Abstract
Purpose
To test the hypothesis that autologous chondrocyte implantation (ACI) has a better treatment effect than microfracture (MF), and increasing superiority over the years, when performed under similar patient-specific and defect-specific conditions.
Methods
We scanned four electronic databases for controlled clinical trials or controlled prospective observational studies. We conducted random-effects meta-analyses of equivalent data using standardized mean differences as the outcome measure of choice at 1, 2, and 5-year follow-up. We assessed heterogeneity with the I 2 index and publication bias with funnel plots and Kendall’s tests.
Results
Our literature search revealed six study populations (nine papers) which satisfied our eligibility criteria. Overall, 399 patients aged between 16 and 60 years with 1–10 cm2 chondral defects were available. The MF and the ACI study groups were well matched regarding patient baseline characteristics. For all papers, microfracture was performed according to Steadman, whereas three generations of ACI were applied. When all were combined, non-significant superiority of ACI over MF was revealed; surprisingly, this superiority decreased over the years. However, our meta-analyses combining solely second and third-generation ACI revealed significant standardized differences, becoming smaller over the years, but always representing a large effect. Nevertheless, our approximate estimate of the difference between the treatment effects provoked by second and third-generation ACI and by MF is not indicative of clinically relevant superiority of ACI over MF at 5-year follow-up.
Conclusions
Both series of meta-analyses (combining either all ACI modifications or solely the second and third generations of ACI) suggest that the treatment effects resulting from ACI and MF converge over the years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is prone to damage from acute high-energy trauma and from repetitive shear and torsional forces applied to the surface. Therefore, chondral lesions are a common pathology of the knee joint. Reparative and restorative techniques are available to treat them surgically. In 1980, reparative microfracture (MF), a single-stage arthroscopic technique, was developed by Steadman [1]. After careful removal of the calcified cartilage layer and debridement of the lesion until a perpendicular healthy defect rim is formed, an awl is used to make multiple holes in the subchondral bone plate. These so-called microfractures enable bone marrow cells to migrate into the cartilage defect and to create a “super clot” that eventually matures into firm repair tissue. Because of its minimally invasive approach, technical simplicity, limited surgical morbidity and low cost, MF has gained popularity over the past two decades. It is now a common first-line treatment for patients with full-thickness cartilage defects of the knee. The 2.7 million knee arthroscopies performed in the US and in Europe in 2007 included 1.8 million cartilage procedures, which included 450,000 microfractures and 50,000 autologous chondrocyte implantations (ACI) [2]. Regenerative ACI is a two-stage biological approach. In an initial arthroscopy a cartilage biopsy is harvested from healthy cartilage of the affected knee and sent for chondrocyte culture. Three to six weeks later an arthrotomy is performed to debride the cartilage lesion and to implant the expanded chondrocytes which start to fill the defect by producing a matrix. Since 1987, when the first ACI was performed by Brittberg [3] many modifications have appeared. In the first generation of ACI, the cultured cells are simply injected under a periosteal patch or a resorbable bi-layer collagen membrane that is sutured to the edges of the prepared chondral defect. Second-generation ACI incorporates biodegradable polymers as three-dimensional temporary scaffolds for the in vitro growth of living cells and their subsequent implantation into the defect site [4, 5]. In third-generation ACI a quality assessment is performed by means of highly specific marker analysis before implantation [6].
MF and ACI are performed to relieve symptoms and restore function by covering the chondral lesion with high-quality tissue that is integrated into the native surrounding cartilage and fulfills all needed mechanical functions, thus enabling the patient to return to his/her pre-injury activity level. In consequence, the quality of the defect fill provided by the techniques seems to be the decisive factor; good or excellent clinical outcomes are directly correlated with hyaline-like defect fill, whereas a fibrous fill is correlated with poorer clinical results [7]. Microfracture induces the growth of a fibrocartilaginous repair tissue, the function of which might deteriorate over time [8, 9]. By surgically implanting healthy cartilage cells into the damaged area of the knee joint, autologous chondrocyte implantation can restore its integrity with hyaline-like cartilage, with a hybrid of fibrocartilage and hyaline-like tissue (with chondrocytes organized in isogeneic groups and with proteoglycans and glycosaminoglycans in the extracellular matrix), or with fibrocartilaginous material containing type-I and type-II collagen and therefore resulting in good and stable clinical results [7]. However, the histological outcome of ACI is highly dependent on the timing of the biopsy. Statistical analysis suggests that if the time after implantation doubles the likelihood of hyaline-like defect fill increases by more than fourfold (p < 0.001) [10], because of its ongoing maturation. These findings might give the impression that ACI is superior to MF in any case and that this superiority even increases over the years. To test this hypothesis, the objective of our study was to compare clinical outcomes after MF and ACI by statistically summarizing the differences between the mean treatment effects after equal follow-up periods.
