Abstract
Chronic graft-versus-host disease (cGVHD) is a multi-organ disease that occurs post-hematopoietic stem cell transplantation, with the mouth being one of the most frequently affected organs. In 2009, the German–Austrian–Swiss working party on bone marrow and blood stem cell transplantation held a consensus conference to define clinical management of cGVHD. The consensus conference aimed to summarize the literature on diagnosis and topical treatment options for oral cGVHD and to provide recommendations for clinical practice, including routine dental and oral care as well as monitoring for secondary malignancies and bisphophonate-induced osteonecrosis of the jaw.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral care is an important component in the management of chronic graft-versus-host disease (cGVHD). In this article, we provide background information about oral cGVHD, focusing on treatment and supportive care. Our goal is to inform oral care providers about current knowledge of cGVHD therapy with particular emphasis on recent progress. This literature review was presented as part of the 2009 Consensus Conference on clinical practice in chronic GVHD held in Regensburg, Germany, which summarized the currently available evidence for diagnosis, first-line, second-line, and topical treatments and provided practical guidelines for the use of treatment modalities.
The Consensus Conference was organized under the auspices of the German working group on bone marrow and blood stem cell transplantation (DAG-KBT) and the German Society of Hematology and Oncology (DGHO), the Austrian Stem Cell Transplant Working Group of the Austrian Society of Hematology and Oncology, the Swiss Blood Stem Cell Transplantation Group (SBST), and the German–Austrian Paediatric Working Group on SCT (PÄD-AG-KBT).
Methods
Guideline development based on evidence
The evaluation of evidence and the subsequent recommendations were graded according to the publication by Couriel et al. [1]. Since evidence for the majority of treatment options in cGVHD is sparse and primarily category C (optional use), additional subgroups are specified in Tables 1 and 2 to indicate the appropriate treatment contexts and types of available evidence. Strength of recommendation and evidence levels were first rated by an expert panel and subsequently reviewed by all participants in the consensus process.
Following the background summary about cGVHD (sections “Chronic GVHD” to “Treatment of Mucosal cGVHD in the Oral Cavity”), the guidelines for topical treatment of mucosal oral cGVHD (sections “Topical Treatment of Oral Mucosal cGVHD” to “Topical Supportive Therapy for Mucosal cGVHD”), salivary gland cGVHD (sections “Treatment for Salivary Gland Oral cGVHD” to “Supportive Therapy for Salivary Gland cGVHD”), scleroderma-like oral cGVHD (section “Treatment for Scleroderma-Like Oral cGVHD (Fibrosis)”), and for dental and oral care (sections “Dental Health Care” to “Secondary Malignancies”) are presented.
Panel composition
Our panel consisted of five cGVHD-involved health care professionals, including an oral medicine specialist, a dentist, two hematologists, and an epidemiologist.
Conflict of interest and financial disclosure
The costs for the workshop and administrative assistance in guideline preparations were paid from an educational grant provided by the University of Regensburg. In addition, the conference was supported by the Jose Carreras Foundation project “Competence center GvHD Regensburg.” No representatives from any of the companies providing grants attended the expert panel. Each panel member completed a conflict of interest disclosure form, revealing all relationships with pharmaceutical companies that could be affected by the development and publication of these guidelines.
Chronic GVHD
Chronic GVHD is a major complication of allogeneic hematopoietic cell transplantation (HCT) with a prevalence that varies from 25% to 80% in long-term survivors; 5-year survival rates are as low as 40% for patients with severe multisystem cGVHD [2, 3].
Chronic GVHD has symptoms resembling autoimmune and other immunologic disorders, such as scleroderma, Sjögren syndrome, primary biliary cirrhosis, wasting syndrome, bronchiolitis obliterans, immune cytopenias, and chronic immunodeficiency.
Symptoms usually present within 2 years after allogeneic HCT and are often preceded by a history of acute GVHD. Manifestations of cGVHD may be restricted to one single organ or may be widespread. Chronic GVHD can lead to debilitating consequences, e.g., joint contractures, loss of sight, end-stage lung disease, or mortality resulting from recurrent or life-threatening infections due to profound chronic immunosuppression [4].
The pathogenesis of cGVHD is poorly understood [4, 5]. Donor-derived immunocompetent T cells have been shown to react against tissue after allo-HCT, directly or through exaggerated inflammatory responses [6, 7]. Current concepts about the pathogenesis of cGVHD include the persistence of alloreactive T cells, a Th1–Th2 shift of the cellular immune response (cGVHD seems to be mediated prominently by the Th2 cytokine response), defective peripheral and central tolerance mechanisms (i.e., failure of control by regulatory T cells and/or impaired negative selection of T cells in the thymus), replacement of antigen presenting cells (APC) of the host by APCs of the donor leading to indirect antigen presentation of allo-antigens, an increasing role of B cells producing auto- and allo-antibodies against the host and nonspecific mechanisms of chronic inflammation leading to fibrosis of involved organs [8]. Recently, an innovative study suggested a model for cGVHD pathogenesis in which the local production of type I interferon (IFN) by plasmacytoid dendritic cells plays a central role. Secretion of type I IFN by activated plasmacytoid dendritic cells induces production of IL-15. Type I IFN and IL-15 activate T cells that manifest the receptor for MIG (monokine induced by gamma-interferon), named CXCR3. Alloreactive CXCR3 T cells migrate into oral mucosal tissues due to chemotaxis to MIG. These T cells enter the epithelial layer and mediate keratinocyte apoptosis. Sustained activation by cytokines secreted by T effector cells may lead to perpetuation of the alloimmune process [9].
Oral cGVHD
The oral cavity is the most frequent site of involvement for bone marrow transplantation and the second most frequently involved site for peripheral blood stem cell transplantation [10]. Chronic GVHD may involve various oral tissues (Table 3). Recognition of oral cGVHD changes may contribute to improved post-HCT medical management, particularly when the only site or the worst site of cGVHD involvement is in the oral tissues.
Clinically, oral cGVHD is characterized by mucosal hyperkeratotic lesions, erythema and inflammation, atrophy, pseudomembranous ulcerations, fibrosis, salivary gland dysfunction, and taste disorders in patterns reminiscent of autoimmune disorders, such as lichen planus, lupus, systemic sclerosis, and Sjögren-like syndrome [3, 11–13].
