Abstract
Metallothioneins (MTs) are small cysteine-rich proteins coordinating various transition metal ions, including ZnII, CdII, and CuI. MTs are ubiquitously present in all phyla, indicating a successful molecular concept for metal ion binding in all organisms. The plant MT Ec-1 from Triticum aestivum, common bread wheat, is a ZnII-binding protein that comprises two domains and binds up to six metal ions. The structure of the C-terminal four metal ion binding βE domain was recently described. Here we present the structure of the N-terminal second domain, γ-Ec-1, determined by NMR spectroscopy. The γ-Ec-1 domain enfolds an M II2 Cys6 cluster and was characterized as part of the full-length Zn6Ec-1 protein as well as in the form of the separately expressed domain, both in the ZnII-containing isoform and the CdII-containing isoform. Extended X-ray absorption fine structure analysis of Zn2γ-Ec-1 clearly shows the presence of a ZnS4 coordination sphere with average Zn–S distances of 2.33 Å. 113Cd NMR experiments were used to identify the MII-Cys connectivity pattern, and revealed two putative metal cluster conformations. In addition, the general metal ion coordination abilities of γ-Ec-1 were probed with CdII binding experiments as well as by pH titrations of the ZnII and CdII forms, the latter suggesting an interaction of the γ domain and the βE domain within the full-length protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
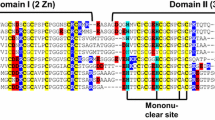
Metallothioneins (MTs) are low molecular mass (2–10 kDa) and Cys-rich proteins with a preference for the coordination of metal ions with d 10 electron configuration, e.g., ZnII, CuI, and CdII [1]. Their occurrence is reported throughout the animal kingdom, in plants, in several eukaryotic microorganisms, as well as in some prokaryotes [2]. The plant MT Ec-1 from wheat consists of 81 amino acids. All of the 17 Cys and two His residues are involved in the coordination of six divalent metal ions that are arranged in two separate metal-binding domains (Fig. 1) [3].
Amino acid sequence of full-length wheat Ec-1 with the Cys-rich metal ion coordinating regions highlighted in gray (top) as well as a schematic representation giving the sort and number of coordinating amino acids. The N-terminal Cys-rich region harbors the Zn2Cys6 cluster described herein, and the central and C-terminal regions together form the Zn4βE domain
Ec-1 is the first and so far only plant MT which has been successfully isolated from plant material [4]. Ec-1 is most abundant in wheat embryos and is present in the ZnII form [5]. Unlike most MTs, Ec-1 also recruits two His residues for ZnII binding, a feature so far only observed in a cyanobacterial MT form [6]. This ligand specificity usually distinguishes MTs from ZnII-binding enzymes, where the ZnII coordination sphere often consists of a mixture of sulfur-, nitrogen-, and oxygen-donor ligands. In consequence, the resulting metal clusters show a limited variety of possible structures. This is demonstrated by the fact that only two basic cluster arrangements have been structurally described for divalent metal ions so far: The M II3 Cys9 cluster of the β domain of vertebrate, crustacean, and echinoderm MTs and the M II4 Cys11 cluster of the α domain from vertebrate and echinoderm MTs (e.g., Protein Data Bank codes 4MT2, 1DMC, 1DME, 1QJK, and 1QJL [7–9]). The Zn4Cys9His2 cluster of the cyanobacterium Synechococcus PCC 7942 is structurally related to the Zn4Cys11 cluster with exchange of two terminal thiolate ligands for imidazole moieties [6]. A Zn3Cys9 cluster with similarity to the M II3 Cys9 cluster mentioned above can be also found in the βE domain of wheat Ec-1, but the ZnII-coordinating residues are interleaved with the two Cys and His ligands of the additional mononuclear ZnII site [10]. This ZnCys2His2 site, although known from certain zinc-finger proteins, was unprecedented in the MT superfamily and is so far uniquely found in the plant Ec proteins. The limited structural variability of the metal ion binding sites in MTs is consistent with the constricted biological functionality known so far, mainly the participation in metal ion homeostasis and detoxification as well as protection against oxidative stress [11–13]. Wheat Ec-1 seems to have a potential role in plant development as inferred from its high abundance during embryogenesis [8]. Interestingly, the only regulatory element found so