Abstract
The aim of the study was to assess the influence of four mandibular protrusion positions, at a constant vertical dimension, on obstructive sleep apnea (OSA). Seventeen OSA patients (49.2 ± 8.5 years) received an adjustable mandibular advancement device (MAD). The patients underwent four polysomnographic recordings with their MAD in situ at, in random order, 0%, 25%, 50%, and 75% of the maximum protrusion. The mean apnea–hypopnea index (AHI) values of the patients differed significantly between the protrusion positions (P < 0.000). The 25% protrusion position resulted in a significant reduction of the AHI with respect to the 0% position, while in the 50% and 75% positions, even lower AHI values were found. The number of side effects was larger starting at the 50% protrusion position. We therefore recommend coming to a weighted compromise between efficacy and side effects by starting a MAD treatment in the 50% protrusion position.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent obstructions of the upper airway, often resulting in oxygen desaturation and arousal from sleep [2]. Continuous positive airway pressure (CPAP) is generally considered the “gold standard” treatment for OSA [10]. However, CPAP is not always tolerated by patients, and it is used less frequently than medically required [21]. As an alternative, oral appliances can be prescribed to prevent upper airway collapse during sleep, especially for mild and moderate cases [18, 26].
In a recent evidence-based review regarding the use of oral appliances in the treatment of OSA, Ferguson et al. [6] indicated that more information is needed about the key design elements of oral appliances that are related to improvements of OSA signs [polysomnographic (PSG) variables] and symptoms (e.g., reports of snoring and excessive daytime sleepiness). According to Ferguson et al. [6], a larger mandibular protrusion will produce a larger decrease in OSA events. However, shortcomings in the available literature and conflicting data do not yet allow definitive conclusions to be drawn [12]. Since the role of vertical opening remains a controversy (i.e., negative or positive influence on the OSA condition [9]), it is of importance to keep this variable constant when investigating the effects of a gradual increase in mandibular advancement on OSA. Therefore, the aim of the present study was to assess the influence of four mandibular protrusion positions, at a constant vertical dimension, on OSA signs and symptoms. The hypothesis thereby was that larger protrusions would yield larger improvements in OSA characteristics.
Materials and methods
Participants
OSA patients were recruited from the Center for Sleep–Wake Disorders at the Slotervaart Medical Center, Amsterdam, The Netherlands. The center's multidisciplinary team consists of a neurologist, ear, nose, and throat (ENT) specialists, pulmonologists, a dentist, a psychologist, and technicians trained in sleep medicine. All patients underwent a thorough medical examination, including a PSG recording (baseline recording; see “Polysomnographic recordings”), as well as an extensive dental examination, including an assessment of the overbite and overjet. The inclusion criteria for participation were age >18 years, an apnea–hypopnea index (AHI) between 5 and 45 events per hour, and two or more OSA symptoms (e.g., reports of snoring and excessive daytime sleepiness [2]). Criteria for exclusion were evidence of respiratory/sleep disorders other than OSA, a body mass index (BMI) > 40, medication usage that could influence respiration or sleep, periodic limb movement disorder, previous treatment with CPAP or a mandibular advancement device (MAD), and reversible morphological upper airway abnormalities (e.g., enlarged tonsils) as assessed by the ENT specialist. Additional dental exclusion criteria were a diagnosis of temporomandibular disorders (based on a functional examination of the masticatory system [4, 19]), untreated periodontal problems, dental pain, and a lack of retention possibilities for the MAD.
The scientific and ethical aspects of the protocol were reviewed and approved by the Medical Ethics Committee of the Slotervaart Medical Center (U/1731/0326).
Mandibular advancement device
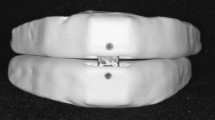
A MAD, set at a constant vertical dimension but with an adjustable protrusion position, was made in the clinic of the Department of Oral Function of ACTA (Fig. 1). This MAD, which has been described in detail previously [1], was slightly modified to make it possible to keep the vertical dimension constant at the different protrusion positions of the MAD. The resulting vertical opening was 6 mm, measured between the first incisors with the MAD in the mouth.
