Abstract
The interaction of Tb3+ and La3+ cations with different photosystem II (PSII) membranes (intact PSII, Ca-depleted PSII (PSII[-Ca]) and Mn-depleted PSII (PSII[-Mn]) membranes) was studied. Although both lanthanide cations (Ln3+) interact only with Ca2+-binding site of oxygen-evolving complex (OEC) in PSII and PSII(-Ca) membranes, we found that in PSII(-Mn) membranes both Ln3+ ions tightly bind to another site localized on the oxidizing side of PSII. Binding of Ln3+ cations to this site is not protected by Ca2+ and is accompanied by very effective inhibition of Mn2+ oxidation at the high-affinity (HA) Mn-binding site ([Mn2+ + H2O2] couple was used as a donor of electrons). The values of the constant for inhibition of electron transport Ki are equal to 2.10 ± 0.03 µM for Tb3+ and 8.3 ± 0.4 µM for La3+, whereas OEC inhibition constant in the native PSII membranes is 323 ± 7 µM for Tb3+. The value of Ki for Tb3+ corresponds to Ki for Mn2+ cations in the reaction of diphenylcarbazide oxidation via HA site (1.5 µM) presented in the literature. Our results suggest that Ln3+ cations bind to the HA Mn-binding site in PSII(-Mn) membranes like Mn2+ or Fe2+ cations. Taking into account the fact that Mn2+ and Fe2+ cations bind to the HA site as trivalent cations after light-induced oxidation and the fact that Mn cation bound to the HA site (Mn4) is also in trivalent state, we can suggest that valency may be important for the interaction of Ln3+ with the HA site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The light absorbed by plants, algae, and cyanobacteria is used by these organisms for water decomposition. This process is carried out by the multicomponent membrane pigment–protein complex called photosystem II (PSII). Water oxidation reaction in PSII supplies electrons to the photosynthetic electron transport chain, triggering the synthesis of high-energy components. The remaining oxygen atoms are a by-product of this reaction. Therefore, the catalytic center oxidizing water [water splitting or oxygen evolving complex (OEC)] carries out the synthesis of an intermolecular bond between two oxygen atoms of two water molecules. Synthetized oxygen is released into the atmosphere and this photosynthetic reaction is practically the only source of O2 in the atmosphere of our planet. The catalytic center of the OEC consists of four manganese cations, one calcium cation, and five oxygen atoms connecting the metal cations [1,2,3]. The detailed structure of a catalytic cluster Mn4CaO5 has been determined at first by X-ray diffraction at 1.9 Å resolution [1] and then with using femtosecond X-ray pulses at 1.95 Å resolution [3]. The latter method allowed to obtain a ‘radiation-damage-free’ structure of Mn/Ca cluster. In subsequent works, a comparative study of the structure of the PSII cores in the dark state (S1) and S3 state (after two flashes) was carried out [4]. The effect of Mn4CaO5 cluster extraction from OEC on the PSII structure was also investigated [5]. Currently, not only is the structure of the PSII core determined, but also more complex formations—the supercomplex of the PSII core with the light-harvesting complex II [6]. The results obtained made it possible to establish that the cluster is coordinated by one imidazole and six carboxyl groups of amino acid residues of the D1 and CP43 proteins of PSII and have four molecules of water, two of which are bound to Mn4 cation and the other two cations bound to Ca2+ cation. The cluster has an irregular cube structure formed by three manganese cations and a Ca2+ cation connected via oxygen bridges. The fourth manganese cation (Mn4) is connected to the cube by two oxygen bridges. However, despite the high resolution of the Mn4CaO5 structure decryption, the mechanism of the water-splitting reaction in PSII remains mostly unclear.
Ca2+ is a necessary cofactor for the water oxidation reaction [7]. A possible role of Ca2+ in this reaction is either “adjustment” of the redox potential of a manganese cluster [8, 9] or binding of one or two substrate water molecules that are oxidized during a catalytic cycle [10]. However, the specific mechanism of Ca2+ participation in the OEC functioning remains unclear [8]. The role of Ca2+ in the water splitting mechanism was investigated in many works with the use of substitution of calcium cation with another metal cation. Ca2+ binding is competitive with divalent cations such as Cd2+ [11], Sr2+ [12], the only metal ion which partially functionally replaces Ca2+, and lanthanides [13, 14]. The substitution of Ca2+ by lanthanide ion (denoted as Ln3+) has proven to be an effective method for investigating the properties of different Ca2+-binding proteins since ions are very effective competitive inhibitors of the Ca2+ site [15]. Various lanthanides were used for studying the Ca-binding site in the OEC of intact PSII [13], Ca-depleted PSII membranes (PSII(-Ca)) [14] and PSII core complexes [16]. Though all lanthanides show very similar chemical properties, significant differences in the action of specific ions on the PSII functioning [14] were revealed. These variations are probably caused by different ion radius. Displacement of Ca2+ by La3+ inhibits the oxygen evolution in intact PSII, preventing the electron donation by Mn cations to YZ [13]. The presence of lanthanides also affects the electron transfer from YZ to P680+ [17]. A lanthanide-substituted OEC displayed a thermoluminescence band arising from S2QA charge recombination, indicating that the Mn cluster is oxidized to the S2 state [14]. EPR studies of Dy3+-substituted PSII also have shown the S1 → S2 transition [16].
Ghanotakis et al. mentioned in their work [13] dedicated to a research of La3+ interaction with Ca-binding site in the OEC of native PSII membranes that Ce3+ and Tb3+ produce the same effect as La3+. In a preliminary study [18], we have investigated the effect of terbium, one of the poorly studied lanthanides, on the native PSII membranes using the fluorescence method. In this paper, we investigated in detail the interaction of Tb3+ (and La3+ for comparison) with different PSII membranes (intact PSII, PSII(-Ca) and Mn-depleted PSII membranes (PSII(-Mn)). Although both Ln3+ cations interact only with Ca2+ site of the OEC in PSII and PSII(-Ca) membranes, we found that in PSII(-Mn) membranes both lanthanide ions tightly bind to another site localized on the oxidizing side and inhibiting very effectively (Ki = 2.10 ± 0.03 µM for Tb3+ and 8.3 ± 0.4 µM for La3+) the donation of electrons by (Mn2+ + H2O2) donor via the high-affinity (HA) Mn-binding site in these membranes.