The Sjögren-like syndrome of cGVHD is characterized by hyposalivation (objectively measurable reduction of saliva secretion) and xerostomia (subjective feeling of dry mouth) due to progressive salivary gland atrophy [11]. Consequences of cGVHD-induced salivary injury include reductions in related salivary functions, such as protection against infection and mechanical and chemical epithelial injuries. Likewise, remineralization of teeth is impaired, and this may lead to dental caries (Table 4). Further consequences are limitations in verbal communication, nutrition, and soft tissue repair [14]. Therefore, cGVHD of salivary glands may cause significant suffering.
Indirectly, mucosal cGVHD can result in damage to the teeth, such as cervical caries, [15] and to periodontal tissues, such as severe gingival recession [16] (Table 4).
Scleroderma-like cGVHD may involve orofacial tissues. Fibrosis and limitation of mouth opening was noted in 73% of patients with and in 26% of patients without cGVHD [11]. These symptoms may result in reduced tongue mobility, impaired speech, and eating difficulties [17]. Typical “purse-string” deformities around the oral cavity may also be present [11].
Chronic GVHD is associated with secondary malignancies, particularly squamous cell carcinomas (SCC) of the oral cavity [18, 19]. In allogeneic HCT recipients, almost all patients experience immune dysregulation and T-cell dysfunction after grafting, especially during the first year. Malignant tumor cells may develop and proliferate because of the toxicity of the conditioning regimen, prolonged immunosuppression, the failure of immune surveillance mechanisms, the lack of T cell-mediated regulatory suppression that normally controls the proliferation of B lymphocytes, or ongoing inflammatory irritation from cGVHD [19, 20].
Viral (mainly, herpes simplex virus), fungal (mainly Candida), and bacterial infections of the oral mucosa are frequently superimposed in patients with cGVHD. This may be in part due to the dryness and immunosuppression. Appropriate diagnostic procedures should be performed to differentiate between oral cGVHD and other mucosal conditions.
Chronic GVHD has additional consequences for oral tissues (Table 4). The signs detailed in Table 4 are associated with symptoms of pain, sensitivity, and dryness to various extents. The indirect effect of oral cGVHD has a tremendous impact on patients’ quality of life. Practical implications will be specified as part of the review about oral care in cGVHD patients below.
In pediatric patients clinical features of oral cGVHD are similar to adults, but children rarely report dry mouth, taste alteration, and difficulties of swallowing. [21]. It should be kept in mind that reduction of oral intake or the need of increased drinking during eating can often be the only symptom. Therefore, parents and patients have to be asked specific questions on symptoms relevant to oral cGVHD.
Clinical tools for the diagnosing and scoring of oral cGVHD
For correct diagnosis, the National Institute of Health (NIH) divided signs and symptoms of oral cGVHD into diagnostic and distinctive signs [4]. Diagnostic signs are sufficient to establish the diagnosis of cGVHD and include lichen planus features, hyperkeratotic plaques, and restriction of mouth opening by skin sclerosis. Distinctive signs support the diagnosis of cGVHD, but the presence of such signs alone is insufficient to establish the diagnosis of cGVHD. Distinctive signs include xerostomia (defined by the NIH as dryness), mucoceles, mucosal atrophy, pseudomembranes, and ulcers. In case of the presence of distinctive signs only, the diagnosis can be supported by histologic, radiologic, or laboratory evidence of GVHD in other organs. If diagnosis of cGVHD is confirmed at other affected organs, a biopsy from the oral mucosa is not necessary for the diagnosis of oral cGVHD. A biopsy is suggested in patients without any diagnostic oral signs and lack of additional manifestations of cGVHD and recommended if oral malignancy is suspected. Oral mucosal cGVHD is histologically characterized by “dyskeratotic” epithelial cells, apoptosis and lichenoid inflammatory infiltrates under the basement membrane. Immunohistopathologically, the subepithelial infiltrate in oral cGVHD consists predominantly of CD3+ T cells (mostly CD3+CD8+) and CD68+ cells of macrophage/dendritic origin [9]. Salivary gland changes are histologically characterized by the presence of lymphocytic infiltration (slight predominance of CD8 versus CD4) of the glandular parenchyma and periductal tissue. With progression of the salivary cGVHD, acinar destruction and fibrosis can be observed.
For activity assessment and response criteria, the NIH Consensus Development Project defined four different types of manifestations of oral cGVHD that should be used for scoring the severity of oral cGVHD (Fig. 1) [22]: lichen planus-like lesions, erythema, ulcerations, and mucoceles. Validation tests for this scale showed its applicability, although some practice is required for its proper use. Furthermore, some refinements have been suggested [23, 24].
NIH scale for response assessment [22]
The NIH Consensus Development Project also recommended collection of patient-reported measures. Patients score their oral pain, dryness (subjective decrease in oral wetness), and sensitivity (irritation resulting from normally tolerated spices, foods, liquids, or flavors) on a 0-to-10 scale. The cGVHD symptom scale also contains a subscale for the mouth.
Treatment of mucosal cGVHD in the oral cavity
Oral cGVHD management focuses primarily on ameliorating symptoms (for instance pain, sensitivity, and oral dryness), maintaining oral function and restoring mucosal integrity by treating even minimally symptomatic ulcerative lesions [3].
Because cGVHD often affects multiple organs, systemic treatment is indicated in patients with multi-organ involvement. Unfortunately, despite growing significance, only a few well-designed controlled trials of systemic treatments for cGVHD have assessed oral outcomes [25]. Oral cGVHD, however, is often refractory to such systemic therapies; thus, complementary topical treatment is required. If cGVHD only affects the oral mucosa, topical treatment may prevent the severe side effects associated with systemic treatments.
Topical treatment of oral mucosal cGVHD
In general, topical steroid preparations are the mainstay of local treatment for cGVHD, although these drugs are not specifically approved for this indication. Other topical non-steroidal immunomodulators and local oral modalities have also been used for the treatment of cGVHD (Table 5). Patient instruction should be emphasized as many of these drugs are not primarily designed for oral use and swallowing them may cause unwanted systemic effects. Each of these agents will be detailed below. It is noteworthy mentioning that none of the treatment modalities discussed in this paper belongs to recommendation levels A or B.
Topical symptomatic management of oral discomfort and pain are complementary to therapeutic treatment. Individually designed preventive and symptomatic treatment of oral symptoms is important. Topical symptomatic treatment mostly consists of local anesthetics although other modalities have been suggested as well.