Polysomnographic recordings
The study consisted of six PSG recordings per patient: a baseline recording at the hospital, four ambulatory recordings at home, and a follow-up recording at the hospital. The patients received the MAD on average 5 weeks before the first ambulatory recording at home. The patients were instructed to wear the MAD every night upon delivery.
The baseline and follow-up PSG recordings were obtained at the sleep laboratory of the Center for Sleep–Wake Disorders at the Slotervaart Medical Center, where the recordings took place in a dark hospital room, using Siesta hardware and Pro-Fusion software (Compumedics, Abbotsford, Australia). The baseline recording was also part of the inclusion procedure (see “Participants”). On average, the follow-up recording was obtained 39 weeks after the baseline recording, with the MAD in situ in its most effective protrusion position (i.e., the one that resulted in the lowest ambulatory-established AHI value; see below). At follow-up, a complete response to the MAD treatment was defined as a reduction in AHI to less than five events per hour. A partial response was defined as a reduction of at least 50% in AHI as compared to baseline, while the AHI remained at five or more [11].
In between the two sleep laboratory recordings, the patients underwent four ambulatory PSG recordings at home using Monet hardware and Rembrandt software (Medcare Automation B.V., Amsterdam, The Netherlands). These recordings were made with the MAD in situ at 0%, 25%, 50%, and 75% of the maximum protrusion in a random order, with average interval duration of 3 weeks between subsequent recordings.
For both the sleep laboratory recordings and the ambulatory recordings, the mounting was performed at the hospital by a trained coworker. The channels of the recordings consisted of two electroencephalographic leads (C3-A2; O2-A1), two electrooculographic leads, mental surface electromyography, nasal–oral airflow using a thermistor, oximetry, abdominal and thoracic respiratory effort, body position, electrocardiography, leg electromyography (m. tibialis anterior), and a piezoelectric lead for the detection of snoring vibrations.
Prior to each of the six PSG recordings, all participants received sleep hygiene advice, e.g., avoiding copious meals and beverages 3 h before bedtime and creating a good sleeping environment [24]. Each PSG recording was followed by a visit at ACTA, during which the BMI (kilogram per square meter) was determined and the Epworth Sleepiness Scale (ESS [15]) was completed. The participants were also interviewed (1) about their compliance (percent of nights per week of wearing), (2) about possible side effects (nature and number; determined in an open question) of the MAD during the study period, and (3) about the change (increased, unchanged, or decreased) in snoring intensity based on information they obtained from their bed partner. Finally, the visits at ACTA were used to adjust the protrusion position of the MAD according to the random order of the study protocol.
Data analysis
The PSG recordings were scored manually in 30-s epochs, and standard sleep and respiratory outcome variables were obtained [2, 27]. All data analyses were performed under blind conditions.
The baseline and follow-up PSG recordings were analyzed by a single technician trained in sleep medicine. This technician's intra-observer reliability of AHI scoring was excellent, with an intraclass correlation coefficient (ICC) of 0.99; that of sleep scoring was also excellent, with ICC values ranging from 0.94 to 0.99.
The ambulatory PSG recordings were also analyzed by a single, experienced examiner. This examiner's intra-observer reliability of AHI scoring was excellent, with an ICC of 0.96; that of sleep scoring could be qualified as at least fair to good, with ICC values ranging from 0.63 to 0.94.
Statistics
Paired-sample t tests were used to test the null hypothesis of no differences in sleep laboratory-established respiratory and sleep data, BMI, and ESS between the baseline and follow-up PSG recordings. The Bonferroni–Holm technique was used to correct for multiple comparisons [14].