Materials and methods
PSII preparations
Native PSII membranes PSII-enriched membrane fragments (BBY-type) were prepared from market spinach following Ghanotakis and Babcock [19]. The functional and spectral characteristics of these preparations matched the previously reported one [20]. Table S1 of Supplementary Material section shows some characteristics of intact PSII membranes as well as PSII(-Ca) and PSII(-Mn) membranes which were determined mainly in our previous works. Some parameters were measured in several different studies with good agreement with each other. This indicates a fairly good uniformity of the PSII samples we prepare. The preparations were stored at − 80 °C in buffer A, containing 15 mM NaCl, 400 mM sucrose, and 50 mM MES/NaOH buffer (pH 6.5). Samples were thawed in the dark for 1 h at 0 °C before treatment or measurement. Chlorophyll concentrations were determined in 80% acetone, according to the method of Porra et al. [21].
Ca2+ depletion Calcium ions, PsbP, and PsbQ extrinsic proteins were removed from native PSII membranes together using a buffer solution containing 2 M NaCl, 0.4 M sucrose, and 25 mM MES (pH 6.5) [22]. The PSII preparations were incubated in this buffer at 0.5 mg/ml Chl for 15 min under room light (4–5 µE m−2 s−1) and at room temperature (22 °C). The resulting material was washed twice with buffer A, and re-suspended in buffer A. This preparation is referred to as PSII(–Ca) membranes. We do not use the chelator (EDTA or EGTA) during Ca2+ extraction, since the chelator binds to the Mn cluster changing its EPR signal in S2 state [23] and functional activity of Mn cluster [24]. The addition of the chelator is not necessary for Ca2+ extraction, since the extraction occurs only due to the change of affinity of calcium cation to its binding site when PsbQ and PsbP proteins are extracted [23]. The evidence of Ca extraction from OEC by 2 M NaCl is the inhibition of O2 evolution (the remaining activity may be about 10%) and the restoration of oxygen-evolving function by exogenous Ca2+ cations up to about 70% [24]. The absence of PsbP and PsbQ proteins in similar preparations obtained by us earlier was confirmed by polyacrylamide gel electrophoresis (see Table S1 in Supplementary Material). Mn content in Ca-depleted PSII membranes after NaCl treatment was 4.0 ± 0.2 Mn/RC.
Mn depletion by Tris treatment Manganese depletion was accomplished by incubating thawed PSII membranes (0.5 mg Chl/ml) in 0.8 M Tris–HCl buffer (pH 8.5) for 15 min in room light (4–5 µE m−2 s−1) at room temperature. The membranes were then pelleted by centrifugation in an Eppendorf centrifuge 5415R (16100g × 3 min), washed three times with buffer A and finally re-suspended in buffer A. These membranes, which do not contain any extrinsic proteins (including PsbO polypeptide), Ca2+ ion, and the Mn catalytic cluster, are called PSII(–Mn) membranes. The absence of all extrinsic proteins in similar preparations obtained by us earlier was confirmed by polyacrylamide gel electrophoresis (see Table S1 in Supplementary Material). Residual Mn content in Mn-depleted PSII membranes after Tris treatment was 0.3 ± 0.1 Mn/RC.
PSII preparations activity
DCPIP reduction activity To determine electron transport activity we measured the rate of the exogenous electron acceptor 2,6-dichlorophenolindophenol (DCPIP) photoreduction. XBDROY light diodes (Cree Inc., USA) with the emission maximum of 450 nm providing a saturating light intensity (1500 μE m−2 s−1) were used as the excitation light source. The reduction rate of DCPIP (40 μM) was determined spectrophotometrically from a change in the absorbance at 600 nm using the molar extinction coefficient for the deprotonated form of DCPIP (ε = 21.8 mM−1 cm−1 [25]). The electron transport activity of the native PSII membranes corresponds to 140–150 µmol DCPIP mg Chl−1 h−1. The concentration of Chl in all samples was 20 µg/ml.
O2-evolving activity Kinetics of a photoinduced oxygen evolution by PSII preparations were registered amperometrically using a closed Clark electrode. The measurements were carried out in a thermostatically controlled cell at 25 °C in the presence of 200 μM of an artificial electron acceptor 2,6-dichloro-p-benzoquinone (DCBQ). The oxygen evolution rate was calculated using a linear part of a kinetic curve for the first 10 s after the illumination was turned on. Calibration of a diffusion current magnitude was carried out using the value of the oxygen concentration in water balanced with air (253 μM). XBDROY light diodes were used as the excitation light source providing a saturating light intensity (1500 μE m−2 s−1). The O2-evolving activity of the native PSII membranes ranged from 450 to 550 µmol O2 mg Chl−1 h−1. The concentration of Chl in all samples was 10 µg/ml.
Fluorescence induction kinetics (FIK)
FIKs were measured using a portable Plant Efficiency Analyzer (Hansatech Instruments Ltd., UK). LED sources of excitation light (λmax = 650 nm; spectral range 580–710 nm) were used in the fluorimeter. The time resolution of fluorescence detection was 10 μs (within the initial 2 ms); 1 ms (within the time interval from 2 ms to 1 s); and 100 ms (time interval > 1 s). Fluorescence induction kinetics were measured at actinic (1500 μE m−2 s−1) intensity of the excitation light flux. The fluorescence signal at 50 μs after the application of continuous actinic light was defined as F0. The initial moment of fluorescence detection in the figures corresponds to 50 μs. A logarithmic time scale was used in the figures as it is commonly employed for the presentation of fluorescence induction kinetics. Dark incubation before measurements was 2 min. The concentration of Chl in all samples was 20 µg/ml.
Determination of the Mn content in Ca-depleted PSII membranes
The Mn assays were performed according to the method of Semin and Seibert [26] with minor modifications [27]. The absorbance at 450 nm was used for colorimetric determination of Mn(II) concentrations using 3,3′,5,5′-tetramethylbenzidine as a chromogenic reagent (extinction coefficient of 34 mM−1 cm−1) in the samples [28].
Metal ions treatment of PSII preparations
For the treatment of PSII preparation, we used the following salts: La(CH3COO)3; Cr(NO3)3; AlCl3 dissolved in buffer A with control of pH; 5 мM Tb2(SO4)3 dissolved in buffer A. It should be noted that acetate ion and NO3− anions can inhibit the functional activity of PSII membranes, but in concentration significantly higher than that used in the present work [29, 30]. 50% inhibition of O2-evolving activity is achieved at a concentration of 225 mM, and DCPIP reduction—650 mM sodium acetate [29].