Oral discomfort may be alleviated by treating hyposalivation. Good basic oral care is important to minimize all local factors that could aggravate or trigger oral symptoms of cGVHD. Infections arising from the teeth and mucosa should be prevented as they present significant danger to patients who are on systemic immunosuppressive therapy.
The application of topical therapy in children may require parents’ assistance to improve compliance. An additional aspect of topical treatment in children is the risk of reaching significant systemic drug levels in small infants receiving topical treatment with steroids or CNIs. These aspects will be detailed below.
Topical steroids
This section discusses steroids commonly used for the treatment of oral cGVHD. Although they are not specifically approved for cGVHD, these steroids have been adopted for use in cGVHD based on their well-accepted use for other oral mucosal conditions, particularly for oral lichen planus. Most studies evaluating steroids for the treatment of oral cGVHD are retrospective evaluations or small uncontrolled clinical trials. Budesonide, dexamethasone, triamcinolone acetonide, fluocinonide, clobetasol, betamethasone dipropionate, and prednisolone will be reviewed. Additional topical steroids suggested in the literature are beclomethasone, fluticasone, and halobetasol [3]. Since topical steroids increase the risk of fungal overgrowth, antifungal prophylaxis should be added, preferably using topical non-absorbable preparations.
Budesonide (C-1 III-1)
Budesonide is a corticosteroid with low bioavailability when absorbed through the oral mucosa, which minimizes the risk for systemic side effects. Elad et al. observed a high response rate with budesonide [27]. An early response noted within the first 2 to 3 weeks of treatment was enhanced by cumulative effects during the first month of treatment [27]. Sari et al. published a retrospective study comparing treatment results of topical budesonide mouthwash compared to no topical treatment. In this study, both groups were additionally treated with systemic therapy consisting of combined prednisone and cyclosporine. The overall oral response rate was 83% for the budesonide group versus 36% for the non-budesonide group, a statistically significant difference [28]. In both the Elad and Sari studies, the budesonide dosage was 3 mg dissolved in 5–10 ml saline taken three to four times per day. Pharmacokinetics studies showed that only 2% of a buccal dose of budesonide achieved systemic circulation in healthy individuals and 10% of the dose was absorbed in people with oral cGVHD, probably because of alterations in drug uptake and metabolization [29]. Therefore, no new signs of risk were associated with the buccal administration of budesonide when taking the known safety profile of oral budesonide into consideration.
A phase II trial evaluating the efficacy of oral budesonide has just been completed, and final results are pending. A randomized double blind phase III trial evaluating the efficacy of budesonide in an effervescent tablet formulation in oral manifestations of cGVHD is currently ongoing.
Dexamethasone (C-1 III-2)
Topical dexamethasone was evaluated in a retrospective study of 16 patients with oral manifestations of cGVHD [30]. Treatment consisted of 0.1 mg/ml dexamethasone mouth wash four times a day in combination with antifungal prophylaxis. The majority of patients showed complete or partial remission, demonstrating the efficacy of topical dexamethasone for treating oral manifestations of cGVHD with only few side effects. The use of higher concentrations of dexamethasone (0.4 mg/ml) has also been reported [3]. Currently, a phase II trial headed by the Dana–Farber Cancer Institute (NCT00686855) compares topical dexamethasone with topical tacrolimus.
Topical triamcinolone fluocinonide, clobetasol, betamethasone dipropionate, and prednisolone (C-1 III-3)
Topical preparations of other steroids have been developed and approved for treatment of non-infectious inflammatory conditions of the oral mucosa, including triamcinolone, fluocinonide, clobetasol, betamethasone, and prednisolone. Such preparations include gels, creams, ointments, and powders [31]. These agents, which should be applied to the affected areas of the oral mucosa one to two times per day, have been frequently used for the treatment of oral manifestations of cGVHD although their effectiveness has not yet been formally evaluated. Betamethasone rinse was reported as an effective treatment for oral cGVHD in combination with systemic steroids in one case; thus, the evidence for its efficacy is sparse [32].
Topical tacrolimus (C-2 III-1)
Since tacrolimus has been successfully used in the systemic prevention and treatment of GVHD, it has been successfully applied in the topical treatment of oral manifestations of cGVHD. Eckhardt et al. reported three patients with refractory oral manifestations responding to the oral application of a skin ointment containing tacrolimus [33]. Fricain et al. showed long-term efficacy of topical tacrolimus in three patients with oral lesions of chronic GVHD [34]. Albert et al. investigated the safety and efficacy of topical tacrolimus ointment in children with oral cGVHD (n = 6) [35]. Tacrolimus ointment (0.1%) applied twice daily was well tolerated. Tacrolimus was systemically absorbed, and a complete response or a very good partial response was observed in four patients. Sanchez et al. topically applied 0.1% tacrolimus ointment three times a day for 2 months, which resulted in the complete remission of oral lesions [36]. Tacrolimus plasma levels dramatically increase after an oral topical application [37]. Schlaak et al. used topical tacrolimus to enhance the treatment with extracorporeal photopheresis (ECP) in three patients, which resulted in improvement within 2 months. However, this combined systemic and topical treatment makes it impossible to assess the therapeutic effect of topical tacrolimus [38]. Currently, topical tacrolimus and topical dexamethasone are being compared in a randomized phase II trial (NCT00686855). Since tacrolimus paste has been originally developed for application to the skin, different formulations of tacrolimus are used for oral treatment (mouthwash 0.1% tacrolimus). One cautionary note is that the Food and Drug Administration recently issued a health advisory about increased cancer risk associated with topical tacrolimus treatment of the skin. However, there is no good evidence addressing the malignant potential following topical oral application.
Topical cyclosporine (C-2 III-1)
Topical cyclosporine mouth rinses are frequently used for treating oral manifestations of cGVHD. Epstein et al. retrospectively analyzed the topical use of cyclosporine mouthwash in 11 patients who had failed prior treatment of cGVHD. Seven out of 11 patients responded to treatment [39]. Another trial evaluated the use of cyclosporine administered in an adhesive hydroxypropyl cellulose base in patients with resistant oral lichenoid reactions [40]. In this study, signs and symptoms of ulcerative oral GVHD abated by more than 50% in three of four patients additionally treated with topical cyclosporine. Topical use of cyclosporine may represent a useful adjunctive approach in the management of oral lichenoid reactions although additional research is needed, particularly with regard to dose escalation and placebo-controlled studies. It should be noted that as with tacrolimus, cyclosporine has been associated with an increased risk of secondary neoplasm [19, 41]. However, there is no good evidence addressing the malignant potential following topical oral cyclosporine.
