Abstract
The aim of the present study was to compare newly formed cementum following different types of regenerative therapy in humans. Eighteen patients, each displaying one advanced intrabony defect around teeth scheduled for extraction, were included in this study. The defects were treated with either guided tissue regeneration (GTR), enamel matrix protein derivative (EMD), EMD plus bioactive glass, bovine-derived xenograft (BDX), BDX plus GTR, or BDX plus EMD. After healing, the teeth were removed together with their surrounding soft and hard tissues. Cellular content, presence of artifactual splits between the new cementum and the old one or the dentin surface, and thickness of the new cementum were evaluated. Irrespective of treatment, the new cementum was of a reparative, cellular, extrinsic and intrinsic fiber type. There were no differences in cementum thickness among treatments. These findings indicate that in humans, (a) the new cementum formed after different types of regenerative therapy was, irrespective of the treatment, of a reparative, cellular, extrinsic and intrinsic fiber type, and (b) the regenerative modality does not seem to influence the type of newly formed cementum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The goal of regenerative periodontal therapy is to reform a tooth’s supporting tissues which have been lost following dental trauma or periodontal disease [15]. Histologically, regenerative periodontal therapy should result in the formation of new cementum, new periodontal ligament, and new alveolar bone [15]. In humans, periodontal regeneration has been shown to occur following the use of intra- or extraoral autografts, demineralized freeze-dried bone allografts, guided tissue regeneration (GTR), bovine-derived xenografts (BDX), enamel matrix protein derivative (EMD), growth factors, and various combinations of these techniques [3, 4, 5, 6, 7, 9, 10, 11, 13, 14, 20, 21, 22, 24, 25, 26, 27, 28, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40].
In spite of the key role that formation of new cementum plays in periodontal regeneration, very little is known about its repair and regeneration in humans [1, 29]. So far, the data regarding new cementum following various regenerative modalities in humans are sparse and controversial [11, 13, 19, 20, 21, 32, 33, 34]. Findings from previous studies have indicated that, in humans, the cementum formed after treatment with various types of bone grafts or GTR is mainly of a cellular type, and artifacts (i.e., splits between the new cementum and the old one or the dentin surface) are often present [3, 4, 9, 11, 19, 32, 34]. An enamel matrix protein derivative (EMD) has also been introduced as a new modality for predictably achieving periodontal regeneration [12].
The rationale for using EMD was based on observations from studies on tooth development which have indicated that enamel matrix proteins (EMP), synthesized and secreted by cells of the Hertwig’s epithelial root sheath, induce the differentiation of dental follicle cells into cementoblasts, which in turn may specifically be responsible for the formation of acellular extrinsic fiber cementum (AEFC), the type which mainly participates in tooth anchorage [12]. In those studies, the newly formed cementum appeared to be similar to AEFC, and artifacts were either not or only very sparsely observed [12, 13]. This lack of artifacts was interpreted as additional evidence of the superior quality of EMD-induced cementum to that formed after other regenerative techniques such as GTR [12]. On the other hand, human histological studies evaluating the healing of intrabony defects following treatment with GTR or EMD have indicated that the cementum formed after both treatments was of a predominantly cellular type, with frequent artifacts [32, 33].
Taken together, the data from human biopsies regarding new cementum after different regenerative modalities are still very controversial. Moreover, to the best of our knowledge, none of the available studies has attempted to evaluate this question systematically. Thus, there are virtually no data from human material attempting to quantify parameters such as cementum thickness, cellular content, and the presence of artifacts between the new cementum and the old one or the dentin surface following different regenerative modalities for intrabony defects. Therefore, the aim of the present study was to shed more light on this subject.
Material and methods
Study structure and patients
Eighteen patients suffering from advanced marginal periodontitis and displaying one advanced intrabony defect each were included in the study. All 18 teeth were scheduled for extraction due to advanced periodontitis and/or further prosthetic considerations. All patients volunteered for the study and received verbal and written information about its purpose, possible risks, and the possibility to withdraw at any time. In every case, written informed consent was obtained prior to the start of the study.