far in the upstream 5′ flanking region of the wheat Ec-1 messenger RNA (mRNA) is an abscisic acid responsive element analog [14]. Even more striking than the putative regulation by one of the major plant hormones involved in abscission, grain filling, desiccation, and embryogenesis [15] is the absence of a metal responsive regulatory element [14]. In addition, it is known that 25% of an entire 35S-labeled Cys pool is found in Ec-1 when wheat grain embryo mRNA is expressed in a cell-free expression system [4]. This indicates either a high rate of mRNA translation or a massive accumulation of Ec-1 mRNA and/or a failure of its degradation in the dried embryo, possibly regulated at the transcriptional level. From further studies, using 8K complementary DNA microarray technology, the Ec-1 transcript abundance was shown to correlate with grain dry weight only [16]. This behavior is found for only three other proteins, the defense proteins γ-purothionin and remorin-like protein and asparagine synthetase 2, a “housekeeping” enzyme. In that study, it was further shown that gene expression during wheat grain development can be divided into ten clusters. The Ec-1 transcript belongs to the tenth cluster, which comprises only ten of the 2,295 differentially expressed genes. The Ec-1 transcript level peaks at 35 days after anthesis, hence in the maturation and desiccation state, and disappears abruptly during the first hour of imbibition [14, 16]. Generally, such rapid decline of transcripts is indicative of redundant mRNA remaining after anthesis. This view is supported by the concomitant decrease of the Ec-1 protein level. Yet, despite the wealth of information, the role of Ec-1 still remains elusive.
In the following, we present the first 3D structure of the N-terminal, 24 amino acid residues comprising γ domain of wheat Ec-1 as determined by NMR spectroscopy. In total, three structures of different γ-Ec-1 forms are presented: the CdII and ZnII forms of the separately expressed domain and the Zn2γ-Ec-1 form as part of the full-length protein. All forms contain a M II2 Cys6 cluster, which is unprecedented for any MT. Extended X-ray absorption fine structure (EXAFS) studies confirm such an arrangement. Moreover, the metal ion binding properties of γ-Ec-1 are probed via pH titration and metal ion reconstitution experiments.
Materials and methods
Chemicals and solutions
113CdCl2 and 15NH4Cl were purchased from Cambridge Isotope Laboratories (Innerberg, Switzerland), d 11-tris(hydroxymethyl)aminomethane (d 11-Tris) was purchased from Euriso-top (Saint-Aubin, France), the enzymes used for plasmid construction and protein cleavage were purchased from Promega (Catalys, Wallisellen, Switzerland), Roche (Rotkreuz, Switzerland), GE Healthcare Europe (Glattbruck, Switzerland), or New England Biolabs (Ipswich, MA, USA), Luria–Bertani broth (Miller) was purchased from Chemie Brunschwig (Basel, Switzerland), and Chelex® 100 resin was purchased from Bio-Rad (Reinach, Switzerland). All other chemicals were ACS grade or comparable and were obtained from Sigma-Aldrich Chemie (Buchs, Switzerland), Calbiochem (VWR International, Lucerne, Switzerland), or Acros Organics (Chemie Brunschwig, Basel, Switzerland). All solutions were prepared using degassed Millipore water. If appropriate, solutions were saturated with nitrogen or argon. Whenever complete absence of oxygen was required, Millipore water was degassed by three consecutive freezing–thawing cycles under vacuum.
Synthetic peptide
A synthetic γ-Ec-1 peptide (purity greater than 90%) consisting of the first 25 amino acids of the full-length Ec-1 protein MGCDD KCGCA VPCPG GTGCR CTSAR was purchased from Sigma-Genosys (Haverhill, UK) and used for EXAFS, electrospray ionization mass spectrometry (ESI–MS), pH and metal ion titration experiments, and for the 2D 1H–1H total correlation spectroscopy (TOCSY) and nuclear Overhauser enhancement spectroscopy (NOESY) NMR experiments of the Cd2γ-Ec-1 form. All other experiments were conducted with the peptide overexpressed using the pGEX-4T-gEc1 construct.
Plasmid construction
The complementary DNA sequence encoding for the first 24 amino acids of wheat Ec-1 without the N-terminal translation initiator Met was optimized for Escherichia coli codon usage. Two additional Gly and Ser residues were added to the N-terminus of the protein to ensure optimal thrombin cleavage, yielding the sequence GS GCDD KCGCA VPCPG GTGCR CTSAR.