Oral phototherapy: psoralen sensitizers with ultra-violet type A, ultra-violet type B, or low level laser therapy (C-2 III-1)
Phototherapy refers to both psoralen sensitizers with ultra-violet type A (PUVA) and ultra-violet type B (UVB). The positive results of phototherapy for cutaneous cGVHD led to an evaluation of the efficacy of intraoral PUVA. In a retrospective analysis, seven patients with oral manifestations of resistant cGVHD received intraoral PUVA. Methoxypsoralen was given at a dosage of 0.3 mg/kg 1 h before ultra-violet type A (UVA) exposure. A glass fiber extension of an UVA source was used for manual intraoral application of 0.5 J/cm2. Treatment was given three to four times per week, and the UVA dose was raised by 0.5 J/cm2 when tolerated. Most patients showed complete or partial improvement [30]. The efficacy of intraoral PUVA was confirmed by two case reports [42, 43]. Atkinson et al. investigated PUVA irradiation to the mouth with 0.75 J/cm2 daily four times per week in two patients. A higher dose (0.6 mg/kg) of the photosensitizer 8-methoxypsoralen was given orally. Each patient improved considerably so that the dosage of conventional immunosuppression could be reduced. No flare was observed 1 month after the end of treatment. PUVA irradiation is a useful therapeutic adjunct in cGVHD that affects the mouth, conferring a steroid or cyclosporine sparing effect [44]. Elad et al. reported the efficacy of intraoral UVB, which does not require exposure to a photosensitizing agent, in two patients [45]. Low level laser therapy may be an additional therapy for oral manifestations of cGVHD as reported for skin cGVHD [46]. Another use of light therapy in cGVHD is ECP, which has shown to be effective in oral cGVHD [47]. Yet, ECP is not considered a topical treatment, and thus, it is not within the scope of this review.
Combination protocol (C-2 III-2)
A combination of dexamethasone rinse (0.5 mg/5 ml) and tacrolimus solution (0.5 mg/5 ml) has been studied [48]. Patients were instructed to rinse with both solutions at the same time, using a total of 5.0 ml (2.5 ml dexamethasone, 2.5 ml tacrolimus), for 5 min before expectorating. Patients were allowed to rinse up to four times a day based on severity of symptoms, with most rinsing two to three times a day. The records of 14 patients with oral cGVHD treated with combination protocol were reviewed retrospectively. Pre-to-post treatment changes in subjective and objective measures were evaluated using the NIH scale [22]. Combined topical dexamethasone/tacrolimus therapy appears to be effective in reducing symptoms attributable to oral cGVHD. In addition, it improved the lichenoid scores significantly but did not improve the erythema and ulceration components of oral cGVHD.
Topical azathioprine (C-3 III-2)
Topical azathioprine rinse and gel (5 mg/cm3) were evaluated in a small open-label study in resistant oral cGVHD [49]. Six patients were included, five of whom received systemic therapy at the time of initiation of topical azathioprine. The mean response to topical azathioprine was measured as decrease in surface area ulceration and severity of erythema. Ulcers improved by 58%, erythema by 55%, and pain was reduced by 63%. Systemic azathioprine has been associated with worse survival in systemic application and with oral malignancies [50]. It is difficult to dissect whether the carcinogenic risk associated with azathioprine results from the co-existence of cGVHD or whether oral cancer is a direct result of systemic treatment with azathioprine [51]. The malignant potential of topical azathioprine application is unknown.
Topical thalidomide (C-4)
Topical thalidomide as ointment and rinse is currently being tested in patients with oral cGVHD at the National Institutes of Health (NCT00075023).
Topical supportive therapy for mucosal cGVHD
Supportive oral care of mucosal cGVHD has several goals, mainly relieving pain and discomfort. Additional aspects of supportive oral care are assessment tools for oral pain, xerostomia treatment, infection prevention, and oral care hygiene (see below “Dental Health Care” section).
Local anesthetics (C-1 III-3)
The most commonly used pharmacology agents to relieve oral mucosal pain are topical local anesthetics including lidocaine, tetracaine, and benzocaine [31]. These drugs are available as gels and sprays or prescribed as a mouthwash. None of these drugs has ever been specifically approved for oral cGVHD. Their accepted palliative potential is based on experiences with other oral diseases. Oral viscous lidocaine (2%) is the most popular preparation. It should be kept in mind that lidocaine may diminish taste perception which may compromise food intake. Likewise lidocaine may reduce the gag reflex which may compromise swallowing, particularly in children. Rarely, it may result in a burning sensation. Toxic reactions (muscle cramps, dysrhythmia, hypotonic, and allergic reactions), associated with an accidental overdose (over 240 ml/day), resulted in elevated serum lidocaine concentrations [52]. However, a pharmacokinetics study of lidocaine mouthwash in patients suffering from severe oral mucositis showed a very wide safety range [53].
Additional drugs with anesthetic effects are dyclonine, diphenhydramine, and doxepin [31]. No data are available about their benefit in oral cGVHD. There is an anecdotal report of topical loperamide use, a mu-opioid agonist, which was successful in oral cGVHD [54].
CO2-laser (C-2 III–2)
Elad et al. reported the benefits of CO2 laser treatment for pain control in severe oral cGVHD [55]. The CO2 laser was applied to mucosal lesions using 1 W for 2–3 s/1 mm2. This treatment resulted in a consistent and significant decrease in pain, measured by means of a standard visual analog scale. Although the patient sample was small, these results suggest that the CO2 laser may be used for the alleviation of pain in oral cGVHD [55].
Treatment for salivary gland oral cGVHD
Hyposalivation due to cGVHD is an important cause of oral morbidity after HCT. Singhal et al. investigated the improvement of salivation after pilocarpine application. Pilocarpine, a parasympathomimetic agent with predominantly muscarinic activity, was given orally to 13 patients with moderate (n = 6) or severe (n = 7) hyposalivation due to cGVHD. The median duration of 19 courses of therapy was 73 days. Ten patients (77%) noticed significant improvement in salivation and relief of symptoms, which reached its maximum after a median of 46 days. Eating and speaking difficulties were reduced, and a beneficial effect on oral mucosa and teeth could be observed [56]. Adverse effects, such as dizziness, hypotonia, diarrhea, and dyspnea, should be detected and treated at an early stage.