The study protocol was approved by the ethics committee of Semmelweis University of Medicine in Budapest, Hungary. Two to 3 months before surgery, all patients received oral hygiene instructions and full-mouth supra- and subgingival scaling in order to reduce soft-tissue inflammation to a minimum. Furthermore, to reduce mobility when needed, the teeth were included in temporary bridge reconstructions or splinted with orthodontic wires. Prior to and 6 months after the surgical procedures, plaque index, gingival index [18], pocket depth, gingival recession, and clinical attachment level were recorded (Table 1).
Surgical procedures and postoperative care
All surgical procedures were performed under local anesthesia. Following intracrevicular incisions, mucoperiosteal flaps were raised at both the vestibular and lingual aspects of the teeth. After removing all granulation tissue from the bone defects, the root surfaces were scaled and planed by means of hand and ultrasonic instruments. Notches were prepared in the root surfaces using a small round bur (2 mm in diameter) to indicate the most apical level of the calculus or the bottom of the defect in cases where no calculus was present. Thus, any periodontal ligament tissue which might be present on the root surface coronally from the notch was considered de novo formed connective tissue.
During surgery and after complete removal of granulation tissue from the defects, the following measurements were made: distance from cementoenamel junction to the bottom of the defect (CEJ-BD) and distance from CEJ to the most coronal extension of the alveolar bone crest (CEJ-BC). The intrabony component (INTRA) of the defects was defined as (CEJ-BD)–(CEJ-BC). After thorough rinsing of the wound with sterile saline, three defects each were assigned to the following treatment groups:
-
1.
GTR
-
2.
EMD
-
3.
EMD plus bioactive glass (BG)
-
4.
BDX (bovine-derived xenograft) plus GTR
-
5.
BDX
-
6.
EMD+BDX
In the GTR group, a bioabsorbable membrane (Resolut) (Gore, Flagstaff, Ariz., USA) of appropriate configuration was selected, trimmed, and fitted to the defect in such a manner that the entire defect and 2–3 mm of the surrounding alveolar bone were covered. The membrane was fixed to the affected tooth or neighboring teeth with bioabsorbable sutures (Dexon II) (Davis and Geck, Manati, P.R.).
In the defects receiving treatment with EMD, EMD+BG, or EMD+BDX, the root surfaces were conditioned for 2 min with a 24% ethylenediamine tetra-acetate (EDTA) gel (PrefGel) (Biora, Malmö, Sweden) according to the instructions given by the manufacturer. The EDTA residues were removed by copious rinsing with sterile saline. The EMD gel (Emdogain) (Biora) was then applied to the root surfaces and the defects with a sterile syringe. In defects treated with EMD+BG (Perioglas) (U.S. Biomaterials, Alachua, Fla., USA) or EMD+BDX (Bio-Oss) (Geistlich, Wolhusen, Switzerland), the remaining EMD was mixed with the respective graft material (either BDX or BG). Care was taken not to overfill the defects. At the defects treated with GTR, BDX, or BDX+GTR, no root surface conditioning was performed.
In the BDX group, the defects were filled with the graft material only, whereas in the BDX+GTR group, a bioresorbable collagen membrane of porcine origin (BioGide Perio) (Geistlich) was additionally placed over the defect so as to cover 2–3 mm of the surrounding alveolar bone and ensure stability of the graft material. No sutures or pins were used for membrane fixation or stabilization.
Finally, in all groups, the mucoperiosteal flaps were repositioned coronally and fixed with vertical or horizontal mattress sutures. Postoperative care consisted of administration of antibiotics for 1 week (1 g/day of amoxicillin) and rinsing with 10 ml of a 0.2% chlorhexidine solution twice a day for 6 weeks. The sutures were removed 14 days following surgery. Recall appointments associated with professional tooth cleaning were performed once per week for the first 4 weeks and once per month for the remaining period. No subgingival instrumentation in the operated areas was performed during the entire experimental period of 6 months.
Biopsy removal and histological preparation
Following local anesthesia, paramarginal incisions were performed and full-thickness mucoperiosteal flaps were raised. The teeth were then removed together with their surrounding soft and hard tissues. After postsurgical healing, all patients received complete prosthodontic treatment.