The ambulatory-established respiratory and sleep data, BMI, and ESS were organized in order of increasing amount of mandibular protrusion. For the effect of protrusion, one-way repeated measures analyses of variance (ANOVA) were used. These analyses were preceded by Mauchly's test of sphericity. Subsequently, the Bonferroni–Holm technique was used to correct for multiple comparisons. Finally, least-significant difference (LSD) pairwise comparisons were used for variables that were significant in the ANOVA.
The self-reported variables “compliance” and “number of side effects” per mandibular protrusion position were analyzed using one-way repeated measures ANOVA, preceded by Mauchly's test of sphericity and followed by LSD pairwise comparisons. To evaluate the association between the self-reported snoring intensity and the four mandibular protrusion positions, a chi-square test was conducted.
All statistical tests were performed with the SPSS 12.0 software package (SPSS Inc., Chicago, IL, USA). Probability levels of P < 0.05 were considered statistically significant.
Results
Initially, 20 OSA patients [13 men; mean ± SD (range) age = 49.5 ± 8.1 (37–66) years] were included in the present study. Two patients (a man and a woman) refused one of the ambulatory PSG recordings, viz., the one with the MAD set at 0% of the maximum protrusion, because they felt distressed (feeling of choking) in that position. Three other patients also reported difficulty with breathing in that position, but they nevertheless completed the entire study. For private reasons that were unrelated to the study, one male patient dropped out of the study after the second ambulatory PSG recording. The three patients who did not complete the entire study were excluded from the data analyses. Thus, a total of 17 patients [12 men; mean ± SD (range) age = 49.2 ± 8.5 (37–66) years] completed the study protocol. The mean maximal mandibular protrusion capacity of these 17 patients was 9.6 (SD, 2.1; range, 6–14) mm. These patients had a mean overjet of 3.2 (SD, 2.0; range, 0–9) mm and a mean overbite of 3.0 (SD, 2.2; range, −2–7) mm.
The mean (±SD) values of the respiratory and sleep parameters, BMI, and ESS of the baseline (no MAD in situ) and follow-up (most effective MAD in situ) PSG recordings at the hospital can be gathered from Table 1. The MAD in its most effective protrusion position (viz., the 25% protrusion position in one patient, the 50% position in six patients, and the 75% position in ten patients) resulted in a significant reduction in AHI with respect to baseline (P = 0.000). At an individual level, 12 of the 17 patients showed a complete response to the most effective MAD, while one other patient responded partially.
The mean (±SD) values of the respiratory and sleep parameters, BMI, and ESS of the four ambulatory PSG recordings can be gathered from Table 2. The mean AHI values of the 17 patients differed significantly between the four mandibular positions (F = 20.403; P < 0.000). The individual results are illustrated in Fig. 2. The AHI values in the 25%, 50%, and 75% positions were significantly lower than in the 0% position (P = 0.000–0.001). Moreover, the AHI values in the 50% and 75% positions were significantly lower than those in the 25% position (P = 0.044 and 0.004, respectively). The sleep variables did not differ significantly between the four ambulatory PSG recordings (F = 0.412–1.439; P = 0.662–0.243). Finally, no differences were found in BMI (F = 0.590; P = 0.625) and ESS (F = 0.464; P = 0.709) between the four recordings.
The percentage of nights per week of wearing the MAD (i.e., the compliance) differed significantly between the various mandibular positions (F = 4.589; P = 0.023). For the 0% protrusion position, the compliance rate was 80.1% (SD, 25.6; range, 10–100); for the 25% position, 94.4% (SD, 6.8; range, 81–100); for the 50% position, 96.4% (SD, 6.3; range, 80–100); and for the 75% position, 90.5% (SD, 17.9; range, 30–100). The MAD set at the 0% protrusion position was worn less frequently than the MAD set at the 25% and 50% positions (P = 0.031 and 0.018, respectively). The reasons for non-compliance were the following: forgotten to insert the MAD before going to sleep, illness (e.g., a cold), and transient side effects (see below).
