Abstract
Squamous cell carcinoma (SCC) of the oral cavity is an extremely invasive tumour of stratified squamous epithelium that spreads throughout degradation of the basement membrane (BM) and extra-cellular matrix. Oral verrucous carcinoma (VC) is a rare low-grade variant of oral SCC that penetrates into the subepithelial connective tissue. It also has a different clinical behaviour from classical oral SCC. We investigated the immunohistochemical expression of laminin, laminin-5, collagen IV and fibronectin in VC, severe epithelial dysplasia (SED) and SCC in order to analyse if the patter of these molecules expression contributes to the differences in the biological behaviour of these diseases. The staining pattern of laminin was less intensive in SCC compared with SED and VC, and collagen IV expression was increased in VC compared with SED. Discontinuities of laminin, collagen IV and fibronectin were more evident in SED than in VC. This study indicates that VC has a biological behaviour different from SED or SCC, observable by immunohistochemistry in the BM zone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Verrucous carcinoma (VC) of the oral mucosa is an uncommon rare low-grade variant of squamous cell carcinoma (SCC) [14, 31], comprising approximately 2–10% of all primary invasive oral carcinomas [14, 18, 19, 25, 30]. Most affected patients are elderly males, there is a close relationship with tobacco use [6, 18], and lymph node involvement and distant metastases are rare [14]. VC is histologically characterised by parakeratotic and non-dysplastic epithelium, with a high order of epithelial differentiation and only slight mitotic activity and pleomorphism; broad bulbous epithelial ridges push into the connective tissue but the basement membrane (BM) is uninterrupted [9, 31, 32].
In contrast, SCC can invade and cross tissue barriers, and BM loss is a clear hallmark of invasion [1–5, 16, 17]. The tumour cells attach and proteolyse components of the BM and then migrate through [3, 4, 10, 17, 29]. Thus, the BM is a barrier for tumour invasion, and its destruction is the first stage of invasion [4, 25, 30].
While there are several studies analysing the expression of the BM component of SCC, there are only few recent reports on BM in VC [9, 13, 15], although focal destruction has been noted in one older study [23].
The treatment of oral VC remains controversial; when achievable, most investigators have supported surgical excision as the treatment of choice [11, 14, 19, 33], mainly based on effectiveness of control and not on the potential risk of transforming VC, which can sometimes contain elements of SCC [14].
We have therefore examined the immunohistochemical expression of four BM proteins in VC, SCC and in severe epithelial dysplasia (SED), also known as carcinoma in situ or atypical hyperplasia. To the best of our knowledge, these proteins have never been studied in the BM of VC.
Methods
Forty oral biopsy specimens were retrospectively selected from the files of the Biomedical Sciences and Human Oncology, Oral Medicine Section, at the University of Turin, Italy. All the cases were collected from biopsy take between 1993 and 2000.
Haematoxylin and eosin sections of each specimen were evaluated by light microscopy and blindly re-examined by an expert oral pathologist. The study included 20 cases of oral VC, ten cases of invasive well differentiated SCC and ten cases of SED. The grade of epithelial dysplasia and the degree of differentiation of SCC were determined according to standard criteria [21, 27, 28, 31].
Antibodies
Monoclonal antibodies (MAbs) directed against human fibronectin (clone NCL-FIB 568; Novocastra Laboratories LTD), type IV collagen (clone CIV22; Immunotech, Westbrook, USA), human laminin-5 (γ2; clone D4B5; Chemicon International) and rabbit polyclonal antibodies to laminin (cod. 2233PLA, distributed by TEMA Research S.r.l., Italy) were used. The MAbs against fibronectin and laminin-5 were diluted 1:200 for 60 min at 26°C, whereas the MAbs against type IV collagen and the polyclonal antibodies to laminin were diluted 1:20 and 1:600, respectively, for 60 min at 26°C.
Immunohistochemistry
Four-micrometer-thick sections were mounted on glass slides covered with Vectabond™ bonding agent (Vector Laboratories, Burlingame, CA, USA; SP-1800); all the specimens were deparaffinised and rehydrated, and then the tissue sections were immersed in 3% H2O2 for 8 min in order to block endogenous peroxidase activity. For optimal antigen retrieval, the sections were heated with pressure cooker for microwave (15 min at 608 W and then 15 min at 304 W; for fibronectin and type IV collagen) or digested with pepsin 0.4% (lab Vision Corporation) for 60 min at 37°C (for laminin and laminin-5). After washing in phosphate buffered saline (PBS, Oxid), the specimens were incubated with the primary antibodies and washed in PBS, and then we used a Universal DAKO Labelled Streptavidin-Biotin® 2 System, Horseradish Peroxidase (DAKO LSAB® 2 System, HRP; Dako Chem MateTM). The first incubation was with a biotinylated link antibody (containing anti-rabbit and anti-mouse immunoglobulins) for 12 min and then with peroxidase-labelled streptavidin also for 12 min. Staining was completed after an incubation with the substrate chromogen (DAB: 3,3 diaminobenzidine; Zymed Laboratories) for 6 min; the result was a brown-coloured precipitate at the antigen site. All the sections were counterstained, dehydrated, cleared and mounted.
