed in cells which were treated with EMD and simultaneously exposed to high biomechanical forces (Fig. 2e, f).
Effect of EMD, biomechanical loading, and IL-1β on anti-inflammatory cytokines
Finally, we sought to investigate if EMD, biomechanical forces, or IL-1β can regulate the gene expression of anti-inflammatory cytokines in PDL cells under normal condition. EMD and biomechanical loading caused a significant downregulation of IL-1RN gene expression, whereas IL-1β significantly increased the mRNA expression of this molecule at 1 day (Fig. 3a). At 6 days, the IL-1RN mRNA expression was upregulated by IL-1β and additionally by biomechanical loading but not affected by EMD (Fig. 3b). EMD, biomechanical loading, and IL-1β reduced significantly the mRNA expression of IL-10 at 1 day (Fig. 3c). At 6 days, the IL-10 gene expression was significantly regulated only by IL-1β, which exerted a stimulatory effect (Fig. 3d).
Effect of EMD, biomechanical loading, and IL-1β on IL-1RN and IL-10. Actions of EMD, low (CTSL) and high (CTSH) biomechanical loading, and IL-1β on IL-1RN mRNA expression at 1 day (a) and 6 days (b) and IL-10 mRNA expression at 1 day (c) and 6 days (d). Asterisk, significantly different from control
Discussion
The major and novel finding of this in vitro study is that biomechanical loading and a proinflammatory environment modulate the anti-inflammatory effects of EMD. While the inhibitory actions of EMD on proinflammatory cytokines were enhanced under simulated inflammatory condition, no significant inhibition of these molecules by EMD was observed in the presence of high biomechanical loading. These findings suggest that high occlusal forces may possibly abrogate anti-inflammatory effects of EMD and should therefore be avoided immediately after the application of EMD to achieve best healing results.
Periodontitis represents an inflammatory disease caused by periodontopathogenic microorganisms in the subgingival biofilm, which trigger the production of inflammatory molecules [1–4]. It has been reported that EMD may reduce the incidence of postoperative pain/swelling and accelerate gingival wound healing, suggesting an anti-inflammatory role for EMD in addition to its stimulating effects on periodontal regeneration [12, 15–18]. The sequential stages of healing are inflammation, cell migration and proliferation, matrix deposition, and, finally, tissue remodeling [19, 20]. EMD has been shown to downregulate the expression of genes involved in the early inflammatory phases of healing and to limit the release of proinflammatory cytokines from stimulated blood cells [21, 22]. These in vitro and clinical studies suggest that EMD can accelerate periodontal healing by downregulation of proinflammatory molecules and, thereby, shortening of the inflammatory stage of healing. In the present study, we sought to examine if the anti-inflammatory properties of EMD are altered in the presence of biomechanical and inflammatory signals. First, we studied if EMD would downregulate the expression of proinflammatory mediators under normal condition, i.e., in the absence of biomechanical loading and inflammation. This cell culture condition has been chosen in most in vitro studies on EMD and undoubtedly allowed to assess the full potential of EMD. EMD caused an mRNA downregulation of proinflammatory mediators in the present study up to 6 days, which supports the assumption that EMD may play a role in dampening inflammation.
Next, we wondered how the regulation of inflammatory mediators by EMD would be altered under simulated inflammatory conditions. Interestingly, the EMD-induced effects were similar to those observed at normal condition and even enhanced at 6 days, indicating that the full anti-inflammatory potential of EMD does not develop until cells are exposed to an inflammatory environment. In vivo, cells can face inflammation due to a residual infection at the sites where EMD is to be applied but also because of wounding of the periodontal tissues due to the surgical trauma. In order to mimic an inflammatory environment, cells were treated with the proinflammatory cytokine IL-1β, which is increased in gingiva and gingival crevicular fluid at inflamed sites [24–27]. IL-1β was applied at a concentration of 1 ng/ml, which is in the range of levels usually found in the gingival crevicular fluid of periodontally diseased patients.