Results and discussion
Interaction of Tb 3+ and La 3+ cations with PSII membranes
The lanthanides are very effective inhibitors of OEC in photosystem II and different Ln3+ cations were studied in several works [13, 14, 16, 31, 32]. Already in one of the initial studies [13], it was found that La3+ ions inhibit OEC due to the substitution of Ca2+ for La3+. These researchers have shown that La3+ competes with Ca2+ for binding site on the oxidizing side of PSII membranes. La3+ ion binds to the Ca-binding site more strongly than Ca2+ (the Ki for La3+ was estimated to be 0.05 mM compared to Km for Ca2+ of 0.6 mM [13]). Further proof of this is the fact that external Ca2+ failed to reactivate PSII treated by Ln3+ [31]. However, bound Ln3+ ion can be removed using EDTA and oxygen-evolving activity can be reconstituted by adding back Ca2+ [31]. Most often La3+, which has the ionic radius similar to the ionic radius of Ca2+ (1.17 Å and 1.14 Å respectively), was used in these investigations. Recently, we investigated the effect of Tb3+ ions with similar ionic radius (1.06 Å) [18] on the PSII OEC function. Terbium is one of the poorly studied lanthanides and in this study we probed terbium effects on the OEC by monitoring the fluorescence induction kinetic and oxygen evolution rate. Our results have shown that Tb3+ inhibits O2 evolution at low concentration (50% inhibition is observed at concentration 0.5 mM) competing with Ca, since the addition of exogenous Ca2+ provided a significant protection of OEC against the inhibiting action of Tb3+ [18]. This result indicates that Tb3+ cation as well as La3+ [13] substitutes for Ca2+ cation in the OEC in the native PSII membranes.
In the above-mentioned works, the measurement of Ln3+ inhibition efficiency of PSII functional activity was carried out using O2 evolution registration. Here, we investigated the effect of lanthanide ions on the electron transport rate in PSII, measuring the rate of DCPIP (artificial electron acceptor) reduction spectroscopically. It is necessary to note that these methods are not equivalent for measurement of electron transport in some PSII membranes. For example, in PSII(-Ca) membranes obtained by NaCl washing extraction of Ca2+ together with extrinsic proteins PsbP and PsbQ, O2 evolving activity becomes about 10% from initial value whereas the rate of DCPIP reduction is about 70% [24]. Existence of rather intensive electron transport in PSII(-Ca) membranes is determined by water oxidation in the absence of Ca ion in the OEC not up to oxygen, but only up to H2O2 [33]. The obtained results are shown in Fig. 1 and demonstrate that La3+ is more active in the inhibition of O2 evolution activity (concentration of 50% inhibition is 0.145 mM) than Tb3+ (concentration of 50% inhibition is 0.484 mM, Ki = 323 ± 7 µM). Inhibition constants Ki were determined using Dixon analysis. Inhibition of DCPIP reduction reaction is observed at large concentrations of lanthanides: concentrations of 50% inhibition are equal 0.287 mM for La3+ and 1.21 mM for Tb3+ (Ki = 454 ± 9 µM) (Fig. 1). It means that the replacement of Ca2+ in the OEC with Ln3+ inhibits more the reaction of O2 synthesis than the reaction of water oxidation, i.e., calcium cation participates mainly on the last steps of the catalytic cycle in O2 evolution process.
Effect of Tb3+ (solid symbols) and La3+ (open symbols) on the oxygen evolution (circles) and DCPIP reduction (squares) in the native PSII membranes. The samples were incubated with Tb3+ or La3+ cations for 3 min at room temperature in the dark before measurements of O2 evolution or DCPIP reduction. Concentration of PSII membranes in sample was 20 µg Chl/ml. 100% is the rate of O2 evolution (450–550 µmol O2 mg Chl−1 h−1) or DCPIP reduction (150–180 µmol DCPIP mg Chl−1 h−1) by native PSII membranes in buffer A
Thus, the inhibitory effect of La3+ cations on the oxygen-evolving activity of PSII membranes saturates at about 20% in the 0.7–2 mM region (concentration dependence on Fig. 1) at incubation time 3 min. The saturation level for Tb3+ is about 30% (1.3–2 mM region). We can suggest some versions of a hypothetical explanation for such character of inhibition concentration dependence with saturation at some level of oxygen release. First, Ca2+ depletion effectively inhibits the oxygen evolution [residual activity 10–25% (24 and references therein)]; however, the water oxidation reaction is inhibited significantly less [residual activity 70–75% (24 and references therein)]. Water is oxidized in such membranes to hydrogen peroxide. The generated H2O2 can split producing the molecular oxygen. Possibly, the OEC with Ln3+ cation instead of Ca2+ have increased catalase activity and residual O2 concentration is determined by H2O2 splitting. Second, the concentration dependence of inhibition by Ln3+ cations’ O2 evolution indicates that in some part of PSII membranes, O2 evolution reaction is not inhibited completely. It means that in some part of PSII samples, OEC is more resistant to the Ln3+ cation action, i.e., PSII preparations can be heterogenous. Heterogeneity of the OEC can be determined for example by the next reason. In the dark (during the treatment by Ln3+ cations), about 25% of the PSII complexes are in the S0 state, whereas the remaining 75% are in the S1 state. Extraction of Ca2+ cation from the OEC needs room light [22], i.e., Ca2+ is extracted better when the Mn cluster is in the higher S states. Therefore, we can suggest that the remaining O2 evolution activity (≈20–30%) after the treatment of PSII by Ln3+ is determined by slow extraction of Ca2+ from the S0 state (≈25%) of the OEC.
Interaction of Tb 3+ cations with PSII(–Ca) membranes
Ca2+ cation can be efficiently extracted from the OEC by incubating the PSII membranes under light in a buffer supplemented with highly concentrated NaCl [22]. NaCl-treated PSII membranes lose extrinsic proteins PsbP and PsbQ as well as calcium ions from the OEC. The rate of O2 evolution decreases to less than 10%, but can be restored by addition of Ca2+ ions to a concentration of about 10 mM. Another feature of PSII(–Ca) membranes is the availability of OEC for bulky reductants [34] and cations [34, 35], since the OEC is not protected by extrinsic proteins. The effect of different Ln3+ on the Ca2+-depleted PSII membranes without 24 kDa extrinsic protein (their properties are similar to PSII(-Ca) membranes) was studied by Ono [14] and Ki values of these lanthanoids were determined. In our work, we investigated the effect of Tb3+ on the electron transfer in PSII(–Ca) membranes prepared by NaCl washing. Titration of Tb3+ effect is presented in Fig. 2. After incubation of samples with Tb3+, the rate of oxygen evolution was measured in the presence of 10 mM Ca2+. The curve with solid circles represents the concentration dependence of Tb3+ effect on the oxygen evolution. It shows that Tb3+ inhibits the electron transport in PSII(–Ca) membranes in the same concentration range as in native PSII membranes (compare Figs. 1 and 2). These data indicate that the Tb3+ cation inhibits the O2 evolution due to tightly binding to the Ca-binding site and Ca2+ ion added after incubation of PSII(–Ca) membranes with Tb3+ cannot replace bound terbium cation. However, if Ca2+ cations were present during incubation of membranes with Tb3+, the level of inhibition was significantly smaller (Fig. 2), i.e., Ca2+ ions prevent the interaction of Tb3+ ions with Ca site. The same results were obtained in the case of native PSII membranes: the presence of Ca2+ during La3+ treatment suppressed the inhibition of oxygen evolution [13]. This result shows that Tb3+ ions interact with the Ca-binding site of the OEC in the PSII(–Ca) membranes as in the native membranes. We calculated the inhibition constant for inhibition of electron transport in the PSII(–Ca) membranes by Tb3+ cations (Ki) using the Dixon plot (1/initial rate vs inhibitor concentration; [36]). The estimated Ki is equal to about 139 ± 5 µM. This value is similar to the Ki for inhibition of electron transport in Ca-depleted PSII membranes by La3+ (200 µM) [14].