Methods
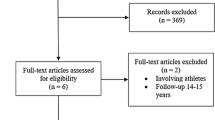
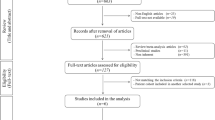
We scanned the electronic databases MEDLINE, EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials to perform a literature search which was completed on March 31, 2013. Because “microfracture” is the generally accepted nomenclature for this technique, it was used as our search term. No language restrictions were applied. For outcome evaluation, clinical scores were regarded as adequate. Our eligibility criteria are presented in Table 1. First, two clinicians separately analyzed the papers identified through data base searching on the basis of title or abstract. Studies clearly failing to meet the selection criteria (original paper, microfracture of the knee, autologous chondrocyte implantation, outcome evaluation) and duplicates were excluded. Subsequently, full-text versions of the remaining 13 papers were obtained for detailed evaluation. Two reviewers, who documented their work by means of standardized forms, independently determined which studies were eligible. For randomized controlled trials they assessed the method of randomization, allocation concealment, blinding, drop-out rates, and the method of statistical analysis. For observational studies they used criteria proposed by Deeks et al. [11]. Discrepancies were resolved by consensus. Finally, data on study characteristics and design, patient age, defect size, and clinical score values (preoperatively and at predefined time points postoperatively) were extracted.
Statistical analysis
We conducted meta-analyses for head-to-head comparison of MF and ACI for trials similar in study populations and outcome assessments. Because eligible studies used a variety of assessment tools, the standardized mean difference (SMD), or, more precisely, the standardized difference between the mean treatment effects (i.e. the differences between the mean pre and postoperative scores) resulting from ACI and MF was appointed as effect size [12] in our meta-analyses. We had to estimate standard deviations of change on the basis of pre–post correlations because only one study reported the standard deviations of the pre–post changes on assessment tools. All meta-analyses were conducted using the package “meta” in the statistic software R [13], applying the random-effects model. For the summary measure SMD Hedges’ adjusted g (calculated as the difference between the two means divided by the pooled standard deviation, with an adjustment for small sample bias) was used for pooling. For each meta-analysis, we performed a test of heterogeneity (I 2 index) and assessed publication bias by use of funnel plots and Kendall’s tests. However, given the small number of component studies in our meta-analyses, these tests have low sensitivity to detect publication bias.
Results
Figure 1 shows the process of selection of eligible studies. Of 1422 citations detected by electronic literature search, nine papers, seven randomized controlled trials and two cohort studies met our eligibility criteria for statistical analysis. Nevertheless, as column 1 of Table 2 indicates, only six patient populations were available: those of Basad et al. [14], Knutsen et al. [8, 9], Kon et al. [15], Kon et al. [16], Saris and co-workers [17–19], and Crawford et al. [20]. However, five of these were evaluated at a variety of predefined time points whereas Kon et al. [16] provided results at 2 years and at a final 7.5-year mean follow-up. Only follow-up periods that could be used for head to head comparison are listed in column 5 of Table 2. Of all papers, microfracture was performed according to Steadman, whereas three generations of ACI were applied (column 3 of Table 2). Overall, 399 patients aged between 16 and 60 years with chondral defects of size 1–10 cm2 were available. They were allocated almost equally to both study groups by Knutsen et al. [8, 9], Kon et al. [15, 16], and Saris and co-workers [17–19]. The MACI™ group of Basad et al. [14] was twice the size of their MF-group because of the combination of the two MACI™ groups planned in the original protocol. Of interest, the randomization process of Crawford et al. [20], generated by a statistician independent of the study, resulted in 9 MF and 21 ACI patients. Of all studies, both study groups were well matched regarding patient baseline characteristics; only slight differences in the duration of symptoms were reported by Basad et al. [14] and Saris and co-workers [17–19]. Generally, associated surgery referred to the treatment of meniscal tears and—except for Crawford et al. [20]—to anterior cruciate ligament reconstruction. Of interest, the rehabilitation protocols of Knutsen et al. [8, 9], Kon et al. [15, 16] Saris and co-workers [17–19], and Crawford et al. [20] were similar but not identical. However, within these studies, the very same regimen was prescribed to both treatment groups. Only the patients of Basad et al. [14] were required to follow a post-operative rehabilitation program appropriate to either ACI or MF.