Immediately upon removal, the biopsies were fixed in 10% buffered formalin, decalcified in EDTA, dehydrated, and fixed in paraffin. Mesiodistal serial sections were cut parallel to the long axes of the teeth with the microtome set at 8 µm. The sections were stained alternatively with hematoxylin and eosin, van Giesson’s connective tissue stain, Ladevig’s connective tissue stain, or the oxytalan-aldehyde-fuchsin-Halmi method [30]. Histological evaluation was performed by one blinded investigator. Only the sections representing central parts of the defects were selected for this purpose. In an attempt to provide quantitative data, the following measurements and semiquantitative analysis were performed: (1) thickness of new cementum in µm, (2) presence of artifacts, and (3) number of cells in the new cementum.
Results
The surgical procedure, postoperative care, clinical results, and linear histologic measurements (i.e., height of new cementum and new bone) for most of the defects included in this study have been reported previously [32, 34, 35, 36]. However, three defects were treated exclusively for this paper (one in the EMD+BDX group and two in the BDX group). Attempts were also made to include defects with comparable clinical and histometric characteristics (Table 1). For this reason, only three defects per group were included. The clinical characteristics of the treated defects prior to and during surgery are presented in Table 1. All treated defects were combined one-to-two-walled defects.
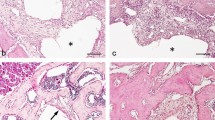
Briefly, the healing following all six different types of regenerative treatment resulted to a varying extent in formation of cementum, periodontal ligament, and bone. Neither ankylosis nor root resorption was observed. The new cementum displayed a predominantly cellular character and comparable thickness in all six treatment groups (Fig. 1, 2, 3, 4, 5, 6) (Table 2). Collagen fibers were observed to run parallel but also to insert into the newly formed cementum, irrespective of the treatment. Artifacts were observed in all biopsies.
Discussion
The results of the present comparative study show that, in humans, the cementum formed after different types of regenerative modalities was, irrespective of the treatment provided, of a reparative, cellular, extrinsic and intrinsic fiber type. The newly formed cementum was of comparable thickness in all six treatment groups, varying from 150.0±57.2 µm to 200.0±29.4 µm. These findings are in contradiction to those from previous investigations which indicated that new cementum formed after EMD treatment was of a predominantly acellular extrinsic fiber type and with no artifacts [13, 20].
Based on the above observations, it was suggested that treatment with EMD may predictably enhance the formation of an acellular type of cementum closely resembling AEFC [12]. However, if this assumption were true, treatment with EMD should promote the formation of acellular cementum, while the use of any other regenerative techniques should yield a cellular (i.e., reparative) type. The present results, however, failed to reveal any difference between the different regenerative modalities in terms of cellular content and presence of artifacts. On the other hand, these findings are in agreement with those from an electron microscopic study evaluating the nature and attachment of cementum formed after GTR treatment in humans [19] revealing that the new cementum bore a close resemblance to cementum formed during spontaneous repair of root resorption.
The present results are also in line with very recent findings from a study investigating the association between Hertwig’s epithelial rooth sheath cells, enamel matrix proteins (EMP), and cementogenesis in porcine teeth. That study failed to demonstrate a causal link between EMP and the formation of AEFC [2]. In this context, it should also be pointed out that results from previous studies on cementogenesis in humans indicate that the formation of AEFC is an extremely slow process.
Zander and Hürzeler [41] measured the cementum thickness in 233 teeth from patients between 11 and 76 years old and concluded that, over decades, the appositional rate of AEFC is on average 1.7–3.9 µm/year. Comparable findings were reported by Dastmalchi et al. [8], who calculated that in erupted human premolars and molars, AEFC increases in thickness by an average of 2.9 µm/year, while Bosshardt and Schroeder [1] showed that, in human premolars, it grew in thickness by 2.0–2.5 µm/year.
In contrast to the slow formation of AEFC, cellular cementum forms very rapidly. It was suggested that the reason for cell (cementocyte) incorporation into the reformed cementum may be dependent upon the speed of cementum formation [1]. This view seems to be corroborated by the present results, in which a rather high amount of new cementum was formed in a relatively short period of time (6 months).
Together with the data from the literature, the present findings indicate that, in humans, AEFC does not seem to form predictably after any of the regenerative modalities used. Furthermore, the fact that no differences in cementum thickness and cellular content were observed between the six different regenerative therapies may indicate that, once the process of periodontal wound healing is initiated, the resulting cementum is, irrespective of treatment modality, always of a reparative, cellular, extrinsic and intrinsic fiber type.