Effect of Tb3+ (curve with solid circles) on the oxygen evolution in the PSII(-Ca) membranes. PSII(-Ca) membranes (10 µg Chl/ml) were incubated for 3 min at room temperature in the dark, in a buffer A and the indicated concentration of Tb3+ and then were assayed for oxygen evolution activity in the presence of 10 mM Ca2+ and 0.2 mM DCBQ (solid circles). The curve with open circles shows the Tb3+ effect on the oxygen evolving activity when 10 mM Ca2+ was included during terbium treatment. Control activity was 320–390 µmol O2 mg Chl−1 h−1 in the presence of 10 mM Ca2+
Figure 3 shows the time-dependent change of O2 evolution in PSII(–Ca) membranes during treatment with Tb3+ cations. Sample membranes at 0.25 mg Chl/ml were incubated in the dark with 1.0 mM Tb3+ in the presence of 30 mM Ca2+ (solid circles) or its absence (half open circles) for the indicated time at room temperature. O2 evolution activity was measured in the presence of 10 mM Ca2+ after 50-fold dilution. Time dependence for Tb3+ is similar to the time dependence for La3+ determined by Ghanotakis et al. [13]. It is necessary to note that the protective effect of Ca2+ in the time course experiment (Fig. 3) is more pronounced than in the results presented in Fig. 2 (concentration dependence). The possible reason for this can be a significant difference in the number of Tb3+ cations per reaction center (RC). This ratio is equal to 1000 in the time course experiment (Fig. 3) and 50,000 in the concentration experiment (Fig. 2). It means that in the experiment presented in Fig. 2, Tb3+ cations in large concentration can partially destroy the Mn cluster. The release of Mn from OEC during incubation of PSII membranes with La3+ was reported by Ghanotakis et al. [13].
The time course of Tb3+ cation interaction with Ca2+-binding site in PSII(-Ca) membranes. Samples (0.25 mg Chl/ml) were incubated with 1 mM Tb3+ (solid circles), 1 mM Tb3+ plus 30 mM Ca2+ (half open circles) and no addition (open circles) for various times in the dark at room temperature. O2 evolution activity was measured in the presence of 10 mM Ca2+ after 50-fold dilution of sample suspension. 100% activity represents 320–390 µmol O2 mg Chl−1 h−1 for Ca-depleted PSII membranes in the presence of 10 mM Ca2+.
Interaction of Tb 3+ and La 3+ cations with PSII(–Mn) membranes
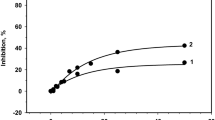
PSII membranes treated with Tris at alkaline pH for OEC extraction have no Mn and Ca ions as well as all three extrinsic proteins and are not able to split water evolving the molecular oxygen. However, such membranes can oxidize exogenous electron donors under light and in the presence of exogenous electron acceptor like DCPIP and therefore the function of electron transport chain can be investigated. PSII(–Mn) membranes can be useful to study the localization of an inhibition site—is it OEC or, for example, the acceptor side of PSII. In the present work, we studied the effect of Tb3+ and La3+ ions on the electron transport chain in PSII(–Mn) membranes prepared by Tris treatment. Electron transport was supported by an exogenous electron donor (Mn2+ + H2O2). This electron donor donates electrons only via the HA Mn-binding site [37,38,39,40]. Electron transfer was probed by measurement of DCPIP reduction. Unexpectedly, we found that Tb3+ and La3+ ions inhibit DCPIP reduction supported by (Mn2+ + H2O2) electron donor at very small concentration (Fig. 4). The concentration of 50% inhibition is about 1 µM for Tb3+ ion (Fig. 4a) and about 3 µM for La3+ ion (Fig. 4b), compared with the concentrations of 50% OEC inhibition in the native PSII membranes—484 µM for Tb3+ and 145 µM for La3+ (Fig. 1). We calculated the inhibition constant for Tb3+ and La3+ cations inactivating electron transport supported by (Mn2+ + H2O2) donor in the PSII(–Mn) membranes using the Dixon plot. Estimated Ki values for inhibition of electron transport are equal to 2.10 ± 0.03 µM for Tb3+ and 8.3 ± 0.4 µM for La3+ (Fig. 5).