The periodontium is subject to complex biomechanical forces during mastication, dental trauma, and functional dental habits. A more recent study focused on the relationship between occlusal loading and periodontitis on an individual tooth level and demonstrated an association between occlusal overloading caused by untreated occlusal discrepancies and the progression of periodontitis [31, 32]. Notably, elimination of tooth overloading significantly reduced the progression of periodontitis over time. These findings implicate that biomechanical forces can interfere with the progression and treatment of periodontitis. Therefore, we also studied how biomechanical forces would affect the anti-inflammatory potential of EMD observed under normal and inflammatory conditions. While EMD downregulated the IL-1β and IL-8 mRNA expression in the presence of low biomechanical forces, the inhibitory actions of EMD on the gene expression of these cytokines were abrogated when cells were exposed to high biomechanical forces. Moreover, expression and synthesis of IL-6 were significantly upregulated by EMD. These findings suggest that the anti-inflammatory potential of EMD is lost under high biomechanical loading conditions. Therefore, it is tempting to speculate that high occlusal loading may lead to a delayed and less-favorable healing as compared to low occlusal loading at EMD-treated teeth after surgery.
Since upregulation of anti-inflammatory molecules may represent another mechanism whereby EMD can inhibit inflammation, we also studied the effect of EMD on IL-1RN and IL-10. Interestingly, EMD did not stimulate either IL-1RN or IL-10 mRNA expression at 1 and 6 days, which suggests that the anti-inflammatory actions of EMD may mainly be mediated by downregulation of proinflammatory mediators but not upregulation of anti-inflammatory molecules. Notably, the mRNA expression of IL-1RN and IL-10 was mainly enhanced in the presence of IL-1β, which may represent a negative feedback loop.
In the present study, we examined the effect of EMD on the gene expression of IL-1β, IL-6, IL-8, COX-2, IL-RN, and IL-10 because these molecules are thought to play a key role in periodontal inflammation and have been shown to be produced by PDL cells [1–4]. In addition, previous studies had revealed that some of these molecules are regulated by EMD in blood cells [22, 23]. However, other inflammatory mediators are also very likely involved in the pathogenesis of periodontitis and whether these molecules are also regulated by EMD has yet to be determined.
In order to study the influence of biomechanical loading on possible anti-inflammatory effects exerted by EMD, tensile strain was applied. In a clinical setting, PDL cells are subject to complex forces, i.e., tension, compression, and shear. Whether compressive, shear, and even combined forces exert similar effects as observed for tensile strain alone in our experiments has yet to be elucidated.
PDL cells were incubated with 0.1 mg/ml of EMD because this concentration had been used by several investigators before and ensured that our data were comparable with those in other studies [21, 33–35]. However, the ability of high biomechanical forces to abrogate the anti-inflammatory effects of EMD, as seen in the present study, may depend on the EMD concentration applied.
It has been reported that EMD exploits for its effects in the SMAD and ERK signal transduction pathways [36, 37]. In the present study, EMD caused an upregulation of the IL-6 mRNA expression via NF-κB but not SMAD signaling. However, since incubation with the NF-κB inhibitor did not completely suppress the IL-6 upregulation by EMD, additional pathways seem to be involved in the actions of EMD. Furthermore, the mechanisms whereby biomechanical strain and IL-1β modulate EMD effects are yet to be elucidated. Agarwal and co-workers have shown that high biomechanical forces can exert proinflammatory effects like IL-1β by using NF-κB [38]. However, in the present study, high biomechanical strain and IL-1β differed in their influence on the anti-inflammatory effects of EMD, suggesting that different regulatory mechanisms play a role. It is also conceivable that the anti-inflammatory effects of EMD are regulated at receptor level by biomechanical forces and IL-1β. So far, the involved receptors for EMD are not fully known. Certainly, receptors for transforming growth factor and bone morphogenetic proteins are involved in EMD effects, since EMD seems to contain and induce these growth factors [21, 36, 39–41]. Future studies should unravel which receptors are required for the anti-inflammatory effects of EMD and how the subsequent signaling is regulated by biomechanical and inflammatory signals.
In the present study, the regulatory effects of EMD on proinflammatory and anti-inflammatory mediators were mainly examined at transcriptional level. Since IL-6 has recently gained increased attention due to its critical role in T cell differentiation, we especially focused on IL-6 and additionally investigated the regulation of this cytokine at protein level [42–44]. Although the EMD-induced alterations in IL-6 gene expression were paralleled with similar changes at protein level, mRNA levels do not always equate with protein synthesis and secretion. The results for the other molecules, which were only studied at transcriptional level, should therefore be interpreted with caution.