Regarding histological quality, Knutsen et al. [8, 9] did not find any significant differences between the ACI and the MF groups; they reported that ACI biopsy specimens tended to have a more hyaline-like appearance 2 years postoperatively. Clear morphological superiority of the cartilaginous tissue over the scar tissue formed after MF was detected by Saris and co-workers [17, 18] 1 year postoperatively; microfracture resulted in significantly lower histological scores for type II collagen and matrix proteoglycan content [17]. Unfortunately, histological analysis was not performed by Basad et al. [14], Kon et al. [15, 16], and Crawford et al. [20].
For outcome evaluation the Lysholm Score [21] was applied by Knutsen et al. [8, 9] and Basad et al. [14]; Kon et al. [15, 16] and Crawford et al. [20] used the IKDC Score (2000 IKDC Subjective Knee Evaluation Form, Patients’ Part) [22] whereas Saris and co-workers [17–19] adopted the KOOS [23] questionnaire. They determined the overall KOOS rating as the average of sub-scores “function in daily living”, “pain”, “symptoms/stiffness”, and “quality of life”. Because patients were significantly limited in sports activities hardly any relevant data were available in the sub-score “sports” which was therefore excluded from their overall KOOS analysis.
Of all relevant study populations, their mean pre and postoperative score values, their treatment effects, and all available standard deviations are presented in Table 3 (1-year follow-up), Table 4 (2-year follow-up), and Table 5 (5-year follow-up). Particularly noticeable is the fact that solely Knutsen et al. [8, 9] evaluated a (not significantly) higher treatment effect for the MF group. All other available studies favored ACI. Nevertheless, a significant difference in the mean treatment effects of ACI and MF was only shown in the overall KOOS at 3-year follow-up [18] and in the IKDC Score at one, two [20], and 5-year follow-up [15].
Initially, we included all available studies, hence pooling three generations of ACI. Figure 2 graphically displays the results of the individual studies for a follow-up period of 1 year; they were statistically summarized to 1.05 with a 95 % confidence interval of (−1.35; 3.45). The p value of 0.39 and the diamond that crosses the zero line both reveal that no significant overall effect of size could be detected. Referring to the 2-year follow-up (Fig. 3) our meta-analysis computed a non-significant value of 0.38 (p = 0.15) with a 95 % confidence interval of (−0.13; 0.90) for the overall SMD. Finally, at 5-year follow-up (Fig. 4) overall SMD decreased to non-significant 0.28 (p = 0.29) with a 95 % confidence interval of (−0.23; 0.79).
Because solely Knutsen et al. [8, 9] applied a first-generation ACI technique we also conducted meta-analyses omitting their patients. Figures 5, 6, and 7 reveal significant superiority of ACI over MF that declines over time. The overall SMDs at 1, 2, and 5-year follow-up are 2.22 (p = 0.0003), 0.56 (p = 0.0001), and 0.51 (p = 0.0008), respectively. Relying on commonly accepted benchmarks, these values correspond to a large effect (SMD >0.5) [24].
Discussion
Undoubtedly, MF and ACI have both been shown to significantly relieve symptoms and improve function, and both provide better results treating defects on the femoral condyles than in the patellofemoral compartment [25]. However, younger and more active patients, with a shorter duration of preoperative symptoms, fewer surgical procedures before cartilage repair or restoration, smaller isolated defects on the medial femoral condyle, and without concomitant ligamentous instability, meniscal deficiency, or tibiofemoral or patellofemoral malalignment, can expect the best outcome, irrespective of the technique used [26]. All studies included in our meta-analyses focused on treatment groups with comparable demographic data. Therefore, different outcomes have to be based on the two procedures themselves and not on patient or defect-specific factors.