The resulting construct was cloned into the pGEX-4T expression vector (GE Healthcare) using the BamH1 and EcoR1 restriction sites, and the construct identity (pGEX-T4-gEc1) was subsequently verified by DNA sequencing. The constructed plasmid was transferred into the protease-deficient E. coli expression strain BL21(DE3).
Protein expression and purification
γ-Ec-1 was overexpressed in the form of the glutathione S-transferase (GST)–MT fusion protein according to the GST purification manual (GE Healthcare). After induction with 1 mM isopropyl-β-d-thiogalactopyranoside at an optical density at 600 nm of 1, cells were harvested after 4–7 h at 37 °C and lysed by sonification. The supernatant was loaded on a pre-equilibrated GST-affinity column (GE Healthcare). After washing, the fusion protein was stripped from the column using 10 mM glutathione and cleaved with 1 unit thrombin per milligram of GST–MT fusion protein for 60 h at 25 °C. Final purification of γ-Ec-1 was performed by size-exclusion chromatography using a Superdex 30 pg column (GE Healthcare), and the molecular identity was verified by ESI–MS (Fig. 2). Crucial for GST–MT protein cleavage with thrombin was the demetalation of the fusion protein with 10 mM EDTA during the GST-affinity column washing step. All chromatographic steps were conducted in 100 mM phosphate-buffered saline at pH 7.3. Purified and completely oxidized γ-Ec-1 was dialyzed twice against 20 mM Tris/HCl pH 8.0, lyophilized, and stored at −80 °C. Average yields were 4 mg of purified protein per liter of cell culture medium. The full-length Ec-1 protein was prepared as described elsewhere [3].
Apoγ-Ec-1 preparation
Apoγ-Ec-1 was prepared freshly prior to each experiment. Typically 1–3 mg of oxidized γ-Ec-1 was incubated with 200 mM dithiothreitol in a 100 mM Tris/HCl solution (pH 8.0) for 1 h prior to acidification to pH 2 with 1 M HCl. The sample was applied to a G10 size-exclusion column (GE Healthcare) pre-equilibrated with 10 mM HCl and eluted under constant argon flow. The residual ZnII, CdII, and CuI/II content of apoγ-Ec-1 was below the detection limit of flame atomic absorption spectroscopy (F-AAS) (0.001 ppm). Prior to the subsequent metal ion reconstitution step, the solution of apoγ-Ec-1 was argon-saturated for 1 h in a N2-flushed glove box and the protein concentration was determined via thiol quantification using the 2,2′-dithiodipyridine assay [17].
Preparation of Zn2γ-Ec-1, Zn6Ec-1, and Cd2γ-Ec-1
For all experiments 2 or 6 Eq of metal ions were titrated to the respective apo form in a N2-flushed glove box. Subsequently, the pH was raised to 8.6 using Tris/HCl or d 11-Tris/HCl for the NMR samples. Reconstituted samples were dialyzed against a 20 mM solution of the respective Tris/HCl buffer or 5 mM NH4Ac for ESI–MS measurements and were concentrated by lyophilization.
pH titrations followed by UV spectroscopy
800 μL of the respective reconstituted Ec-1 form (approximately 10 μM each) in 1 mM Tris/HCl pH 8.6 and 10 mM NaCl were titrated with diluted HCl as described in [3]. For the concurrent titration of both domains, γ-Ec-1 and βE-Ec-1 were mixed in equimolar amounts. Plots of molar absorptivity at 230 nm for the ZnII forms and at 250 nm for the CdII-loaded species against pH were fitted with the program Origin 7.0® (OriginLab, Northampton, MA, USA) using three different functions, considering either one or two common apparent pK a values for the Cys residues in the presence of the respective metal ions as described in [3, 18].
Titration of apoγ-Ec-1 with CdII
For each titration point, 90 μL of 32 μM apoγ-Ec-1 were mixed with the appropriate amount of a 1.25 mM CdCl2 solution in a N2-purged glove box. The pH was raised to approximately 8.6 with 100 mM Tris that had been pretreated with Chelex 100, resulting in samples with 20 μM Ec-1, 20 mM Tris/HCl, and 10 mM NaCl. Samples were transferred into cuvettes, sealed, and UV spectra were recorded.