For the duration of the study, all patients reported (mild) transient side effects due to wearing of the MAD. Apart from hypersalivation and a feeling of a dry mouth (xerostomia), the side effects that might possibly be related to jaw position were the following: tenderness in the masseter muscle region upon awakening (n = 12), sensitive teeth upon awakening (n = 9), uncomfortable wearing (n = 9), feeling of changes in occlusion upon awakening (n = 7), and difficulty swallowing with the MAD in situ (n = 4). The number of reported side effects differed significantly between the various mandibular positions (F = 4.467, P = 0.023); they were reported more frequently with the MAD in situ set at the 50% and 75% protrusion positions than at the 0% and 25% positions (P = 0.011–0.038).
Finally, a decrease in self-reported snoring intensity was found more frequently with the MAD in situ set at the 50% and the 75% protrusion positions (χ 2 = 6.0 and 14.0; P = 0.050 and 0.001, respectively).
Discussion
In this study, the influence of four mandibular protrusion positions, at a constant vertical dimension, on OSA signs and symptoms was assessed. Our hypothesis was that larger protrusions would yield larger improvements in OSA characteristics.
Vertical dimension
Two of the 20 initially recruited patients refused to sleep with the MAD set at 0% of the maximum protrusion because they felt too distressed in that position (i.e., they perceived difficulty in breathing). Three more patients perceived breathing difficulties with the MAD in the 0% position as well, but they were nonetheless willing and able to sleep with the appliance in situ. A possible explanation for these perceived difficulties in breathing is that, in OSA patients, airway patency is reduced during sleep, particularly in the supine position when the jaw is more open and the posterior displacement of the tongue and hyoid bone tends to be more pronounced [3, 23, 29]. Via this mechanism, it could be possible that, following an increase in vertical dimension without protrusion (viz., with an MAD set at the 0% protrusion position), the mandible rotates posteriorly, thereby reducing airway patency even more than while sleeping without an intraoral appliance in situ [8, 22]. However, since only five out of the 20 initially recruited patients experienced breathing difficulties, the validity of this possible mechanism needs to be assessed in future studies.
Mandibular protrusion position
The present results show that the MAD set at 50% and 75% of the maximum protrusion yielded significantly lower values of the AHI than the MAD in the 25% protrusion position. Furthermore, all three protrusion positions resulted in lower AHI values than the 0% position. These findings suggest a dose dependency, i.e., larger protrusions yielded larger improvements in OSA, which is in line with our hypothesis. Tegelberg et al. [30], who reported that the 50% and 75% protrusion positions were equally effective in groups of mild–moderate OSA patients, did not study smaller protrusion positions, so that a dose dependency could not be derived from their study. Other studies, however, do show indications of a dose dependency, thereby corroborating the present findings. For example, Kato et al. [17] found that each 2 mm of mandibular advancement gave a 20% improvement in the number of nocturnal desaturations. Likewise, Walker-Engström et al. [31] observed that the efficacy of MAD treatment in a severe OSA group was significantly higher with a more pronounced advancement compared with less advancement. Thus, a dose dependency seems to be a consistent finding in the literature. The present study, however, is the first to demonstrate this for a wide range of protrusion positions (including the 0% position) at a fixed vertical dimension and by using a random order for the mandibular positions as an important aspect of the study design.
Although self-reported compliance has been suggested to overestimate the actual use of oral appliances, covert compliance monitoring has shown excellent agreement between objective and subjective compliance [20]. Reviewing self-report data, Ferguson et al. [6] found a median use of 77% of nights in the studies with a 1-year follow-up. In the present study, compliance rates of up to 97% were found. The reason for this relatively high compliance, especially for the 25% and 50% protrusion positions, may be the fact that, during the study period, the patients frequently visited ACTA to be interviewed about, among others, the frequency of wearing. This regular contact with the examiner could have motivated the patients to use their MAD on an almost nightly basis. A possible explanation for the finding that MADs set at the 25% and 50% protrusion positions were worn more frequently than those set at the 0% position is that patients were probably less willing to use the 0% protrusion position, among others, due to the perceived difficulties with breathing in that position (see above).