Our three meta-analyses including all available studies favored ACI over MF at 1, 2, and 3-year follow-up with a decreasing overall standardized difference in means. Because these differences are not significant (p ≥ 0.15) we have to reject our hypothesis; ACI may not be regarded as superior to MF in general.
Undoubtedly, first-generation ACI has a variety of limitations related to the complexity and morbidity of the technique (e.g. the handling of a delicate liquid suspension of chondrocytes, the need to make a hermetic seal using sutures to avoid cell leakage) [27]. Therefore, we performed another series of meta-analyses, omitting the patients of Knutsen et al. [8, 9]; we calculated significant overall SMDs that decreased from 1 to 3 and from 3 to 5-year follow-up, all representing a large effect. Nevertheless, a large effect need not be regarded as clinically relevant. Because it has been suggested that any differences in outcome resulting from a hyaline-like rather than fibrocartilaginous defect fill may be quite subtle and may only reveal themselves after many years of follow-up (5–10 years) [28] we tried to quantify the superiority of ACI over MF that was revealed by our meta-analysis referring to the 5-year follow-up. We estimated the standard deviation within groups, measured as overall KOOS points, using the standard deviations of the pre-post changes; they had been solely provided by D.B.F. Saris on our request. According to its definition, we multiplied overall Hedges’ g with the standard deviation within groups. This product turned out to be 9.9 overall KOOS points with (4.1; 15.6) as the relevant 95 % confidence interval; this is our approximate estimate of the expectable difference between the mean treatment effects provoked by ACI and MF. Because eight to 10 KOOS points have been calculated for a minimum perceptible clinical improvement (the difference on the measurement scale associated with the smallest change in the health status noticeable by the patient) [29], at least our approximate estimate of the lower limit of the 95 % confidence interval does not represent a clinically significant superiority of ACI (referring to the second or third generation) over MF. Moreover, our results suggest that the outcomes provoked by ACI and MF converge on long-time follow-up.
Surprisingly, fibrocartilaginous repair tissue (mean stiffness 1.5 ± 0.35 N) may enable adequate patient-reported outcomes although solely hyaline-like defect fill (mean stiffness 3.0 ± 1.1 N) [7] corresponds approximately to native articular cartilage; its stiffness ranges from 2.4 ± 0.8 N (located in the medial plateau of the tibia) to 5.6 ± 1.2 N (located in the lateral condyle of the femur) [30]. In consequence, appropriate patient allocation to ACI or MF is crucial. In our opinion not only patient and defect-specific characteristics but also patient demands on his/her knee as a result of daily routine and recreational activities should be considered when deciding between ACI and MF for a particular cartilage lesion. Of course, fibrocartilage has limited resistance to sheer and compressive loads. Therefore, for larger defects or high-demand patients MF may not adequately relieve symptoms and restore function [31]; in consequence, ACI with its potential long-term benefits because of its hyaline-like defect fill should be performed. Of interest, lesions larger than 4 cm2 have significantly worse clinical results than lesions smaller than 4 cm2 when treated with microfracture, whereas defect size does not significantly affect the outcome after ACI [9, 15]. An additional reason for not routinely applying microfracture for larger lesions is that use of ACI as a second-line procedure after MF results in less favorable and less predictable outcome [32]. However, rehabilitation for ACI is extensive and demanding and achieving the ultimate clinical benefits may be delayed by at least six to 12 months for some patients [31]. In contrast, MF involves less surgery, thus rehabilitation is easier, enabling faster recovery and a faster return to competition by athletes [16]. Finally, in health care the cost must be taken into consideration. Mainly because of chondrocyte cell cultivation the cost of ACI is approximately ten times that of MF [28].
Limitations of our study include the fact that several means and ranges of score values for the patients of Knutsen et al. [8, 9] and Crawford et al. [20] had to be deduced from histograms and box plots. Furthermore, only two studies presented score values 1, 2, and 5 years postoperatively. We are aware that our meta-analyses and our approximate calculation must be interpreted cautiously. Undoubtedly, large, well-designed, long-term multicenter studies are needed to achieve adequate study populations and power to enable clearly defined indications; they would aid determination of which patients will benefit most from MF and those for whom there will be no permanent improvement. By allocating the latter to ACI early failures could probably be avoided.