Mass spectrometry
Samples of Zn2γ-Ec-1 in 100 mM NH4Ac (pH 8) were treated with 2 Eq ZnII or 4 Eq CdII and injected directly or with a prior acidification step into an Ultima API quadrupole time-of-flight spectrometer (Waters, UK). As a solvent, 10 mM NH4Ac in 50% MeOH (pH 7.5) or 50% acetonitrile with 0.2% formic acid (pH 2–3) was used. Scans were accumulated and further processed with the software MassLynx 3.5 (Micromass). Deconvolution of mass spectra was done by applying the maximum entropy algorithm of the MassLynx tool MaxEnt1. Electrospray parameters were capillary voltage 2.8 V, cone voltage 60 V, and source temperature 80 °C.
X-ray absorption spectroscopy
To determine the average ZnII-binding motif, K-edge X-ray absorption spectra of Zn2γ-Ec-1 were recorded at beamline D2 of the EMBL Outstation Hamburg at DESY, Germany, as described in [19]. Data reduction, such as background removal, normalization, and extraction of the fine structure, was performed with KEMP [20] assuming a threshold energy of E 0,Zn = 9,662 eV. The extracted K-edge EXAFS data were converted to photoelectron wave vector k-space and weighted by k 3. Initial evaluation of the spectra by ABRA [21], which is based on EXCURV [22], included a systematic screening of approximately 400 potential binding motifs. The subsequent meta-analysis identified structural zinc sites, and thus in the final refinement the total number of ligands was fixed at four. The absence of multiple scattering contributions indicative of the binding of imidazole rings to the ZnII ion limited the refinement to the following parameters for each structural model: the atomic distances (R), the Debye–Waller factors (2σ 2), and a residual shift of the energy origin (EF) were refined, minimizing the fit index (Φ). An amplitude reduction factor (AFAC) of 1.0 was used throughout the data analysis.
NMR spectroscopy
Zn 152 N-γ-Ec-1, Cd 152 N-γ-Ec-1, 113Cd2γ-Ec-1, and Zn 156 N-Ec-1 samples were prepared as described above. The lyophilized proteins were dissolved in 10% D2O/90% H2O, 15 mM d 11-Tris/HCl pH 6.9, and 50 mM NaCl to a final concentration of 1 mM protein for 1H–NMR and 3 mM protein for 113Cd-NMR studies. 1H–NMR experiments to elucidate the protein backbone structure were recorded at 25 °C with Bruker Avance 700- and 600-MHz spectrometers. 113Cd-NMR experiments to investigate the binding sites of the CdII ions were performed with a Bruker DRX 500-MHz spectrometer. Assignment of resonances in Cd2γ-Ec-1 and Zn6Ec-1 was performed using 3D 15N-resolved TOCSY [23, 24] and NOESY [25, 26] spectra recorded with 80- and 120-ms mixing times, respectively. Distance restraints were derived from the 120-ms mixing time 3D 15N-resolved NOESY and 2D NOESY experiments. Resonance assignments for the Zn2γ-Ec-1 domain were conducted using 2D TOCSY and 2D NOESY spectra with 80- and 120-ms mixing times, respectively. Additionally, a 120-ms mixing time 3D 15N-resolved NOESY spectrum as well as the information from the Cd2γ-Ec-1 and Zn6Ec-1 forms were used to validate the assignment. Distance restraints were again derived from the 3D 15N-resolved NOESY and 2D NOESY experiments. In all cases zero-quantum interference in the spectra was suppressed using an appropriate filter [27, 28]. 15N,1H correlation maps were derived from a gradient-enhanced [15N,1H] heteronuclear single quantum coherence (HSQC) experiment using the Rance–Palmer trick for sensitivity enhancement [29, 30]. 1D 113Cd-NMR and 2D [113Cd,1H]-HSQC spectra, as well as 2D [113Cd,113Cd] correlation spectroscopy (COSY) experiments were recorded to investigate the metal cluster [31]. 3 J[Hβ,Cd] couplings derived from a 2D [113Cd,1H]-HSQC spectrum allowed the individual Cd-Cys connectivities to be established.