Taken together, our findings show for the first time that biomechanical loading and a proinflammatory environment modulate the anti-inflammatory effects of EMD. While anti-inflammatory actions of EMD were enhanced under simulated inflammatory conditions, high biomechanical loading abrogated the anti-inflammatory effects of EMD. Within the limits of this in vitro study, we conclude that high occlusal tooth loading may reduce the anti-inflammatory effects of EMD and, thereby, lead to a delayed and/or less-favorable healing immediately after surgery.
References
Graves DT, Cochran D (2003) The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 74:391–401
Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ (2007) Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res 86:306–319
Noguchi K, Ishikawa I (2007) The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontol 2000 43:85–101
Bartold PM, Cantley MD, Haynes DR (2010) Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000 53:55–69
Deschner J, Arnold B, Kage A, Zimmermann B, Kanitz V, Bernimoulin JP (2000) Suppression of interleukin-10 release from human periodontal ligament cells by interleukin-1beta in vitro. Arch Oral Biol 45:179–183
Long P, Hu J, Piesco N, Buckley M, Agarwal S (2001) Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res 80:1416–1420
Garrett S (1996) Periodontal regeneration around natural teeth. Ann Periodontol 1:621–666
Heitz-Mayfield L (2005) How effective is surgical therapy compared with nonsurgical debridement? Periodontology 2000 37:72–87
Bosshardt DD (2008) Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol 35(8 Suppl):87–105
Cochran DL, King GN, Schoolfield J, Velasquez-Plata D, Mellonig JT, Jones A (2003) The effect of enamel matrix proteins on periodontal regeneration as determined by histological analyses. J Periodontol 74:1043–1055
Giannobile W, Somerman M (2003) Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol 8:193–204
Jepsen S, Heinz B, Jepsen K, Arjomand M, Hoffmann T, Richter S, Reich E, Sculean A, Gonzales JR, Bödeker RH, Meyle J (2004) A randomized clinical trial comparing enamel matrix derivative and membrane treatment of buccal Class II furcation involvement in mandibular molars. Part I: study design and results for primary outcomes. J Periodontol 75:1150–1160
Venezia E, Goldstein M, Boyan B, Schwartz Z (2004) The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta-analysis. Crit Rev Oral Biol Med 15:382–402
Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV (2009) Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst Rev (4):CD003875
Mirastschijski U, Konrad D, Lundberg E, Lyngstadaas SP, Jorgensen LN, Agren MS (2004) Effects of a topical enamel matrix derivative on skin wound healing. Wound Repair Regen 12:100–108
Okuda K, Miyazaki A, Momose M, Murata M, Nomura T, Kubota T, Wolff LF, Yoshie H (2001) Levels of tissue inhibitor of metalloproteinases-1 and matrix metalloproteinases-1 and -8 in gingival crevicular fluid following treatment with enamel matrix derivative (EMDOGAIN). J Periodontal Res 36:309–316
Tonetti MS, Fourmousis I, Suvan J, Cortellini P, Brägger U, Lang NP, European Research Group on Periodontology (ERGOPERIO) (2004) Healing, post-operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. J Clin Periodontol 31:1092–1098
Wennström JL, Lindhe J (2002) Some effects of enamel matrix proteins on wound healing in the dento-gingival region. J Clin Periodontol 29:9–14
Aukhil I (2000) Biology of wound healing. Periodontol 2000 22:44–50
Häkkinen L, Uitto VJ, Larjava H (2000) Cell biology of gingival wound healing. Periodontol 2000 24:127–152
Parkar M, Tonetti M (2004) Gene expression profiles of periodontal ligament cells treated with enamel matrix proteins in vitro: analysis using cDNA arrays. J Periodontol 75:1539–1546
Myhre AE, Lyngstadaas SP, Dahle MK, Stuestøl JF, Foster SJ, Thiemermann C, Lilleaasen P, Wang JE, Aasen AO (2006) Anti-inflammatory properties of enamel matrix derivative in human blood. J Periodontal Res 41:208–213
Sato S, Kitagawa M, Sakamoto K, Iizuka S, Kudo Y, Ogawa I, Miyauchi M, Chu EY, Foster BL, Somerman MJ, Takata T (2008) Enamel matrix derivative exhibits anti-inflammatory properties in monocytes. J Periodontol 79:535–540