Side effects in relation to MAD usage are frequently reported [12]. These effects are usually described as mild and acceptable; resolution normally occurs within several days or weeks when the appliance is used regularly and its fit adjusted occasionally [6, 12]. In the present study, the patients reported several mild, transient side effects caused by the MAD during the study period, which, in some cases, led to a less frequent use of the appliance. Due to its design, a MAD transmits forces upon the mandible via the dental arches. In addition, the mandibular part of the temporomandibular joint is forced out of its natural resting position during overnight use of the MAD. The nature of the side effects observed in this study, like sensitive teeth, masseter muscle tenderness, and a feeling of changes in occlusion upon awakening, might (at least in part) be due to these mechanisms [7]. Consequently, more side effects could be expected at larger protrusion positions. Indeed, side effects were reported more frequently with the 50% and 75% protrusion positions than with the smaller mandibular protrusions.
Most effective protrusion position
In the present study, the most effective MAD resulted in a decrease in AHI to normal levels (i.e., less than five events per hour) in 12 of the 17 patients. This success rate of approximately 70% is higher than the average success rates of 52% and 54% reported by Ferguson et al. [6] and Hoffstein [13], respectively. A possible reason for the differences in success rates between the present study and previous ones may be sought in methodological disparities, e.g., differences not only in baseline patient characteristics like OSA severity and BMI and in the amount of mandibular protrusion of the MAD but also in the definition of treatment success. In the present study, only objective variables were considered when determining treatment success. However, since patients primarily report to physicians with subjective complaints of OSA, a uniform definition of treatment success that includes not only objective criteria (e.g., AHI and oxygenation) but also subjective symptoms (e.g., self-reports of snoring and sleepiness) needs to be established [6].
Unlike other studies [13], the ESS did not improve with the most effective MAD in situ in the present group of OSA patients. A similar finding of persisting daytime sleepiness was reported in the studies by Engleman et al. [5] and Neill et al. [25]. In the present study, the number of respiratory arousals did reduce significantly with MAD treatment. Since arousals are known to change the sleep architecture and an increase in arousals may yield daytime sleepiness as one of its possible consequences [28], it was assumed that the daytime sleepiness would improve with the reduced number of respiratory arousals. Possibly, there is a delay in the effect of improved sleep architecture on daytime sleepiness. This remains to be studied in future research. Another possible explanation is the relatively low ESS score at baseline of on average 12.2 (out of a maximum possible score of 24). This value is close to the sleepiness threshold of 10, so that there is little left to be gained [16].
In the present study sample, a relatively small protrusion of the mandible (viz., 25% of the maximum protrusion) already resulted in a significant reduction of the AHI with respect to the 0% position, while at the 50% and 75% protrusion positions, even lower AHI values were found, suggesting the existence of a dose dependency. The number of side effects is larger starting at the 50% protrusion position. Within the limitation of the present study, we therefore recommend coming to a weighted compromise between efficacy and side effects by starting a MAD treatment in the 50% protrusion position. Only when this position does not result in an AHI reduction and/or a satisfactory relief of symptoms may more advancement of the mandible to 75% of the maximum protrusion be considered.