Effect of different Tb3+ (a) and La3+ (b) concentrations on the light-dependent DCPIP reduction in PSII(-Mn) membranes with (Mn2+ + H2O2) system (open circles) or DPC (solid circles) as artificial electron donors. Concentration of PSII(-Mn) membranes in sample was 20 µg Chl/ml. 100% is the rate of DCPIP reduction (120 or 90 µmol DCPIP mg Chl−1 h−1) by PSII(-Mn) membranes in buffer A with (Mn2+ + H2O2) donor system or DPC respectively. A 5 mM Tb2(SO4)3 (10 mM Tb3+) solution was prepared using buffer A. Prior to measurements, preparations were incubated with Tb3+ or La3+ for 2 min in the dark at room temperature
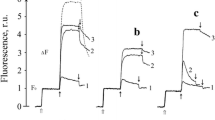
The effective inhibition of Mn2+ oxidation at the HA site by Tb3+ and La3+ cations is supported by the measurement of fluorescence induction kinetics (FIK) presented on Fig. 6a, b respectively. In native PSII samples, the FIK curve exhibits three characteristic points corresponding to F0 (point O), the yield of fluorescence when QA is reduced (point J), and the Fmax level (point P), where the plastoquinone pool (which quenches fluorescence in the oxidized form [41]) is also reduced. Extraction of the Mn cation from the OEC significantly changes the FIK curve, where a new peak K, close to point J seen in native PSII membranes, appears and indicates QA reduction [42]. However, the fluorescence yield subsequently decreases after peak K since there is no additional electron transfer from the donor side of PSII to continue to reduce QA, and QA is rapidly oxidized by QB (Fig. 6a, b, curves 1). Addition of Tb3+ or La3+ cations to PSII(–Mn) membranes does not influence the shape of the FIK curve (Fig. 6a, b, curves 2), whereas in the presence of (Mn2+ + H2O2) electron donor FIK is significantly changed (Fig. 6a, b, curves 3). In this case, the FIK is similar to the kinetics of native PSII membranes with intact electron transport. The shape of the FIK curve in PSII(–Mn) membranes incubated with Tb3+ or La3+ before addition of (Mn2+ + H2O2) donor is closer to the kinetics in PSII(–Mn) membranes without donor (Fig. 6a, b, curves 4). These results indicate that Ln3+ cation inhibits the Mn2+ oxidation at the PSII(-Mn) donor side. It is necessary to note that FIK measurement was carried out without artificial electron acceptor, allowing to eliminate possible artifacts due to reduction/oxidation of acceptor DCPIP.
Effect of Tb3+ (a) and La3+ (b) on the chlorophyll a fluorescence induction curves for dark adapted PSII(-Mn) membranes (20 μg Chl/ml) in buffer A. Curves 1 in a and b represent FIK in PSII(-Mn) membranes. Curves 2 in a and b represent FIK in PSII(-Mn) membranes incubated with Tb3+ (2 mM) or La3+ (2 mM), respectively, for 2 min at room temperature in dark before measurement. Curves 3 in a and b: FIK in PSII(-Mn) membranes with exogenous electron donor (Mn2+ + H2O2) (2 µM and 3 mM respectively). Curves 4 in a and b: FIK in PSII(-Mn) membranes after incubation with Tb3+ (2 mM) or La3+ (2 mM), respectively, in the presence of exogenous electron donor (Mn2+ + H2O2) during measurement
Thus, the inhibition of electron transport in PSII(-Mn) membranes by lanthanoid cations has very high efficiency and Ki values of this reaction are significantly smaller than Ki value for inhibition of electron transport in PSII(-Ca) membranes (Ki is 140 µM for Tb3+ or 200 µM for La3+) [14]. Since the inhibition of electron transport in intact PSII and PSII(-Ca) is carried out by binding of Ln3+ cations to Ca-binding site, we can conclude that the binding site for Tb3+ and La3+ in PSII(-Mn) membranes is not Ca-binding site. This conclusion is confirmed by the results of the next experiment where, before incubation of PSII(-Mn) membranes, we added together Tb3+ cations (10 µM) and Ca2+ cations (the indicated concentration). After incubation in dark at room temperature for 3 min, the sample was centrifuged and after washing the membranes were suspended in buffer A. Electron transport activity of treated membranes was measured using (Mn2+ + H2O2) donor and DCPIP as acceptor of electrons. The obtained results are presented in Fig. 7. These data demonstrate that Ca does not affect the interaction of Tb3+ with the inhibition site and provide additional evidence that Tb3+ binding site is different from Ca-binding site. It is necessary to note that our preliminary studies have shown that Tb3+ cation binds to the inhibition site very tightly and cannot be removed by centrifugation of samples.
Ca2+ effect on the inhibition of electron transport in PSII(-Mn) membranes by Tb3+ cations. PSII(-Mn) membranes (40 μg Chl/ml) were incubated with 10 μM Tb3+ and indicated Ca2+ concentration for 3 min in the dark at room temperature. The membranes were then pelleted by centrifugation (16100 g × 5 min) at 4 °C and re-suspended in buffer A (20 μg Chl/ml). The rate of electron transport was measured as DCPIP reduction in the presence of artificial electron donor (Mn2+ + H2O2)
The donation of electrons by (Mn2+ + H2O2) is realized through the HA Mn-binding site [37,38,39,40]. In the process of donation, Mn2+ cation binds to this site and the bound cation is oxidized by YZ·, then Mn3+ is reduced by H2O2. Therefore, it is reasonable to suggest that Ln3+ cations inhibit the Mn2+ oxidation by interacting with the HA site. It is known that another electron donor, DPC, also donates electrons via HA site along with donation through the low affinity site [39, 43]. DPC at 200 µM donates to both sites at the same rate, but addition of several µM MnCl2 non-competitively inhibits DPC photooxidation only at the HA site [43,44,45]. The result is a 50% decrease in the rate of DCPIP photoreduction. This effect has been used as the “DPC inhibition assay” for investigation of the HA site [43, 45, 46]. It was shown with using of this test that Mn2+ cations inhibit the process of DPC oxidation at the HA site with Ki = 1.5 µM [46]. The inhibition of DPC oxidation at the HA site was observed also for Fe2+ cations in the same range of concentration (several µM) [47]. Thus, Mn2+ and Fe2+ cations are similar to Tb3+ and La3+ cations concerning the concentration required for inhibition and Ki values.
Although in these investigations Mn2+ and Fe2+ cations were used, the inhibition of the HA site is realized by trivalent cations since the inhibition of HA site is observed only under illumination which provides oxidation of these cations [43, 47, 48]. Taking into account the fact that Ln3+ cations are also trivalent cations and that Mn cation bound to the HA site (Mn4) is also in trivalent state [3], we can suggest that valency can be important for interaction of metal cations with the HA site. Therefore, we studied the influence on the HA site of some other metal cations and presented the results in the Table 1. We found that trivalent cations like Cr3+ and Al3+ are less effective inhibitors than Ln3+. Divalent cation Cd2+ is almost inactive, but at the same time apparent Ki for Zn2+ is about 18 µM and 33 µM for Co2+ [46]. Thus, the available data do not clearly support the idea of relationship between metal cation valency and its efficiency in the inhibition of the HA site.