Hönig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F (1989) Increased interleukin-1 beta (IL-1 beta) concentration in gingival tissue from periodontitis patients. J Periodontal Res 24:362–367
Hou L, Liu C, Chang W (1994) Increased interleukin-1 beta levels in gingival crevicular fluid of Chinese periodontal patients. J Formos Med Assoc 93:99–103
Mathur A, Michalowicz B, Castillo M, Aeppli D (1996) Interleukin-1 alpha, interleukin-8 and interferon-alpha levels in gingival crevicular fluid. J Periodontal Res 31:489–495
Preiss D, Meyle J (1994) Interleukin-1 beta concentration of gingival crevicular fluid. J Periodontol 65:423–428
Deschner J, Rath-Deschner B, Reimann S, Bourauel C, Götz W, Jepsen S, Jäger A (2007) Regulatory effects of biophysical strain on rat TMJ discs. Ann Anat 189:326–328
Rath-Deschner B, Deschner J, Reimann S, Bourauel C, Jäger A, Götz W (2009) Regulatory effects of biomechanical strain on the IGF system in human periodontal cells. J Biomech 42:2584–2589
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta C(T)) method. Methods 25:402–408
Harrel SK, Nunn ME, Hallmon WW (2006) Is there an association between occlusion and periodontal destruction?: yes—occlusal forces can contribute to periodontal destruction. JADA 137:1380–1392
Harrel SK, Nunn ME (2001) The effect of occlusal discrepancies on periodontitis. II. Relationship of occlusal treatment to the progression of periodontal disease. J Periodontol 72:495–505
Hoang AM, Oates TW, Cochran DL (2000) In vitro wound healing responses to enamel matrix derivative. J Periodontol 71:1270–1277
Rincon J, Haase H, Bartold P (2003) Effect of Emdogain on human periodontal fibroblasts in an in vitro wound-healing model. J Periodontal Res 38:290–295
Schwarz F, Rothamel D, Herten M, Sculean A, Scherbaum W, Becker J (2004) Effect of enamel matrix protein derivative on the attachment, proliferation, and viability of human SaOs(2) osteoblasts on titanium implants. Clin Oral Investig 8:165–171
Takayama T, Suzuki N, Narukawa M, Tokunaga T, Otsuka K, Ito K (2005) Enamel matrix derivative stimulates core binding factor alpha1/Runt-related transcription factor-2 expression via activation of Smad1 in C2C12 cells. J Periodontol 76:244–249
Zeldich E, Koren R, Nemcovsky C, Weinreb M (2007) Enamel matrix derivative stimulates human gingival fibroblast proliferation via ERK. J Dent Res 86:41–46
Agarwal S, Long P, Seyedain A, Piesco N, Shree A, Gassner R (2003) A central role for the nuclear factor-kappaB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J 17:899–901
Johnson DL, Carnes D, Steffensen B, Cochran DL (2009) Cellular effects of enamel matrix derivative are associated with different molecular weight fractions following separation by size-exclusion chromatography. J Periodontol 80:648–656
Suzuki S, Nagano T, Yamakoshi Y, Gomi K, Arai T, Fukae M, Katagiri T, Oida S (2005) Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-{beta}. J Dent Res 84:510–514
Okubo K, Kobayashi M, Takiguchi T, Takada T, Ohazama A, Okamatsu Y, Hasegawa K (2003) Participation of endogenous IGF-I and TGF-beta 1 with enamel matrix derivative-stimulated cell growth in human periodontal ligament cells. J Periodontal Res 38:1–9
Hirano T (2010) Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir. Proc Jpn Acad B Phys Biol Sci 86:717–730
Blanchard F, Duplomb L, Baud’huin M, Brounais B (2009) The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev 20:19–28
Gaffen SL, Hajishengallis G (2008) A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res 87:817–828
Acknowledgments
This study was supported by a grant from the German Research Foundation (Clinical Research Unit 208/TP4) and the Medical Faculty of the University of Bonn. We would like to thank Marcel Drolshagen, Katharina Reifenrath, and Susanne Reimann for their great support.
Conflicts of interest
EMD was provided by Straumann AG (Switzerland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nokhbehsaim, M., Deschner, B., Winter, J. et al. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1β in vitro. Clin Oral Invest 16, 275–283 (2012). https://doi.org/10.1007/s00784-010-0505-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0505-8