Immunohistochemical evaluation
The intensity and distribution of immunoreactions were examined on the BM of the VC and SED and on the remaining BM and around the cancer cell nests of the SCC. The staining pattern of the four antibodies was evaluated semi quantitatively and graded as follow: +++, continuous linear staining, definitely coloured; ++, linear staining, moderate coloured; +, weak staining; +/−, very weak staining; −, negative staining. The term “discontinuous” (for VC and SED) was used to indicate a fragmented and not always linear staining along the BM, whereas the term “variable” (for the SCC) indicated different staining intensity in the BM in different fields of the same case or destroyed BM in some areas. The distribution of extra-cellular matrix is the around blood vessels, submucosal salivary glands, nerves and fascicles of muscle; these structures were used as internal positive controls.
Statistical analyses
The results were statistically compared using the Fisher’s exact test for qualitative variables and the Mann–Whitney test for the quantitative data. p values less than 0.05 were taken as significant. All analyses were performed using SPSS® software.
Results
The mean age of the unrelated 40 patients (20 men and 20 women) was 67.65 years. The mean for SED was 66.3 years; mean for VC was 65.75 years; and mean for SCC was 70.9 years.
Immunohistochemical findings
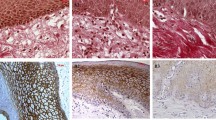
In Table 1 and Fig. 1 are summarised and showed the immunohistochemical findings. The staining pattern of laminin was less defined in SCC compared with SED (p = 0.041) and VC (p = 0.017). Although staining appeared less intensive in SED than VC, the difference was not statistically significant. BM laminin staining was more discontinuous in SED than VC (p = 0.002), and similar results were found for type IV collagen (p = 0.025) and fibronectin (p = 0.03).
Immunoperoxidase staining of ESD (a, c, f), VC (b, d, g) and SCC (e, h). Type IV collagen stained more strongly and was more defined in VC than in ESD (b and a, respectively). The staining pattern of laminin decreased in SCC compared with ESD and VC (e, c and d, respectively). Laminin-5 staining patterns were similar for ESD, VC, SCC (f, g, h). Interestingly, a particularly strong stromal network was seen at the invading front of SCC (h). (Original magnification ×10)
Type IV collagen stained more strongly and was more defined in VC than in SED (p = 0.048), while, between VC and SCC and between SCC and SED, the stain intensity was approximately the same. The variability of stain intensity in BM of SCC is due to a weak or moderate positivity alternates with no stain; probably, the areas with the stain were near the foci of true invasion, in which there was no collagen IV.
Fibronectin staining patterns were similar for all the different lesions, with considerable negative staining in the BM zone. However, the expression of fibronectin was significantly increased in the stromal tissue of 50% of the SCC but only in 10% of cases of VC. Moreover, in some VC and SED, there was localization in and between basal layer cells.
Laminin-5 staining patterns were similar for all the three different lesions. Interestingly, a particularly strong stromal network was seen at the invading front of SCC. Laminin-5 antibody marked some cells in the basal epithelial layer in two specimens of SED and one of VC. Along the BM, laminin-5 was always linear and never discontinuous, contrary to the findings with other antibodies.