For investigation of the possible binding of Ln3+ cations to the HA site, we used “DPC inhibition assay”. According to this test, the inhibition of the HA site by micromolar concentration of inhibitors interacting with the HA site like Mn2+ [43, 45, 46] or Fe2+ [47, 49] is accompanied by inhibition of DPC oxidation only via the HA site. Considering that DPC donates at equal rates to HA site and low-affinity site, 100% inhibition of the HA site corresponds to a 50% decrease in the rate of DCPIP photoreduction by DPC (since after 100% inhibition of the HA site DPC continue to donate electrons via only the low affinity site). In our experiments, we found that at Ln3+ concentration which completely inhibits Mn2+ oxidation at the HA site, the rate of DCPIP reduction supported by DPC decreases by up to approximately 50% (Fig. 4). These results provide additional evidence that bound Ln3+ cations inhibit the HA site Mn-binding site. However, it should be noted that there is some difference in “DPC inhibition assay” for Mn2+or Fe2+ and Tb3+or La3+. Inhibition of DPC and (Mn2+ + H2O2) donor oxidation begins in fact at the same concentration of Mn2+or Fe2+, whereas in the case inhibition by Tb3+or La3+ these concentrations do not coincide (Fig. 4). Inhibition of DPC oxidation by lanthanoid cations starts when about 70–80% of the HA site is blocked by the cations. It can be suggested that probably the Mn3+/Fe3+ binding site partially does not coincide with the binding site for Tb3+/La3+.
Used in our study, the OEC-depleted PSII membranes are interesting objects for investigation of photosynthesis mechanisms (for example, for the study of one of the most important processes in photosynthesis—photoactivation). Recently, Zouni group [5] for the first time obtained the structure of PSII without Mn4CaO5 cluster (at 2.55 Å resolution) using T. elongatus PSII crystals. Unexpectedly, they found that extraction of Mn cluster is not accompanied by rearrangement of the metal-coordinating residues, i.e., HA Mn-binding site is in starting state and ready to bind metal cation. This may be important for effective binding of Ln3+ cation. OEC-depleted PSII crystals are able to bind Mn ions and the initial stages of photoactivation were investigated [5]. However, low resolution (4.5 Å) does not allow yet to draw certain conclusions about the structure of the partially reconstructed cluster. In this regard, the fact that we have found the high-efficient binding of Ln3+ cations with the HA Mn-binding site may be of some interest. The point is that Ln3+ cations have very good X-ray scattering properties [50]. Taking into account the possibility of Ln3+ cations binding to the HA site, they can be used as probes in X-ray crystallography instead of Mn ions to increase resolution. A similar approach was successfully applied by Kawakami et al. [51], who investigated Br− anions instead of Cl− anions. It should also be noted that HA Mn-binding site in the apo-PSII is occupied by Mn4 in the native PSII as has been proven by Asado and Mino with the use of pulsed EPR [52]. Binding metal cation is coordinated with axial ligands D170 and E333 in the D1 polypeptide which can effectively bind Ln3+ cations, since it is known that lanthanides typically bind ionically via oxygen-containing sidechains [50]. Based on the above data, we propose a hypothetical model for binding the lanthanide cation to the HA Mn-binding site (Fig. 8). This model is founded on the structure of PSII(-Mn) crystals obtained by Zouni group [5] (PDB:5MX2).
Proposed binding center of Ln3+ ions in PSII without Mn4CaO5 cluster. The model is based on the structure of apo-PSII obtained by Zhang et al. [5] (PDB:5MX2). Amino acid residues forming the ligand environment of metal cluster and the residue of redox-active tyrosine YZ are shown. The structure is built using the VMD program (v. 1.9.3)
Conclusions
It was shown in several investigations that lanthanide ions inhibit the OEC in PSII [13, 16,17,18, 31, 32] and PSII(-Ca) [14] membranes. Kinetic analysis suggests that lanthanides function as a mixed-type competitor for Ca2+ cation included in the OEC [13]. Unexpectedly in our study, we found that lanthanide cations (Tb3+ and La3+) strongly bind at the oxidizing side of PSII(-Mn) membranes in the dark. This binding cannot be prevented by addition of Ca2+ ions like the interaction of Ln3+ cations with PSII [13] and PSII(-Ca) [14] membranes. Bound Ln3+ cations inhibit the oxidation of Mn2+ cations of the electron donor system (Mn2+ + H2O2) via the HA Mn-binding site with Ki = 2.1 µM for Tb3+ ion and about 8.3 µM for La3+. It should be noted that Mn2+ cations inhibit the DPC oxidation via the HA site with similar Ki = 1.5 µM [46]. Inhibition of electron donation by (Mn2+ + H2O2) donor is accompanied by inhibition of DPC oxidation up to 50%. These results indicate that Ln3+ cations bind directly or close to the HA Mn-binding site in PSII(-Mn) membranes. Taking into account the fact that Mn2+ and Fe2+ cations bind to HA site as trivalent cations after light-induced oxidation and that Mn cation bound to the HA site (Mn4) is also in trivalent state, we can suggest that valency may be important for interaction of Ln3+ with the HA site.