Sequence-specific resonance assignment was performed using the method developed by Wüthrich and coworkers [32]. Assignments were achieved on the basis of information from 2D TOCSY, NOESY, 2D [15N,1H]-HSQC, 3D 15N-resolved NOESY, and 3D 15N-resolved TOCSY experiments. The 2D and 3D spectra were evaluated with the programs XEASY [33] and CARA [34], respectively. As a first step, the spin systems were identified in the 2D TOCSY or 3D 15N-resolved TOCSY experiments. Subsequently, spin systems were linked on the basis of nuclear Overhauser enhancement (NOE) information derived from 2D NOESY and 3D 15N-resolved NOESY. Once longer stretches had been identified, they were mapped onto the sequence of γ-Ec-1.
For the structure calculations, NOE peaks were picked and integrated using the program XEASY for 2D experiments and CARA for 3D experiments employing identical lower integration thresholds. Torsion angle dynamics [35] were performed with the noeassign [36] algorithm of the program CYANA 2.1 [37]. Structure calculations were started from 100 conformers with randomized torsion angle values. The 20 conformers with the lowest final target function value were further subjected to restrained energy minimization in explicit solvent against the AMBER force field [38] using the program OPALp [39, 40]. The resulting structures were deposited in the Protein Data Bank under accession codes 2I61 and 2I62. Structure figures were generated with the program MOLMOL [41].
Results and discussion
Initial quantification of metal ion binding
Reconstituted forms of γ-Ec-1 with ZnII and CdII were analyzed for their metal ion content using F-AAS and the 2,2′-dithiodipyridine assay and yielded MII to thiol group ratios of 1:3, indicating the binding of two divalent metal ions per protein. The proposed metal stoichiometry was confirmed by mass spectrometry. For this 2 Eq of ZnII or 4 Eq of CdII ions were added to a solution of Zn2γ-Ec-1, and the resulting mixture was analyzed by ESI–MS at either acidic or neutral pH. The spectrum at acidic pH shows the apoγ-Ec-1 species as expected, whereas at neutral pH exclusively Zn2γ-Ec-1 or Cd2γ-Ec-1 is observed despite the addition of an excess of the respective metal ion (Fig. 2).
To corroborate this result, apoγ-Ec-1 was titrated with increments of CdII and UV spectra were recorded (Fig. 3). They show the formation of the typical ligand-to-metal charge transfer bands at 245–250 nm indicative of CdII coordination to thiolate groups.
a UV spectra of the stepwise reconstitution of apoγ-Ec-1 with CdII ions showing the evolution of the S → CdII ligand-to-metal charge transfer bands around 250 nm. b Molar absorptivity at 225, 250, and 265 nm against the number of equivalents of CdII ions added, with the maximum value being reached in all cases after addition of 2 Eq
These bands increase in absorptivity up to the addition of 2 Eq of CdII and remain constant thereafter. It had already been shown that the full-length Ec-1 protein is able to coordinate six divalent metal ions and that four of them can be accommodated in the C-terminal βE domain [3, 10]. That the remaining two metal ions are bound within the N-terminal γ domain was evidenced by ESI–MS measurements on a proteolytically digested Zn6Ec-1 sample revealing the presence of a Zn2γ-Ec-1 species [19]. In the same publication, a [113Cd,113Cd]-COSY spectrum of 113Cd6Ec-1 showed cross-peaks between two 113Cd signals that originate from metal ions coordinated within the γ domain and led to the proposal of a Cd2Cys6 cluster. The results with the separate γ-Ec-1 peptide presented here clearly demonstrate that the binding ability for two divalent metal ions is not restricted to the domain within the full-length protein. This validates our experimental approach to use the separate γ-Ec-1 sequence for the in-depth spectroscopic characterization of the γ domain of wheat Ec-1.
pH titrations of Zn2γ-Ec-1 and Cd2γ-Ec-1
pH titrations followed by UV spectroscopy were performed to investigate the pH-dependent metal ion release. Although not tantamount, the apparent pK a values of the Cys residues obtained in presence of the respective metal ion are a good indication for the relative binding affinity of the metal ion to the MT. A number of apparent pK a values for different ZnII and CdII MTs were recently reviewed [13]. In the context of the study presented here, the pH stability of γ-Ec-1 in comparison to the full-length protein and the βE domain is of special interest. The UV spectra of the pH titration of Zn2γ-Ec-1 and Cd2γ-Ec-1 are depicted in Fig. 4 as are the plots of molar absorptivity at 230 nm (ZnII form) and 250 nm (CdII form) against the respective pH values.