References
Aarab G, Lobbezoo F, Wicks DJ, Hamburger HL, Naeije M (2005) Short-term effects of a mandibular advancement device on obstructive sleep apnoea: an open-label pilot trial. J Oral Rehabil 32:564–570
American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Cartwright RD (1984) Effect of sleep position on sleep apnea severity. Sleep 7:110–114
Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6:301–355
Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, Mackay TW, Douglas NJ (2002) Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med 166:855–859
Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W (2006) Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 29:244–262
Fritsch KM, Iseli A, Russi EW, Bloch KE (2001) Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Crit Care Med 164:813–818
Gagnon Y, Mayer P, Morisson F, Rompré PH, Lavigne GJ (2004) Aggravation of respiratory disturbances by the use of an occlusal splint in apneic patients: a pilot study. Int J Prosthodont 17:447–453
George PT (2001) Selecting sleep-disordered-breathing appliances: biomechanical considerations. J Am Dent Assoc 132:339–347
Giles T, Lasserson T, Smith B, White J, Wright J, Cates C (2006) Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev Issue 3:CD001106
Gotsopoulos H, Chen C, Qian J, Cistulli PA (2002) Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 166:743–748
Hoekema A, Stegenga B, de Bont LG (2004) Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea–hypopnea: a systematic review. Crit Rev Oral Biol Med 15:137–155
Hoffstein V (2007) Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath 11:1–22
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Statist 6:65–70
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Johns MW (1993) Daytime sleepiness, snoring and obstructive sleep apnea: the Epworth sleepiness scale. Chest 103:30–36
Kato J, Isono S, Tanaka A, Watanabe T, Araki D, Tanzawa H, Nishino T (2000) Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest 117:1065–1072
Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Owens J, Pancer JP, American Academy of Sleep (2006) Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 29:240–243
Lobbezoo F, van Selms MK, John MT, Huggins K, Ohrbach R, Visscher CM, van der Zaag J, van der Meulen MJ, Naeije M, Dworkin SF (2005) Use of the research diagnostic criteria for temporomandibular disorders for multinational research: translation efforts and reliability assessments in The Netherlands. J Orofac Pain 19:301–308
Lowe AA, Sjöholm TT, Ryan CF, Fleetham JA, Ferguson KA, Remmers JE (2000) Treatment, airway and compliance effects of a titratable oral appliance. Sleep 23:S172–S178
McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ (1999) Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 159:1108–1114
Meurice JC, Marc I, Carrier G, Sériès F (1996) Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med 153:255–259
Miyamoto K, Ozbek MM, Lowe AA, Sjöholm TT, Love LL, Fleetham JA, Ryan CF (1999) Mandibular posture during sleep in patients with obstructive sleep apnoea. Arch Oral Biol 44:657–664
Morin CM (2005) Psychological and behavioral treatments for primary insomnia. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 4th edn. Saunders, Philadelphia, pp 726–737
Neill A, Whyman R, Bannan S, Jeffrey O, Campbell A (2002) Mandibular advancement splint improves indices of obstructive sleep apnoea and snoring but side effects are common. N Z Med J 115:289–292
Ng AT, Gotsopoulos H, Qian J, Cistulli PA (2003) Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med 168:238–241
Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Brain Information Service/Brain Research Institute, UCLA, Los Angeles
Roehrs T, Carskadon MA, Dement WC, Roth T (2005) Daytime sleepiness and alertness. In: Kryger M, Roth T, Dement WC (eds) Principles and practice of sleep medicine. Elsevier, Amsterdam, pp 39–50
Strollo PJ, Atwood CW, Sanders MH (2005) Medical therapy for obstructive sleep apnea–hypopnea syndrome. In: Kryger MH, Roth T, Dement WC (eds) Priniciples and practice of sleep medicine, 4th edn. Saunders, Philadelphia, p 1053
Tegelberg A, Walker-Engström ML, Vestling O, Wilhelmsson B (2003) Two different degrees of mandibular advancement with a dental appliance in treatment of patients with mild to moderate obstructive sleep apnea. Acta Odontol Scand 61:356–362
Walker-Engström ML, Ringqvist I, Vestling O, Wilhelmsson B, Tegelberg A (2003) A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep Breath 7:119–130
Acknowledgments
The authors would like to thank the staff of the Center for Sleep–Wake Disorders of Slotervaart Medical Center in Amsterdam, The Netherlands for their assistance with this work. The Netherlands Institute for Dental Sciences (IOT) supported this work.
Conflict of interest
All authors declare that they have no conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aarab, G., Lobbezoo, F., Hamburger, H.L. et al. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Invest 14, 339–345 (2010). https://doi.org/10.1007/s00784-009-0298-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-009-0298-9