Abbreviations
- Chl:
-
Chlorophyll
- DCBQ:
-
2,6-Dichloro-p-benzoquinone
- DCPIP:
-
2,6-Dichlorophenolindophenol
- DPC:
-
Diphenylcarbazide
- HA:
-
High-affinity Mn-binding site
- MES:
-
2-(N-Morpholino)-ethanesulfonic acid
- Ln3 + :
-
Lanthanide ions
- OEC:
-
Oxygen-evolving complex
- PSII:
-
Photosystem II
- PSII(-Ca):
-
Ca2+-depleted PSII membranes
- PSII(-Mn):
-
Mn-depleted PSII membranes
- RC:
-
Reaction center
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60. https://doi.org/10.1038/nature09913
Najafpour MM, Renger G, Hołyńska M, Moghaddam AN, Aro EM, Carpentier R, Nishihara H, Eaton-Rye JJ, Shen J-R, Allakhverdiev SI (2016) Manganese compounds as water-oxidizing catalysts: from the natural water-oxidizing complex to nanosized manganese oxide structures. Chem Rev 116:2886–2936. https://doi.org/10.1021/acs.chemrev.5b00340
Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen J-R (2015) Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517:99–103. https://doi.org/10.1038/nature13991
Young ID, Ibrahim M, Chatterjee R, Gul S, Fuller FD, Koroidov S, Brewster AS, Tran R, Alonso-Mori R, Kroll T, Michels-Clark T, Laksmono H, Sierra RG, Stan CA, Hussein R, Zhang M, Douthit L, Kubin M, de Lichtenberg C, Pham LV, Nilsson H, Cheah MH, Shevela D, Saracini C, Bean MA, Seuffert I, Sokaras D, Weng TC, Pastor E, Weninger C, Fransson T, Lassalle L, Brauer P, Aller P, Docker PT, Andi B, Orville AM, Glownia JM, Nelson S, Sikorski M, Zhu DL, Hunter MS, Lane TJ, Aquila A, Koglin JE, Robinson J, Liang MN, Boutet S, Lyubimov AY, Uervirojnangkoorn M, Moriarty NW, Liebschner D, Afonine PV, Waterman DG, Evans G, Wernet P, Dobbek H, Weis WI, Brunger AT, Zwart PH, Adams PD, Zouni A, Messinger J, Bergmann U, Sauter NK, Kern J, Yachandra VK, Yano J (2016) Structure of photosystem II and substrate binding at room temperature. Nature 540:453–457. https://doi.org/10.1038/nature20161
Zhang M, Bommer M, Chatterjee R, Hussein R, Yano J, Dau H, Kern J, Dobbek H, Zouni A (2017) Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. eLife 6:e26933. https://doi.org/10.7554/eLife.26933
Wei X, Su X, Cao P, Liu X, Chang W, Li M, Zhang X, Liu Z (2016) Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature 534:69–74. https://doi.org/10.1038/nature18020
Yocum CF (1991) Calcium activation of photosynthetic water oxidation. Biochim Biophys Acta 1059:1–15. https://doi.org/10.1016/S0005-2728(05)80182-3
Shamsipur M, Pashabadi A (2018) Latest advances in PSII features and mechanism of water oxidation. Coord Chem Rev 374:153–172. https://doi.org/10.1016/j.ccr.2018.07.006
Bang S, Lee Y-M, Hong S, Cho K-B, Nishida Yu, Seo MS, Sarangi R, Fukuzumi S, Nam W (2014) Redox-inactive metal ions modulate the reactivity and oxygen release of mononuclear non-haem iron(III)–peroxo complexes. Nat Chem 6:934–940. https://doi.org/10.1038/nchem.2055
McEvoy JP, Brudvig GW (2006) Water-splitting chemistry of photosystem II. Chem Rev 106(11):4455–4483. https://doi.org/10.1021/cr0204294
Waggoner CM, Yocum CF (1990) Calcium activated oxygen evolution. In: Baltscheffsky M (ed) Current research in photosynthesis. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-0511-5_167
Ghanotakis DF, Babcock GT, Yocum CF (1984) Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted photosystem II preparations. FEBS Lett 167:127–130. https://doi.org/10.1016/0014-5793(84)80846-7
Ghanotakis DF, Babcock GT, Yocum CF (1985) Structure of the oxygen-evolving complex of Photosystem II: calcium and lanthanum compete for sites on the oxidizing side of Photosystem II which control the binding of water-soluble polypeptides and regulate the activity of the manganese complex. Biochim Biophys Acta 809:173–180. https://doi.org/10.1016/0005-2728(85)90060-X
Ono T (2000) Effects of lanthanide substitution at Ca2+-site on the properties of the oxygen evolving center of photosystem II. J Inorg Biochem 82:85–91. https://doi.org/10.1016/S0162-0134(00)00144-6
Kretsinger RH, Nelson DJ (1976) Calcium in biological systems. Coord Chem Rev 18:29–124. https://doi.org/10.1016/S0010-8545(00)82054-8
Lee C-I, Lakshmi KV, Brudvig GW (2007) Probing the functional role of Ca2+ in the oxygen-evolving complex of photosystem II by metal ion inhibition. Biochemistry 46:3211–3223. https://doi.org/10.1021/bi062033i
Bakou A, Ghanotakis DF (1993) Substitution of lanthanides at the calcium site(s) in photosystem II affects electron transport from tyrosine Z to P680+. Biochim Biophys Acta 1141:303–308. https://doi.org/10.1016/0005-2728(93)90057-M
Loktyushkin AV, Lovyagina ER, Semin BK (2019) Interaction of terbium cations with the donor side of photosystem II in higher plants. Moscow Univ Biol Sci Bull 74:81–85. https://doi.org/10.3103/S009639251902007X
Ghanotakis DF, Babcock GT (1983) Hydroxylamine as an inhibitor between Z and P680 in photosystem II. FEBS Lett 153:231–234. https://doi.org/10.1016/0014-5793(83)80154-9
Dunahay TG, Staechelin LA, Seibert M, Ogilvie PD, Berg SP (1984) Structural biochemical and biophysical characterization of four oxygen-evolving photosystem 2 preparations from spinach. Biochim Biophys Acta 764:179–193. https://doi.org/10.1016/0005-2728(84)90027-6
Porra RJ, Tompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll a and chlorophyll b extracted with 4 different solvents—verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Ono T, Inoue Y (1990) Abnormal redox reactions in photosynthetic O2-evolving centers in NaCl/EDTA-washed PS II. A dark-stable EPR multiline signal and an unknown positive charge accumulator. Biochim Biophys Acta 1020:269–277. https://doi.org/10.1016/0005-2728(90)90157-Y
Boussac A, Zimmermann J-L, Rutherford AW (1990) Factors influencing the formation of modified S2 EPR signal and the S3 EPR signal in Ca2+-depleted photosystem II. FEBS Lett 277:69–74. https://doi.org/10.1016/0014-5793(90)80811-V
Semin BK, Davletshina LN, Ivanov II, Rubin AB, Seibert M (2008) Decoupling of the processes of molecular oxygen synthesis and electron transport in Ca2+-depleted PSII membranes. Photosynth Res 98:235–249. https://doi.org/10.1007/s11120-008-9347-5
Armstrong JM (1964) The molar extinction coefficient of 2,6-dichlorophenol indophenol. Biochim Biophys Acta 86(1):194–197. https://doi.org/10.1016/0304-4165(64)90180-1
Semin BK, Seibert M (2009) A simple colorimetric determination of the manganese content in photosynthetic membranes. Photosynth Res 100:45–48. https://doi.org/10.1007/s11120-009-9421-7