Conclusion
Both series of meta-analyses (combining either all ACI modifications or solely second and third generations of ACI) could not reveal clinically relevant superiority of ACI over MF. ACI and MF are complementary procedures. Decision making must take patient objectives, physical demands, compliance, and patient and defect-specific factors into consideration.
References
Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21:761–8.
Stommen J. Small companies, big ideas. ODT. http://www.odtmag.com/. Delporte C, Barbella M; 2010.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95.
Kon E, Delcogliano M, Filardo G, Montaperto C, Marcacci M. Second generation issues in cartilage repair. Sports Med Arthrosc Rev. 2008;16:221–9.
Kon E, Filardo G, Di Matteo B, Perdisa F, Marcacci M. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res. 2013;2:18–25.
Gobbi A, Bathan L. Biological approaches for cartilage repair. J Knee Surg. 2009;22:36–44.
Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12.
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigson TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–12.
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86A:455–64.
Gikas P, Morris T, Carrington R, Skinner J, Bentley G, Briggs T. A correlation between the timing of biopsy after autologous chondrocyte implantation and the histological appearance. J Bone Joint Surg Br. 2009;91:1172–7.
Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:1–173.
Ziegler A, Lange S, Bender R. Systematische Übersichten und Meta-Analysen. Dtsch Med Wochenschr. 2004;129:T11–5.
Team RDC. R: a language and environment for statistical computing; 2011. http://www.r-projectorg.
Basad E, Ishaque B, Bachmann G. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomized study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–27.
Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Am J Sports Med. 2009;37:33–41.
Kon E, Filardo G, Berruto M, Benazzo F, Zanon G, Della Villa S, Marcacci M. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39:2549–57.
Saris DBF, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–46.
Saris DBF, Vanlauwe JV, Almquist JV, Verdonk R, Bellemans J, Luyten FP. Treatment of symptomatic cartilage defects of the knee. Characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37:10S–19S.
Vanlauwe J, Saris DBF, Victor J, Almqvist KF, Bellemans J, Luyten FP. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee. Am J Sports Med. 2011;39:2566–74.
Crawford DC, DeBerardino TM, Williams RJ 3rd. NeoCart, an autologous cartilage tissue implant compared with microfracture for treatment of distal femoral cartilage lesions. J Bone Joint Surg Am. 2012;94:979–89.
Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–4.
ICRS. http://www.cartilage.org.
KOOS. http://www.koos.nu.
Bortz J, Döring N. Forschungsmethoden und Evaluation für Human- und Sozialwissenschaftler. Berlin: Springer; 2006.
Gomoll AH, Farr J, Gillogly SD, Kercher JS, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470–90.
Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220–33.
Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, Pignotti E, Marcacci M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med. 2009;37(Suppl):156S–66S.
Cameron P, Wright C, Wale JMG, Hart J, Morgan DFN. Matrix-induced autologous chondrocyte implantation (MACI) and autologous chondrocyte implantation (ACI). In: Ageing DoHa, ed. Canberra: Medical Services Advisory Committee; 2010.
Roos EM, Lohmander LS. The Knee Injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64.
Lyyra T, Kiviranta I, Väätäinen U, Helminen HJ, Jurvelin J. In vivo characterization of indentation stiffness of articular cartilage in the normal human knee. J Biomed Mater Res. 1999;48:482–7.
Batty L, Dance S, Bajaj S, Cole BJ. Autologous chondrocyte implantation: an overview of technique and outcomes. ANZ J Surg. 2011;18:18–25.
Minas T, Gomoll AH, Rosenberger R, Royce R, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902–8.
Acknowledgments
We would like to thank Helmut Schlumprecht for performing the statistical analyses, Daniel B.F. Saris for providing unpublished data, and Florian Kutscha-Lissberg for reviewing the literature.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negrin, L.L., Vécsei, V. Do meta-analyses reveal time-dependent differences between the clinical outcomes achieved by microfracture and autologous chondrocyte implantation in the treatment of cartilage defects of the knee?. J Orthop Sci 18, 940–948 (2013). https://doi.org/10.1007/s00776-013-0449-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-013-0449-3