Representative UV spectra of the titration of a Zn2γ-Ec-1 and b Cd2γ-Ec-1 with increasing amounts of HCl. c Molar absorptivity at 230 nm for the ZnII form and at 250 nm for the CdII form versus pH. To allow better comparability, the values obtained for the apo forms in both titrations were shifted to zero and in addition the plot of the ZnII form was normalized to the values obtained for the CdII form. Curve fits were performed with equations considering one (dashed lines) or two (solid lines) apparent pK a values as described in the electronic supplementary material
Fitting of the data was performed as described in [6, 21], and the results are presented in more detail in the electronic supplementary material. As the pK a values obtained for both Zn2γ-Ec-1 and Cd2γ-Ec-1 were significantly higher than determined for the respective βE and full-length forms (Fig. 5), also a titration of an equimolar mixture of both domains was performed. The resulting pK a values for the mixed domains lie in between the values obtained for the respective γ and βE domains, but are still significantly higher than the values for the full-length Ec-1 forms (Fig. 5).
Hence, it appears that not the mere presence but rather the close proximity of the respective other domain leads to the observed increased pH stability of the full-length protein. Although possible, it seems unlikely that the nature of the five amino acids SGAAA between the γ domain and the βE domain, which were removed in the separately expressed domains, has a major influence as the residues are neither charged nor especially bulky or hydrophobic. So far, also no indications for any sort of interactions between the two domains have been observed, judging from the lack of corresponding NOE signals in the NMR experiments. Furthermore, 15N dynamics data, in particular values of the 15N{1H}-NOE, indicate that the linker comprising residues 26–30 is fully flexible [10]. One possible explanation for the low pK a values of the full-length protein is that the spatial proximity of the respective other domain leads to a reduced solvent accessibility and hence to a deferred metal ion displacement by protons. Alternatively, formation of intermediate species in the full-length protein at decreasing pH values could occur, slowing down the metal ion release process, i.e., migration of metal ions between the two domains or even transient generation of a new cluster arrangement. However, such species would not be detected in the structural investigation presented here, as the NMR experiments were performed at pH 6.9, whereas metal ion release only starts below pH 6.5 for the ZnII form and below pH 5.5 for the CdII form. It is interesting to note that the stabilizing effect of the respective other domain is obviously much more pronounced for the γ domain, whereas the βE domain shows within the error limits the same apparent pK a values as the full-length protein. Shifts in pK a values are subtle probes of thermodynamic protein stability, and our data indicate that the γ domain is intrinsically less stable than the βE domain when in isolation, although both are structured and capable of metal binding.
An additional surprising result was obtained when the pH titration data for Zn2γ-Ec-1 and Cd2γ-Ec-1 were evaluated more closely. The absorptivity values obtained from the curve fit with the equation considering two apparent pK a values reveal a decrease by approximately one third for the first protonation step, characterized by pK a2, i.e., Δε 2,900 ± 400 M−1 cm−1 for Zn2γ-Ec-1 and Δε 7,200 ± 1,700 M−1 cm−1 for Cd2γ-Ec-1, and a decrease by two thirds for the second step, characterized by pK a1, i.e., Δε 5,700 ± 400 M−1 cm−1 for Zn2γ-Ec-1 and Δε 17,300 ± 1,700 M−1 cm−1 for Cd2γ-Ec-1 (see the electronic supplementary material). Disregarding the contribution of bridging thiolate ligands to the ligand-to-metal charge transfer bands, which is considerably less than the contribution of terminal thiolate ligands, the absorptivity decreases suggest the loss of two terminal metal–thiolate bonds in the first step and of four metal–thiolate bonds in the second step. This suggests that in the first step one metal ion is released and hence the contribution of two terminal and two bridging metal–thiolate bonds is lost, whereas in the second step the second metal ion is released and hence the contribution of the residual four metal–thiolate bonds disappears. As a result, the two metal ions in γ-Ec-1 seem to be released at pH values approximately 0.6–0.7 units apart and this might also be an indication that γ-Ec-1 contains two metal ion binding sites with different affinities.
Extended X-ray absorption fine structure spectroscopy of Zn2γ-Ec-1
EXAFS spectra were recorded to analyze the coordination environment of the ZnII ions in Zn2γ-Ec-1. The results reveal the presence of four sulfur ligands and no contribution of lighter ligands with nitrogen- or oxygen-donor atoms within the error limits. The Zn–S distances of 2.332(3) Å are in the normal range for ZnII coordination by thiol ligands (Fig. 6, Table 1).