Nagler et al. demonstrated that a 2-week application of oral pilocarpine of 30 mg/day resulted in normalizing the altered salivary biochemical and immunological composition in patients with cGVHD. Oral pilocarpine reduced and normalized elevated Na, Mg, total protein, albumin, EGF, IgG, and IgA concentrations in both resting and stimulated conditions. The ability of oral pilocarpine to normalize and reverse salivary biochemical and immunological alterations induced by cGVHD parallels its known stimulatory effect on salivary flow rates [57]. Yet, these studies were open trials, and the only randomized controlled trial was inconclusive with regard to the efficacy of pilocarpine in cGVHD [58].
Cevimeline is a parasympathetic agent with a similar safety and efficacy profile to pilocarpine [59–62]. Cevimeline has been reported to be effective in the treatment of Sjögren syndrome and in a small-case series of cGVHD patients [63].
Other cholinergic drugs are bethanecol and pyridostigmine; however, no trials investigating these agents as sialogogues in cGVHD have been conducted so far. Similarly, no studies exist about mucolytic agents in patients with cGVHD.
Supportive therapy for salivary gland cGVHD
Artificial saliva [64] (pH neutral, non-demineralizing, and caries-protective by fluoride) or salivary gland stimulation with sugar-free gum can improve xerostomia to additionally facilitate food intake and deglutition [3, 65].
A novel biotechnology, i.e., an electro-stimulator mounted on a removable intraoral appliance, has been recently tested for the palliation of xerostomia [66]. This appliance aims at stimulating salivation by applying electrical excitation to nerves in the area of the lower wisdom tooth next to the lingual nerve. A randomized placebo-controlled study is currently focusing on patients with cGVHD (personal communication by S. Elad).
Treatment for scleroderma-like oral cGVHD (fibrosis)
Information about treatment of perioral cutaneous scleroderma-like involvement and mucosal fibrosis is scarce, and treatment results are often inadequate. First-line treatment is physical therapy with a mouth-opener for a few weeks. In extreme cases, surgery may be required to allow food intake and routine dental care. Clinical experience is based on studies in patients with scleroderma and not limited to oral involvement. Some anti-fibrotic drugs (for example halofuginone) have been evaluated [67]. It is noteworthy that scleroderma-like cutaneous cGVHD may respond to systemic immunosuppression like ECP, rituximab, imatinib, and mTOR inhibitors. However, the effect of these treatment options on the perioral and oral tissues fibrosis was assessed only for ECP [47].
Dental health care
Since oral cGVHD has an indirect effect on dental and periodontal tissues (Table 4), special attention should be given to preventing dental and periodontal complications. The importance and potential therapeutic benefit of maintaining oral care was suggested based on the link between periodontal infection and induction of systemic pro-inflammatory cytokines which may trigger cGVHD [3].
Mucosal cGVHD is also associated with pain, which reduces compliance to oral hygiene protocols, hence increasing the risk of dental decay and periodontal disease. Furthermore, the main effect of cGVHD on salivary glands is hyposalivation, which leads to rampant dental caries.
Therefore, dental health care teams should provide instructions on oral prophylaxis and hygiene and encourage patients to maintain meticulous oral hygiene. Hygiene should preferably start before transplantation and continue after diagnosis of oral cGVHD. Likewise, dental intervention should aim at eliminating active dental or oral infections whenever necessary. Periodontal maintenance should become routine. As part of prophylactic dental treatment, sealing of anatomical dental fissures may prevent dental caries.
It should be noted that long-term effect of hematopoietic stem cell transplantation in pediatric patients on the dental development may lead to disturbances in dental root development, microdontia, or dental agenesis and malocclusions [68, 69]. These developmental disturbances may further complicate the dental treatment [70]. Often such dental disturbances lead to malocclusions which require orthodontic treatment. Orthodontic appliances frequently cause trauma to the oral mucosa which may aggravate oral cGVHD. Therefore, orthodontic appliance-induced trauma should be ruled out, and removal of the orthodontic appliance should be considered.
Topical fluorides should be applied for stabilizing incipient caries and chlorhexidine solutions may be applied to reduce the bacterial load in the oral cavity [3, 64]. Several new remineralizing agents are available today. Some agents are based on calcium-phosphate solutions, others on casein protein. These products are indicated for patients with a dry mouth; however, these agents have never been specifically evaluated for patients with salivary glands cGVHD. Finally, basic oral care should be augmented with diet modifications, particularly with a reduced sugar intake [3].
In patients with fibrosis of the oral tissues, restriction of mouth opening limits access for dental intervention. In severe cases, dental treatment may become extremely complicated.
Most dental treatments are postponed until mucosal cGVHD is ameliorated. When dental treatment becomes possible, it should be adjusted to the systemic medical condition. Normal counts of white blood cells and platelets should be confirmed. If the patient is neutropenic or thrombocytopenic or both, dental treatment should be adjusted accordingly [71].
The question of the necessity of antibiotics in patients with cGVHD has been discussed in the literature although no specific data are available [71]. Because of the lack of a clear link between dental procedure-related bacteremia and catheter infection, no guideline routinely recommends antibiotics before dental treatments. The current American Heart Association protocol for the prevention of infective endocarditis after oral procedures can be used to identify the few patients requiring antibiotics and the antibiotic protocol to be used [72]. Antibiotic prophylaxis before invasive oral procedures should be recommended for patients with immunosuppression. In contrast, prolonged steroid treatment is not an automatic indication for antibiotic prophylaxis before dental treatment [73].
Bisphosphonate-related osteonecrosis of the jaw
As patients with cGVHD are often treated with long-term steroids, they may develop osteopenia or osteoporosis and receive bisphosphonates. Bisphosphonate-related osteonecrosis of the jaws (BRONJ) is a severe oral complication with a significant impact on oral function that may lead to marked maxillofacial mutilation [74]. The most common clinical presentation is pain, swelling, necrotic bone exposure, and secreting fistula. The underlying indication for HCT in most of these BRONJ patients is multiple myeloma. Therefore, the estimated incidence of BRONJ in patients post-HCT is probably similar to the incidence of BRONJ in patients with multiple myeloma reported to be 4.5–15% after intravenous bisphosphonates therapy [75–78]. In Germany, the incidence of BRONJ was 28% in patients treated with bisphosphonates [79].