The fact that splits occurred in all treatment groups may indicate that, in humans, the type of regenerative therapy itself does not seem to influence (i.e., reduce) significantly the occurrence of such artifacts. It should be kept in mind that the significance of such histological artifacts for the clinical outcome of treatment is still controversially discussed in the literature.
While some authors consider the presence of splits between the new cementum and the old one or dentin to result mainly from the decalcification process during histological preparation and do not necessarily reflect poor quality of the regenerated tissues [17], others have interpreted similar findings as representing a weakness which might negatively affect the supporting apparatus of the tooth [23]. It is, however, unknown to what extent the type of new cementum and the presence of artifacts may affect the clinical outcome of the therapy.
In this context, a recent monkey experiment compared the susceptibility of GTR-regenerated periodontal attachment after ligature-induced periodontitis with that of pristine periodontium [16]. The histologic analysis indicated that the root surfaces treated with GTR were covered by newly formed cementum of the reparative, cellular, extrinsic and intrinsic fiber type, while the cementum on pristine roots was mainly AEFC. However, the results failed to show that teeth with a periodontal attachment apparatus formed by GTR are more susceptible to periodontitis than those with pristine periodontium.
It is also important to emphasize that, to the best of our knowledge, no other studies have attempted systematically to evaluate and compare newly formed cementum following different regenerative therapies, and thus direct comparisons with other studies are not possible.
Conclusions
The present findings indicate that in humans (1) the new cementum formed after six different types of regenerative therapy was, irrespective of treatment, of a reparative, cellular, extrinsic and intrinsic fiber type and (2) the regenerative modality does not seem to influence the type of newly formed cementum.
References
Bosshardt DD, Schroeder HE (1996) Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec 245:267–292
Bosshardt DD, Nanci A (2004) Hertwig’s epithelial root sheath, enamel matrix proteins, and initiation of cementogenesis in porcine teeth. J Clin Periodontol 31:184–192
Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, Stevens M, Romberg E (1989) Histologic evaluation of new attachment apparatus formation in humans. Part II. J Periodontol 60:675–682
Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, Stevens M, Romberg E (1989) Histologic evaluation of a new attachment apparatus formation in humans. Part III. J Periodontol 60:683–693
Camelo M, Nevins M, Schenk R, Simion M, Rasperini G, Lynch S, Nevins M (1998) Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent 18:321–331
Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M (2003) Periodontal regeneration in human class II furcations using purified recombinant human platelet-derived growth factor (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent 23:213–225
Cortellini P, Clauser C, Pini Prato GP (1993) Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol 64:387–391
Dastmalchi RA, Polson A, Bouwsma O, Proskin H (1990) Cementum thickness and mesial drift. J Clin Periodontol 17:709–713
Dragoo MR, Sullivan HC (1973) A clinical and histological evaluation of autogenous iliac bone grafts in humans. I. Wound healing 2 to 8 months. J Periodontol 44:599–613
Froum SJ, Kushnek L, Scopp IW, Stahl SS (1983) Healing responses of human intraosseous lesions following the use of deridement, grafting and citric acid root treatment. I. Clinical and histologic observations six months post surgery. J Periodontol 54:67–76
Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J (1986) New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol 13:604–616
Hammarström L (1997) Enamel matrix, cementum development and regeneration. J Clin Periodontol 24:658–668
Heijl L (1997) Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J Clin Periodontol 24:693–696
Hiatt WH, Schallhorn RG, Aaronian AJ (1978) The induction of new bone and cementum formation. IV. Microscopic examination of the periodontium following human bone and marrow allograft, autograft and non-grafted periodontal regenerative procedures. J Periodontol 49:495–512
Karring T, Lindhe J, Cortellini P (2003) Regenerative periodontal therapy. In: Lindhe J, Karring T, Lang NP (eds) Clinical periodontology and implant dentistry. Blackwell, Oxford, pp 650–704
Kostopoulos L, Karring T (2004) Susceptibility of GTR-regenerated periodontal attachment to ligature-induced periodontitis. An experiment in the monkey. J Clin Periodontol 31:336–340