EXAFS (left) and corresponding Fourier transform (right) of Zn2γ-Ec-1. The EXAFS is dominated by a single frequency, originating from sulfur backscattering. In the refinement, no other first-shell contribution could be identified. In line with this result, the Fourier transform is dominated by a single peak at 2.3 Å. The additional peak above 3 Å is refined as a metal–metal contribution, indicative of bridging sulfur ligands. The corresponding parameters are given in Table 1
Assuming the presence of a Zn–Zn interaction with a distance of 3.163(6) Å improves the fit index significantly from 0.2988 to 0.2141. However, the error range for the corresponding average number of ligands is relatively high. These findings together with the results from the concentration and ESI–MS measurements presented above strongly suggest the formation of a Zn2Cys6 metal–thiolate cluster with four terminal and two bridging thiolate groups. Such a cluster is in accordance with the Cd2Cys6 cluster proposed to be formed in 113Cd6Ec-1 on the basis of the [113Cd,113Cd]-COSY spectrum mentioned already [19].
NMR solution structures of the separate Zn2γ-Ec-1 and Cd2γ-Ec-1 peptides
Except for the first two amino acids Gly and Ser, which were engineered to improve proteolytic cleavage of the GST-fusion protein by thrombin, all residues could be identified by 3D 15N-resolved NOESY and TOCSY NMR experiments in case of the CdII form or by 3D 15N-resolved NOESY, 2D TOCSY, and [15N,1H]-HSQC experiments for the ZnII form. Experiments with the NMR-active 113Cd nucleus were performed to probe the metal ion coordination sphere. [113Cd,1H]-HSQC spectra allow the observation of cross-peaks based on 3 J couplings between the Hβ protons of the Cys residues and the respective coordinated CdII ions. In theory, each of the 12 Hβ protons of the six Cys residues present in the peptide should display a 3 J coupling to one 113CdII ion, or in the case of bridging thiolate ligands, to two CdII ions. As shown in Fig. 7, indeed most of the Cys residues correlate with metal ions; however, not two as expected but rather three possible bridging Cys residues were identified.
The 1D 113Cd NMR spectrum of Cd2γ-Ec-1 shows two doublets representing the two CdII ions. The 2D [113Cd,1H]-HSQC NMR spectrum allows two possible solutions for the bridging Cys residues: Cys-9/Cys-3 and Cys-9/Cys-21. The assignment of cross-peaks to Cys residues is schematically depicted at the bottom
Despite differences in size and electronegativity, CdII and ZnII forms of MTs have been interchangeably used for structural studies [31, 42, 43]. In the case of the γ-Ec-1 domain, such an assumption is justified by the reasonable agreement between proton chemical shifts in the two forms (Fig. 8).
Chemical shift assignment was based on the sequence-specific sequential resonance assignment procedure developed by Wüthrich and coworkers [32]. Overall, the completeness of proton assignment was 99%. No long-range NOEs reflecting contacts of residues from the C-terminal and N-terminal regions were observed in the NOESY spectra. Pro-12 (in contrast to Pro-14) was shown to be connected via a cis peptide bond to the previous residue in both metal isoforms (Fig. S1), as deduced from comparably strong NOEs of the sequential α protons. The comparison of backbone amide 1H and 15N chemical shifts for residues of the γ domain indicates that differences as large as 0.4 or 1.1 ppm are observed between the Zn and Cd species, respectively (Fig. S2). The largest differences are not limited to residues that are involved in metal coordination, but also include some of the residues in the small loop regions. A comparison of the amide proton and nitrogen chemical shifts of Zn2γ-Ec-1 with the values previously determined for the full-length Zn6Ec-1 protein reveals that chemical shift differences are negligible and limited to the terminal residues (Fig. S2). The latter are expected to be different owing to the slightly N-terminally modified sequence (GlySer instead of Met) or owing to the additional presence of the C-terminal βE domain in the full-length species. As mentioned already, a somewhat surprising result of the analysis is that although the analysis of the pK a values indicated differences between corresponding segments in the isolated γ domain compared with the full-length protein, no such differences could be detected in the backbone amide chemical shifts. We speculate that there may be contacts between the two domains that, however, are very transient (and possibly also unspecific) in nature.