Several risk factors for BRONJ have been identified. The dominant factor is the intravenous route of administration and longstanding use of bisphosphonates, which led to the publication of preventive protocols. All preventive protocols recommend dental evaluations and treatment before the initiation of intravenous bisphosphonate therapy [80, 81]. Elimination of such dental foci of infection or unrestorable teeth will minimize the need for later surgical interventions, thus minimizing the risk of BRONJ. After the initiation of bisphosphonates, elective dento-alveolar surgery should be avoided by all means [81, 82]. If surgical intervention is unavoidable, it should be preceded by antibiotic prophylaxis [81, 83]. Good surgical technique is important to facilitate repairs. Prevention also includes educational programs to increase awareness for BRONJ among patients and health care providers. A comprehensive review of BRONJ and treatment guidelines is published in the 2009 position paper of the American Association of Oral and Maxillofacial Surgeons [80, 84].
The treatment goals in patients with an established diagnosis of BRONJ are to eliminate pain, control infection, and minimize the progression or occurrence of bone necrosis.
Secondary malignancies
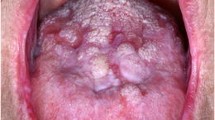
Long-term survivors of HCT have an increased risk of developing secondary malignancies including SCC of the oral mucosa [18, 85]. Curtis and Demarosi reported the increased risk of developing SCC of the oral cavity after HCT to be associated with cGVHD [18, 19]. Therefore, annual screenings for oral malignancies should be a standard for cGVHD patients. The median time from HCT to solid tumor is 7 years. Surveillance includes a thorough examination of the oral cavity and the neck. Photo-documentation, imaging techniques, visualization equipment, or advanced methods for histocytological evaluation (brush biopsy) may be used to facilitate early diagnosis [86–88]. Distinguishing cGVHD from a malignant transformation is a clinical challenge to the examiner (Fig. 2). When a mucosal lesion is suspected to be malignant a biopsy should be taken.
Summary
Oral cGVHD presents differently in various oral tissues. A comprehensive treatment approach for oral cGVHD needs to address mucosa, salivary glands, musculoskeletal tissues, teeth, periodontium, and combined oral function. Current treatment recommendations rely on clinical experience of respected authorities or very small controlled trials rather than rigorously controlled trials. Optimal treatment involves interdisciplinary team work, and the treatment plan should address the type of oral cGVHD manifestation. Topical treatments are of special clinical value if oral mucosal cGVHD is resistant to systemic treatment or if only the mouth is involved. A variety of topical treatments have been reported to have positive effects, and further research is warranted. Dental treatment plan is adjusted to the effects of cGVHD on the oral tissues and the status of immunosuppression.
References
Couriel D, Carpenter PA, Cutler C et al (2006) Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 12(4):375–396
Baird K, Pavletic SZ (2006) Chronic graft versus host disease. Curr Opin Hematol 13:426–435
Schubert MM, Correa ME (2008) Oral graft-versus-host disease. Dent Clin North Am 52(1):79–109
Filipovich AH, Weisdorf D, Pavletic S et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11(12):945–956
Sullivan KM (2004) Graft vs. host disease. In: Blume KG, Forman SJ, Appelbaum FR (eds) Thomas’ hematopoietic cell transplantation, 3rd edn. Blackwell, Malden, pp 635–664
Ferrara JL, Reddy P (2006) Pathophysiology of graft-versus host disease. Semin Hematol 43:3–10
Martin RW 3rd, Farmer ER, Altomonte VL et al (1995) Lichenoid graft-vs-host disease in an autologous bone marrow transplant recipient. Arch Dermatol 131:333–335
Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373(9674):1550–1561
Imanguli MM, Swaim WD, League SC et al (2009) Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 113(15):3620–3630
Flowers ME, Parker PM, Johnston LJ et al (2002) Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood 100(2):415–419
Woo SB, Lee SJ, Schubert MM (1997) Graft-vs.-host disease. Crit Rev Oral Biol Med 8:201–216
Schubert MM, Sullivan KM, Morton TH et al (1984) Oral manifestations of chronic graft-versus-host disease. Arch Intern Med 144:1591–1595
Schubert MM, Sullivan KM (1990) Recognition, incidence, and management of oral graft-versus host disease. NCI Monogr 9:135–143
Fox PC, Van der Ven P, Sonies BC, Weiffenbach JM, Baum BJ (1985) Xerostomia: evaluation of a symptom with increasing significance. J Am Dent Assoc 110:519–525
Porter SR, Scully C, Hegarty AM (2004) An update on the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:28
da Fonseca MA, Murdoch-Kinch CA (2007) Severe gingival recession and early loss of teeth in a child with chronic graft versus host disease: a case report. Spec Care Dentist 27(2):59–63
Lawley TJ, Peck GL, Moutsopoulos HM, Gratwohl AA, Deisseroth AB (1977) Scleroderma, Sjögren’s-like syndrome, and chronic graft-versus-host disease. Ann Intern Med 87:707–709
Curtis RE, Rowlings PA, Deeg HJ et al (1997) Solid cancers after bone marrow transplantation. N Engl J Med 336(13):897–904
Demarosi F, Lodi G, Carrassi A et al (2005) Oral malignancies following HSCT: graft versus host disease and other risk factors. Oral Oncol 41(9):865–877
Otsubo H, Yokoe H, Miya T et al (1997) Gingival squamous cell carcinoma in a patient with chronic graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84(2):171–174
Treister NS, Woo SB, O’Holleran EW, Lehmann LE, Parsons SK, Guinan EC (2005) Oral chronic graft-versus-host disease in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11(9):721–731
Pavletic SZ, Martin P, Lee SJ et al (2006) Measuring therapeutic response in chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 12(3):252–266
Elad S, Zeevi I, Or R et al (2010) Validation of the National Institute of Health (NIH) Scale for oral chronic graft versus host disease (cGVHD). Biol Blood Marrow Transplant 16(1):62–69, Epub 2009 Sep 3
Treister N, Stevenson K, Kim H (2009) Oral chronic graft-versus-host disease scoring using the NIH consensus criteria. Biol Blood Marrow Transplant 16(1):108–114, Epub 2009 Sep 17
Imanguli MM, Pavletic SZ, Guadagnini JP et al (2006) Chronic graft versus host disease of oral mucosa: review of available therapies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(2):175–183