Semin BK, Davletshina LN, Ivanov II, Seibert M, Rubin AB (2012) Rapid degradation of the tetrameric Mn cluster in iluminated, PsbO-depleted photosystem II preparations. Biochemistry (Moscow) 77:152–156. https://doi.org/10.1134/S0006297912020058
Serrat FB (1998) 3,3′,5,5′-Tetramethylbenzidme for the colorimetric determination of manganese in water. Microchim Acta 129:77–80. https://doi.org/10.1007/BF01246852
Lovyagina ER, Semin BK (2016) Mechanism of inhibition and decoupling of oxygen evolution from electron transfer in photosystem II by fluoride, ammonia and acetate. J Photochem Photobiol B 158:145–153. https://doi.org/10.1016/j.jphotobiol.2016.02.031
Lovyagina ER, Belevich NP, Semin BK (2016) Inhibition of photosystem II electron transport chain by ammonia and “decoupling effect”. Modern trends in biological physics and chemistry (BPPC) 1:95−98. https://pureportal.spbu.ru/files/9280949/Proceedings_BPPC_2016_Vol_1.pdf#page=96
Bakou A, Buser C, Dandulakis G, Brudvig G, Ghanotakis DF (1992) Calcium binding site(s) of photosystem II as probed by lanthanides. Biochim Biophys Acta 1099:131–136. https://doi.org/10.1016/0005-2728(92)90209-K
Riggs-Gelasco PJ, Mei R, Ghanotakis DF, Yocum CF, Penner-Hahn JE (1996) X-ray absorption spectroscopy of calcium-substituted derivatives of the oxygen-evolving complex of photosystem II. J Am Chem Soc 118:2400–2410. https://doi.org/10.1021/ja9504505
Semin BK, Davletshina LN, Timofeev KN, Ivanov II, Rubin AB, Seibert M (2013) Production of reactive oxygen species in decoupled, Ca2+-depleted PSII and their use in assigning a function to chloride on both sides of PSII. Photosynth Res 117:385–399. https://doi.org/10.1007/s11120-013-9870-x
Ghanotakis DF, Topper JN, Youcum CF (1984) Structural organization of the oxidizing side of photosystem II. Exogenous reductants reduce and destroy the Mn-complex in photosystems II membranes depleted of the 17 and 23 kDa polypeptides. Biochim Biophys Acta 767(3):524−531. https://doi.org/https://doi.org/10.1016/0005-2728(84)90051-3
Semin BK, Seibert M (2016) Substituting Fe for two of the four Mn ions in photosystem II—effects on water-oxidation. J Bioenerg Biomembr 48:227–240. https://doi.org/10.1007/s10863-016-9651-2
Segel IW (1993) Enzyme kinetics behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley, New York
Inoue H, Wada T (1987) Requirement of manganese for electron donation of hydrogen peroxide in Photosystem II reaction center complex. Plant Cell Physiol 28:767–773. https://doi.org/10.1093/oxfordjournals.pcp.a077357
Boussac A, Picaud M, Etienne A-L (1986) Effect of potassium iridic chloride on the electron donation by Mn2+ to photosystem II particles. Photobiochem Photobiophys 10:201–211
Semin BK, Davletschina LN, Aleksandrov AYu, Lanchinskaya VYu, Novakova AA, Ivanov II (2004) pH-dependence of iron binding to the donor side of photosystem II. Biochemistry (Moscow) 69:410–419. https://doi.org/10.1023/B:BIRY.0000022066.38297.8a
Ono T-A, Mino H (1999) Unique binding site for Mn2+ ion responsible for reducing an oxidized YZ tyrosine in manganese-depleted photosystem II membranes. Biochemistry 38:8778–8785. https://doi.org/10.1021/bi982949s
Pospíšil P, Dau H (2000) Chlorophyll fluorescence transients of photosystem II membrane particles as a tool for studying photosynthetic oxygen evolution. Photosynth Res 65:41–52. https://doi.org/10.1023/A:1006469809812
Strasser BJ (1997) Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth Res 52:147–155. https://doi.org/10.1023/A:1005896029778
Hsu B-D, Lee J-Y, Pan R-L (1987) The high-affinity binding site for manganese on the oxidizing side of Photosystem II. Biochim Biophys Acta 890:89–96. https://doi.org/10.1016/0005-2728(87)90072-7
Preston C, Seibert M (1991) The carboxyl modifier 1-ethyl-3-[3-(dimethylamino) propyl] carbodiimide (EDC) inhibits half of the high-affinity manganese-binding site in photosystem II membrane fragments. Biochemistry 30:9615–9624. https://doi.org/10.1021/bi00104a008
Seibert M, Tamura N, Inoue Y (1989) Lack of photoactivation capacity in Scenedesmus obliquus LF-1 results from loss of half the high-affinity manganese-binding site: relationship to the unprocessed D1 protein. Biochim Biophys Acta 974:185–191. https://doi.org/10.1016/S0005-2728(89)80371-8
Ghirardi ML, Lutton TW, Seibert M (1996) Interactions between diphenylcarbazide, zinc, cobalt, and manganese on the oxidizing side of photosystem II. Biochemistry 35:1820–1828. https://doi.org/10.1021/bi951657d
Semin BK, Ivanov II, Rubin AB, Parak F (1995) High-specific binding of Fe(II) at the Mn-binding site in Mn-depleted PSII membranes from spinach. FEBS Lett 375:223–226. https://doi.org/10.1016/0014-5793(95)01215-Z
Hoganson CW, Ghanotakis DF, Babcock GT, Yocum CF (1989) Mn2+ reduces Y+ in manganese-depleted photosystem II preparations. Photosynth Res 22:285–293. https://doi.org/10.1007/BF00048306
Semin BK, Ghirardi ML, Seibert M (2002) Blocking of electron donation by Mn(II) to YZ• following incubation of Mn-depleted photosystem II membranes with Fe(II) in the light. Biochemistry 41:5854–5864. https://doi.org/10.1021/bi0200054
Boggon TJ, Shapiro L (2000) Screening for phasing atoms in protein crystallography. Structure 8:R143–R149. https://doi.org/10.1016/s0969-2126(00)00168-4
Kawakami K, Umena Y, Kamiya N, Shen J-R (2009) Location of chloride and its possible functions in oxygen-evolving photosystem II revealed by X-ray crystallography. PNAS 106:8567–8572. https://doi.org/10.1073/pnas.0812797106
Asada M, Mino H (2015) Location of the high-affinity Mn2+ site in photosystem II detected by PELDOR. J Phys Chem B 119:10139–10144. https://doi.org/10.1021/acs.jpcb.5b03994
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767. https://doi.org/10.1107/S0567739476001551
Semin BK, Davletshina LN, Rubin AB (2019) Effect of sucrose-bound polynuclear iron oxyhydroxide nanoparticles on the efficiency of electron transport in the photosystem II membranes. Photosynth Res 142:57–67. https://doi.org/10.1007/s11120-019-00647-4
Acknowledgements
We are grateful to Dr. S. Vassiliev (University of New Brunswick, Canada) for technical comments and editorial advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lovyagina, E.R., Loktyushkin, A.V. & Semin, B.K. Effective binding of Tb3+ and La3+ cations on the donor side of Mn-depleted photosystem II. J Biol Inorg Chem 26, 1–11 (2021). https://doi.org/10.1007/s00775-020-01832-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01832-w