Discussion
Extra-cellular matrix (ECM) constitutes an environment of active structural proteins and influence tumour behaviour [26]. Degradation of BM occurs when carcinomas start to invade the stroma, and it has been suggested that this is essential for metastasis [8]. Loss of the components of basement membrane is characteristic in malignant lesions; a possible explanation for this disappearance could be a failure either in the synthesis, secretion or assembly of basement membrane components or an active degradation of BM by tumoral cells. VC has a biological behaviour different from that of conventional carcinomas, and recent data suggest that the less aggressive nature may be connected to its matrix metalloproteinases expression (MMP) profile [15]. The results of the present study indicate dissimilar expression of some proteins of the basement membrane in various conditions. The staining pattern of basement membrane of the VC was more often continuous than in the preneoplastic lesion and was always well expressed, which might explain the low malignant potential and poor ability to invade surrounding tissues. It has been reported that VC share many of the losses of chromosomal regions present in dysplasia and SCC, but these losses accumulate differently and earlier [22]. This difference is also supported by the different expression of laminin-5 in our series, similar to other findings [20, 24]. The finding of laminin-5 expression at sites of severe dysplasia and microinvasion in vivo, as well as in senescent keratinocytes and young wound-edge keratinocytes in culture, already suggested an unexpected connection between a biological response of normal keratinocytes and that of keratinocytes in lesions, making the transition from premalignant dysplasia to invasive carcinoma. The overexpression of laminin-5 in invasively growing cancer cells could point to a role for this molecule in establishing focal adhesion of cancer cells to the ECM during their migration through surrounding tissues [24]. Normally, constituents of laminin-5 are expressed in squamous epithelium and are associated with anchoring filaments. Laminin-5 is an extra-cellular protein that links the basement membrane via integrins to hemidesmosomes. However, this interaction is sensitive to proteolytic cleavage mediated by MMP, which releases domain III of the laminin-5 γ2 chain and subsequently leads to cell motility [20]. We might suppose that laminin-5 is overexpressed only by invasive malignant cells at the invading front of SCC, supporting this hypothesis with the results of Garzino-Demo and co-workers, who showed the increased expression and diffuse distribution of α6, associates with laminin-5, on the cell surface of many cancers [7]. Moreover, it is also significant to consider a possible overexpression of laminin-5 as a result of fragmentation generated by proteolitic matrix degradation during invasion.
Also, the overexpression of fibronectin was totally different in SCC and VC, indicating this molecule as a marker of tumour invasiveness [8, 12, 26].
Our results indicate that the expression of laminin seems to decrease in neoplastic lesions, as previously reported [8, 9, 12]. Generally, the samples of laminin and collagen IV obtained from VC were more clear, continuous and regular than those obtained from SED. Staining for laminin in VC did not show the epithelial basement membrane to be thin, frequently discontinuous and weakly stained as in SCC.
The results of our study indicate that the oral verrucous carcinoma has a biological behaviour, along the basement membrane, different from SED or SCC and which could be more similar to non-malignant hyperplastic lesions. Of course, future studies, for example on the integrin family, which mediate the adhesion phenomena for the basement membrane proteins or on matrix metalloproteinases, which degrade the ECM, may help us towards a better understanding of the behaviour of the basement membrane in oral neoplastic lesions, including VC, in tumor invasion and metastasis. Moreover, in order to confirm our results, further study of the genetic expression of these proteins will be necessary to fully understand the nature of the difference between VC and SCC in order to achieve new treatment strategies and much still remains to be clarified.
However, in conclusion, the BM proteins expression in VC shows a fully development, more intensive than in preneoplastic lesion, while it is poorly organized in SCC; some BM protein, e.g. laminin and type IV collagen, are good markers for BM integrity; fibronectin and laminin-5 are very interesting protein for their peri-tumoral stromal distribution and could play an important role in the mechanism of tumoral infiltration. Further studies about their role in oral lesions will be needed to confirm and explain this controversial point.