Wolff D, Gerbitz A, Ayuk F et al. (2010) Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. doi:10.1016/j.bbmt.2010.06.015
Elad S, Or R, Garfunkel AA, Shapira MY (2003) Budesonide: a novel treatment for oral chronic graft versus host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95(3):308–311
Sari I, Altuntas F, Kocyigit I et al (2007) The effect of budesonide mouthwash on oral chronic graft versus host disease. Am J Hematol 82(5):349–356
Dilger K, Halter J, Bertz H et al (2009) Pharmacokinetics and pharmacodynamic action of budesonide after buccal administration in healthy subjects and patients with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant 15(3):336–343
Wolff D, Anders V, Corio R et al (2004) Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs.-host disease. Photodermatol Photoimmunol Photomed 20:184–9059
National Cancer Institute (2009) Graft-versus-host disease. www.cancer.gov/cancertopics/pdq/supportivecare/oralcomplications/HealthProfessional/page10
França CM, Domingues-Martins M, Volpe A, Pallotta Filho RS, SoaresdeAraújo N (2001) Severe oral manifestations of chronic graft-vs.-host disease. J Am Dent Assoc 132(8):1124–1127
Eckardt A, Starke O, Stadler M et al (2004) Severe oral chronic graft-versus-host disease following allogeneic bone marrow transplantation: highly effective treatment with topical tacrolimus. Oral Oncol 40(8):811–814
Fricain JC, Sibaud V, Swetyenga N, Tabrizi R, Campana F, Taieb A (2007) Long-term efficacy of topical tacrolimus on oral lesions of chronic graft-versus-host disease. Br J Dermatol 156(3):588–590
Albert MH, Becker B, Schuster FR et al (2007) Oral graft vs. host disease in children—treatment with topical tacrolimus ointment. Pediatr Transplant 11(3):306–311
Sánchez AR, Sheridan PJ, Rogers RS (2004) Successful treatment of oral lichen planus-like chronic graft-versus-host disease with topical tacrolimus: a case report. J Periodontol 75(4):613–619
Conrotto D, Carrozzo M, Ubertalli AV et al (2006) Dramatic increase of tacrolimus plasma concentration during topical treatment for oral graft-versus-host disease. Transplantation 27;82(8):1113–1115, Erratum in: Transplantation. 2007. 83(1):76
Schlaak M, Treudler R, Colsman A, Al-Ali H, Simon JC (2008) Oral graft-versus-host disease: successful therapy with extracorporeal photopheresis and topical tacrolimus. J Eur Acad Dermatol Venereol 22(1):112–113
Epstein JB, Reece DE (1994) Topical cyclosporin A for treatment of oral chronic graft-versus-host disease. Bone Marrow Transplant 13(1):81–86
Epstein JB, Truelove EL (1996) Topical cyclosporine in a bioadhesive for treatment of oral lichenoid mucosal reactions: an open label clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82(5):532–536
Deeg HJ, Socié G, Schoch G et al (1996) Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood 87(1):386–392
Menillo SA, Goldberg SL, McKiernan P, Pecora AL (2001) Intraoral psoralen ultraviolet A irradiation (PUVA) treatment of refractory oral chronic graft-versus-host disease following allogeneic stem cell transplantation. Bone Marrow Transplant 28(8):807–808
Redding SW, Callander NS, Haveman CW, Leonard DL (1998) Treatment of oral chronic graft-versus-host disease with PUVA therapy: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86(2):183–187
Atkinson K, Weller P, Ryman W, Biggs J (1986) PUVA therapy for drug-resistant graft-versus-host disease. Bone Marrow Transplant 1(2):227–236
Elad S, Garfunkel AA, Enk CD et al (1999) Ultraviolet B irradiation: a new therapeutic concept for the management of oral manifestations of graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88(4):444–450
Chor A et al (2004) Successful treatment of oral lesions of chronic lichenoid graft-vs.-host disease by the addition of low-level laser therapy to systemic immunosuppression. Eur J Haematol 72:222–224
Greinix HT, Volc-Platzer B, Rabitsch W et al (1998) Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood 92(9):3098–3104
Mawardi H, Stevenson K, Gokani B et al (2009) Combined topical dexamethasone/tacrolimus therapy for management of oral chronic GVHD. Bone Marrow Transplant 45:1062–1067
Epstein JB, Gorsky M, Epstein MS, Nantel S (2001) Topical azathioprine in the treatment of immune-mediated chronic oral inflammatory conditions: a series of cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91(1):56–61
Sullivan KM, Witherspoon RP, Storb R et al (1988) Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood 72(2):546–554
Curtis RE, Metayer C, Rizzo JD et al (2005) Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood 105(10):3802–3811
Yamashita S, Sato S, Kakiuchi Y et al (2002) Lidocaine toxicity during frequent viscous lidocaine use for painful tongue ulcer. J Pain Symptom Manage 24(5):543–545
Elad S, Cohen G, Zylber-Katz E et al (1999) Systemic absorption of lidocaine after topical application for the treatment of oral mucositis in bone marrow transplantation patients. J Oral Pathol Med 28(4):170–172
Nozaki-Taguchi N, Shutoh M, Shimoyama N (2008) Potential utility of peripherally applied loperamide in oral chronic graft-versus-host disease related pain. Jpn J Clin Oncol 38(12):857–860
Elad S, Or R, Shapira MY et al (2003) CO2 laser in oral graft-versus-host disease: a pilot study. Bone Marrow Transplant 32(10):1031–1034
Singhal S, Powles R, Treleaven J et al (1997) Pilocarpine hydrochloride for symptomatic relief of xerostomia due to chronic graft-versus-host disease or total-body irradiation after bone-marrow transplantation for hematologic malignancies. Leuk Lymphoma 24(5–6):539–543
Nagler RM, Nagler A (2001) The effect of pilocarpine on salivary constituents in patients with chronic graft-versus-host disease. Arch Oral Biol 46(8):689–695
Agha-Hosseini F, Mirzaii-Dizgah I, Ghavamzadeh L et al (2007) Effect of pilocarpine hydrochloride on unstimulated whole saliva flow rate and composition in patients with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant 39:431–434
Grisius MM (2001) Salivary gland dysfunction: a review of systemic therapies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:156–162
Fife RS, Chase WF, Dore RK et al (2002) Cevimeline for the treatment of xerostomia in patients with Sjögren’s syndrome: a randomized trial. Arch Int Med 162:1293–1300
Petrone D, Condemi JJ, Fife R et al (2002) A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum 46(3):748–754
Yasuda H, Niki H (2002) Review of the pharmacological properties and clinical usefulness of muscarinic agonists for xerostomia in patients with Sjögren’s Syndrome. Clin Drug Invest 22(2):67–73