Listgarten MA (1972) Electron microscopic study of the junction between surgically denuded root surfaces and regenerated periodontal tissues. J Periodont Res 7:68–90
Löe H (1967) The Gingival Index, the Plaque Index and the Retention Index system. J Periodontol 38:610–616
Luder HU, Zappa U (1998) Nature and attachment of cementum formed under guided conditions in human teeth. An electron microscopic study. J Periodontol 69:889–898
Mellonig JT (1999) Enamel matrix derivative for periodontal reconstructive surgery: technique and clinical and histologic case report. Int J Periodontics Restorative Dent 19:9–19
Mellonig J (2000) Human histologic evaluation of a bovine-derived xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent 20:19–29
Nabers CL, Reed OM, Hammer JE (1972) Gross and histologic evaluation of an autogenous bone graft 57 months postoperatively. J Periodontol 43:702–704
Nalbandian J, Frank, RM (1980) Electron microscopic study of the junction between surgically denuded root surfaces and regenerated periodontal tissues. J Periodont Res 15:71–89
Nevins ML, Camelo M, Lynch SE, Schenk RK, Nevins M (2003) Evaluation of periodontal regeneration following grafting intrabony defects with Bio-Oss collagen: a human histologic report. Int J Periodontics Restorative Dent 23:9–17
Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE (2003) Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogen bone. J Periodontol 74:1282–1292
Nyman S, Lindhe J, Karring T, Rylander H (1982) New attachment following surgical treatment of human periodontal disease. J Clin Periodontol 9:290–296
Parma-Benfenatti S, Tinti C (1998) Histologic evaluation of new attachment utilizing a titanium-reinforced barrier membrane in a mucogingival recession defect. A case report. J Periodontol 69:834–839
Ross S, Cohen W (1968) The fate of an osseous tissue autograft. A clinical and histologic case report. Periodontics 6:145–151
Schroeder HE (1992) Biological problems of regenerative cementogenesis: synthesis and attachment of collagenous matrices on growing and established root surfaces. Int Rev Cytol 142:1–59
Sculean A, Karring T, Theilade J, Lioubavina N (1997) The regenerative potential of oxytalan fibers. An experimental study in the monkey. J Clin Periodontol 24:932–936
Sculean A, Donos N, Chiantella GC, Windisch P, Reich E, Brecx M (1999) Treatment of intrabony defects with bioabsorbable membranes. A clinical and histologic study. Int J Periodontics Restorative Dent 19:501–509
Sculean A, Donos N, Windisch P, Gera I, Brecx M, Reich E, Karring T (1999) Healing of human intrabony defects following treatment with enamel matrix proteins or guided tissue regeneration. J Periodont Res 34:310–322
Sculean A, Chiantella GC, Windisch P, Donos N (2000) Clinical and histologic evaluation of treatment of intrabony defects with an enamel matrix protein derivative (Emdogain). Int J Periodontics Restorative Dent 20:375–381
Sculean A, Windisch P, Keglevich T, Chiantella GC, Gera I, Donos N (2003) Clinical and histologic evaluation of human intrabony defects treated with an enamel matrix protein derivative combined with a bovine-derived xenograft. Int J Periodontics Restorative Dent 23:47–55
Sculean A, Stavropoulos A, Windisch P, Keglevich T, Karring T, Gera I (2004) Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin Oral Invest 8:70–74
Sculean A, Windisch P, Keglevich T, Gera I (2005) Clinical and histological evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. Int J Periodontics Restorative Dent (in press)
Stahl S, Froum S, Kushner L (1983) Healing responses of human teeth following the use of debridement grafting and citric acid root conditioning. II. Clinical and histologic observations: one year post-surgery. J Periodontol 54:325–338
Vincenzi G, DeChiesa A, Trisi P (1997) Guided tissue regeneration using a resorbable membrane in gingival recession-type defects: a histologic case report in humans. Int J Periodontics Restorative Dent 18:24–33
Yukna RA, Mellonig J (2000) Histologic evaluation of periodontal healing in humans following regenerative therapy with enamel matrix derivative. A 10-case series. J Periodontol 71:752–759
Yukna RA, Salinas TJ, Carr RF (2002) Periodontal regeneration following use of ABM/P-15: a case report. Int J Periodont Rest Dent 22:146–155
Zander HA, Hürzeler B (1958) Continuous cementum apposition. J Dent Res 37:1035–1044
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sculean, A., Stavropoulos, A., Berakdar, M. et al. Formation of human cementum following different modalities of regenerative therapy. Clin Oral Invest 9, 58–64 (2005). https://doi.org/10.1007/s00784-004-0288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-004-0288-x