References
Al-Rawi NH, Talabani NG (2008) Squamous cell carcinoma of the oral cavity: a case series analysis of clinical presentation and histological grading of 1, 425 cases from Iraq. Clin Oral Investig 12:15–18
Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA (1993) Molecular aspects of tumor cell invasion and metastasis. Cancer 71:1368–1383
Bosman FT, Havenith MG, Visser R, Cleutjens JP (1992) Basement membranes in neoplasia. Prog Histochem Cytochem 24:1–92
d'Ardenne AJ (1989) Use of basement membrane markers in tumour diagnosis. J Clin Pathol 42:449–457
Driemel O, Dahse R, Berndt A, Pistner H, Hakim SG, Zardi L, Reichert TE, Kosmehl H (2007) High-molecular tenascin-C as an indicator of atypical cells in oral brush biopsies. Clin Oral Investig 11:93–99
Eberle C, Phillips JL, Tary P, Menck HR (1997) Quality management in the National Cancer Data Base: a re-abstracting study of the Midwest region. J Regist Manage 24:93–97
Garzino-Demo P, Carrozzo M, Trusolino L et al (1998) Altered expression of alpha 6 integrin subunit in oral squamous cell carcinoma and oral potentially malignant lesions. Oral Oncol 34:204–210
Harada T, Shinohara M, Nakamura S, Oka M (1994) An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Arch 424:257–266
Jiang L, Wang S, Chen X (2001) Immunohistochemical and ultrastructural study of basement membrane in oral verrucous carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi 36:308–310
Kamboi M, Mahajan S (2007) Micronucleus—an upcoming marker of genotoxic damage. Clin Oral Investig 11:121–126
Kang CJ, Chang JT, Chen TM, Chen IH, Liao CT (2003) Surgical treatment of oral verrucous carcinoma. Chang Gung Med J 26:807–812
Kannan S, Balaram P, Chandran GJ et al (1994) Alterations in expression of basement membrane proteins during tumour progression in oral mucosa. Histopathology 24:531–537
Kobayashi H, Sagara J, Masumoto J et al (2003) Shifts in cellular localization of moesin in normal oral epithelium, oral epithelial dysplasia, verrucous carcinoma and oral squamous cell carcinoma. J Oral Pathol & Med 32:344–349
Koch BB, Trask DK, Hoffman HT et al (2001) National survey of head and neck verrucous carcinoma: patterns of presentation, care, and outcome. Cancer 92:110–120
Impola U, Uitto VJ, Hietanen J et al (2004) Differential expression of matrilysin-1 (MMP-7), 922 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol 202:14–22
Leblond CP, Inoue S (1989) Structure, composition, and assembly of basement membrane. Am J Anat 185:367–390
Mauch C, Krieg T, Bauer EA (1994) Role of the extracellular matrix in the degradation of connective tissue. Arch Dermatol Res 287:107–114
Neville BW, Day TA (2002) Oral cancer and precancerous lesions. CA Cancer J Clin 52:195–215
Ogawa A, Fukuta Y, Nakajima T et al (2004) Treatment results of oral verrucous carcinoma and its biological behavior. Oral Oncol 40:793–797
Ono Y, Nakanishi Y, Ino Y et al (1999) Clinocopathologic significance of laminin-5 gamma2 chain expression in squamous cell carcinoma of the tongue: immunohistochemical analysis of 67 lesions. Cancer 85:2315–2321
Pindborg JJ, Reichart PA, Smith CJ, Van der Waal I (1997) Histological typing of cancer and precancer of the oral mucosa. Springer, Berlin
Poh CF, Zhang L, Lam WL et al (2001) A high frequency of allelic loss in oral verrucous lesions may explain malignant risk. Lab Invest 81:629–634
Prioleau PG, Santa Cruz DJ, Meyer JS, Bauer WC (1980) Verrucous carcinoma: a light and electron microscopic, autoradiographic, and immunofluorescence study. Cancer 45:2849–2857
Pyke C, Romer J, Kallunki P et al (1994) The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol 145:782–791
Sakr WA, Zarbo RJ, Jacobs JR, Crissman JD (1987) Distribution of basement membrane in squamous cell carcinoma of the head and neck. Hum Pathol 18:1043–1050
Shinohara M, Nakamura S, Harada T, Shimada M, Oka M (1996) Mode of tumor invasion in oral squamous cell carcinoma: improved grading based on immunohistochemical examination of extracellular matrices. Head Neck 18:153–159
Sobin LH, Wittekind CH (1997) TNM classification of malignant tumours, 5th edn. Wiley-Liss, New York
Speight PM (2007) Update on oral epithelial dysplasia and progression to cancer. Head and Neck Pathol 1:61–66
Timpl R, Brown JC (1996) Supramolecular assembly of basement membranes. Bioessays 18:123–132
Tosios KI, Kapranos N, Papanicolaou SI (1998) Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology 33:261–268
Worl Health Organization Classification of Tumours (2005) Pathology & genetics. Head and neck tumours. International Agency for Research on Cancer (IARC). In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) Head and neck tumours. IARC, Lyon, pp 177–180
Yeh CJ (2003) Treatment of verrucous hyperplasia and verrucous carcinoma by shave excision and simple cryosurgery. Int J Oral Maxillofac Surg 32:280–283
Yoshimura Y, Mishima K, Obara S et al (2001) Treatment modalities for oral verrucous carcinomas and their outcomes: contribution of radiotherapy and chemotherapy. Int J Clin Oncol 6:192–200
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arduino, P.G., Carrozzo, M., Pagano, M. et al. Immunohistochemical expression of basement membrane proteins of verrucous carcinoma of the oral mucosa. Clin Oral Invest 14, 297–302 (2010). https://doi.org/10.1007/s00784-009-0296-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-009-0296-y