Carpenter PA, Schubert MM, Flowers ME (2006) Cevimeline reduced mouth dryness and increased salivary flow in patients with xerostomia complicating chronic graft-versus-host disease. Biol Blood Marrow Transplant 12(7):792–794
Hahnel S, Behr M, Handel G, Bürgers R (2009) Saliva substitutes for the treatment of radiation-induced xerostomia-a review. Support Care Cancer J 17(11):1331–1343
Majorana A, Schubert MM, Porta F et al (2000) Oral complications of pediatric hematopoietic cell transplantation: diagnosis and management. Support Care Cancer 8(5):353–365
Strietzel FP, Martín-Granizo R, Fedele S et al (2007) Electrostimulating device in the management of xerostomia. Oral Dis 13(2):206–213
Pines M et al (2003) Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol Blood Marrow Transplant 9(7):417–425
Dahllöf G, Jönsson A, Ulmner M, Huggare J (2001) Orthodontic treatment in long-term survivors after pediatric bone marrow transplantation. Am J Orthod Dentofacial Orthop 120(5):459–465
van der Pas-van Voskuilen IG, Veerkamp JS, Raber-Durlacher JE, Bresters D, van Wijk AJ, Barasch A, McNeal S, Gortzak RA (2009) Long-term adverse effects of hematopoietic stem cell transplantation on dental development in children. Support Care Cancer 17(9):1169–1175
American Academy on Pediatric Dentistry Clinical Affairs Committee, American Academy on Pediatric Dentistry Council on Clinical Affairs (2008–2009) Guideline on dental management of pediatric patients receving chemotherapy, hematopoietic cell transplantation, and/or radiation. Pediatr Dent 30(7 Suppl):219–225
Brennan MT, Woo SB, Lockhart PB (2008) Dental treatment planning and management in the patient who has cancer. Dent Clin North Am 52(1):19–37
Wilson W, Taubert KA, Gewitz M et al (2007) Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 138(6):739–745, 747–760
Little JW, Falace DA, Miller CS, Rhodus NL (2008) Dental management of the medically compromised patient, 7th edn. Mosby Elsevier, St. Louis
Ruggiero SL, Drew SJ (2007) Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res 86:1013–1021
Bamias A, Kastritis E, Bamia C et al (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23:8580–8587
Durie BG, Katz M, Crowley J (2005) Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353:99–102
Hoff AO, Toth BB, Altundag K et al (2008) Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23:826–836
Terpos E, Sezer O, Croucher PI et al (2009) The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol 20:1303–1317
Boonyapakorn T, Schirmer I, Reichart PA et al (2008) Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol 44(9):857–869
Ruggiero SL, Dodson TB, Assael LA et al (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67:2–12
Groetz et al (2006) The guidelines of the German society of dentistry and oral medicine (DGZMK). Dtsch Zahnarztl Z 61:510–513
Scully C, Madrid C, Bagan J (2006) Dental endosseous implants in patients on bisphosphonate therapy. Implant Dent 15(3):212–218
Montefusco V, Gay F, Spina F et al (2008) Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma 49(11):2156–2162
Colella G, Campisi G, Fusco V et al (2009) American Association of Oral and Maxillofacial Surgeons position paper: bisphosphonate-related osteonecrosis of the jaws—2009 update: the need to refine the BRONJ definition. J Oral Maxillofac Surg 67(12):2698–2699
Leisenring W, Friedman DL, Flowers ME (2006) Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol 24(7):1119–1126
Trullenque-Eriksson A, Muñoz-Corcuera M, Campo-Trapero J et al (2009) Analysis of new diagnostic methods in suspicious lesions of the oral mucosa. Med Oral Patol Oral Cir Bucal 14(5):E210–E216
Mehrotra R, Hullmann M, Smeets R et al (2009) Oral cytology revisited. J Oral Pathol Med 38(2):161–166
Patton LL, Epstein JB, Kerr AR (2008) Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc 139(7):896–905
Acknowledgments
The authors would like to thank all participating centers at the conference: Rostock, Hamburg, Tuebingen, Ulm, Münster, Hannover, Wiesbaden, Berlin, Freiburg, Vienna, Duesseldorf, Kiel, Oldenburg, Augsburg, Munich, Dresden, Essen, Cologne, Leipzig, Heidelberg, Mainz, Nantes, Paris, Greifswald, Wuerzburg, Jena, Nuernberg, Erlangen, Linz, Regensburg, and Basel.
Conflict of interest
J. Meier, S.Z. Pavletic, H. Greinix, M. Gosau, H. Bertz, and S.J. Lee have no conflict of interests. D. Wolff and S. Elad are paid research consultants for Dr. Falk Pharma GmbH. S. Elad is an unpaid research consultant for Saliwell Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer
1. The Working Group has provided this Consensus Paper on oral cGVHD to inform practitioners, patients, and other interested parties. The Consensus Paper is based on a review of the existing data and the clinical observations of an expert task force.
The Consensus Paper is informational in nature and is not intended to set any standards of care. The Working Group cautions all readers that the strategies described in the Consensus Paper are not intended as practice parameters or guidelines and might not be suitable for every, or any, purpose or application. This Consensus Paper cannot substitute for the individual judgment brought to each clinical situation by the patient’s oral medicine specialist or hematologist. As with all clinical materials, the Consensus Paper reflects the science related to cGVHD at the paper’s development, and it should be used with the clear understanding that continued research and practice could result in new knowledge or recommendations. The Working Group makes no express or implied warranty regarding the accuracy, content, completeness, reliability, operability, or legality of information contained within the Consensus Paper, including, without limitation, the warranties of merchantability, fitness for a particular purpose, and non-infringement of proprietary rights. In no event shall the Consensus Panel be liable to the user of the Position Paper or anyone else for any decision made or action taken in reliance on such information.
2. The Working Group wishes to emphasize that the recommendations in this document represent a wide variety of generally accepted current medical practices. Good clinical judgment and individual circumstances should determine appropriate interventions for specific patients.
Rights and permissions
About this article
Cite this article
Meier, J.KH., Wolff, D., Pavletic, S. et al. Oral chronic graft-versus-host disease: report from the International Consensus Conference on clinical practice in cGVHD. Clin Oral Invest 15, 127–139 (2011). https://doi.org/10.1007/s00784-010-0450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0450-6