Initial structures calculated without addition of explicit metal–Cys(Sγ) restraints (Fig. S3) converge for the amino acid residues Gly-2 to Gly-18. However, the positions of the C-terminal residues and of the thiol groups of the Cys residues deviate substantially. When upper distance restraints were added that enforced tetrahedral geometry and metal–sulphur distances derived from EXAFS experiments, the calculations for the C-terminal part of the structure also converged. Although Cys-9 (numbering according to the amino acid sequence given in Fig. 1) could be unambiguously identified as a bridging Cys residue on the basis of the [113Cd,1H]-HSQC experiment, the nature of the second bridging Cys residue could not be experimentally established. Accordingly, independent structure calculations were performed assuming the bridging residues to be (1) Cys-9 and Cys-21, (2) Cys-9 and Cys-3, and (3) Cys-9 and Cys-13, with all other Cys residues coordinating in a terminal fashion. The calculation using Cys-9 and Cys-13 as bridging residues resulted in no low-energy conformer and was therefore excluded from further analysis. The resulting structures containing the metal cluster arrangements Cys-9/Cys-21 and Cys-9/Cys-3 are representatively shown for Zn2γ-Ec-1 in Fig. 9, and the statistics from the corresponding structure calculations are summarized in Table 2.
a Structure bundle for Zn2γ-Ec-1 showing the Cys-9/Cys-21 metal cluster arrangement. The backbones are shown in gray, the Cys residues of one representative structure in stick mode, and the two corresponding ZnII ions as light-blue spheres. Cys residues are numbered according to their position in the amino acid sequence given in Fig. 1. b Backbone overlay of two representative structures of Zn2γ-Ec-1 with Cys-9/Cys-21 connectivity as in a and with Cys-9/Cys-3 arrangement (olive backbone, Cys residues as green sticks, ZnII ions as dark-green spheres)
Similar to previously determined structures of MTs, the γ domain of Ec-1 is devoid of regular secondary structure. Superposition of backbone atoms result in root mean square deviations of 0.62–0.8 Å for backbone atoms of residues 2–22, and 1.3–1.5 for all heavy atoms, and the values are very similar for the CdII-loaded and ZnII-loaded isoforms (see Table 2). The overall fold resembles a hook, in which the stem part is formed by the segments containing Cys7-9 and Cys19-21, and the loop by the coordination of Cys-13 to the metal. Two loops bridge the latter residue and the next metal-anchoring residues Cys-9 and Cys-19. Coordination of Cys-3 brings the N-terminal segment into proximity. No significant differences in conformation that were unambiguously supported by the NOEs were observed between the CdII-loaded and ZnII-loaded peptides despite the fact that in part substantial chemical shift differences are observed (vide supra). A superposition of the backbone atoms of conformers, in which either Cys-3 or Cys-21 was constrained to be the bridging ligand, revealed that only small structural adaptations were necessary to transform one form into the other. Considering that only upper-distance limits were derived from the NOEs and taking the inherent dynamics of the system as well as the low proton density in MTs, which are largely devoid of regular secondary structure or tertiary contacts, into account, we feel that no sound statements on structural differences of the ZnII-loaded or CdII-loaded species can be made on the basis of the present data. Moreover, the lack of explicit 113Cd-Cys(Hβ) cross-peaks in the [113Cd,1H]-HSQC experiment for these residues, and the fact that the target functions of the calculated conformers are very similar precludes unambiguous determination of the nature of the second bridging Cys residue. Whether this is because the coordination mode in the peptide changes dynamically or whether the NMR data are simply insufficient to describe a unique coordination mode remains unclear presently.
An M II2 Cys6 cluster as identified in the γ domain is unprecedented for MTs so far, but a very similar Zn2Cys6 cluster was previously observed in the transcription factor GAL4 from Saccheromyces cerevisiae [44]. Also here, the metal–Cys connectivities were probed by replacement of ZnII ions by 113CdII. Since the two 113Cd resonances at 669 and 707 ppm are well separated, it was possible to identify the bridging Cys residues using selectively decoupled [113Cd,1H]-HSQC spectra. However, such an experiment is not feasible in the case of Cd2γ-Ec-1 owing to the very small chemical shift difference of only 2 ppm. Nevertheless, two further peculiarities in the NMR spectra corroborate the concomitant presence of two different, probably interchanging cluster arrangements. Firstly, in 113Cd2γEc-1 TOCSY spectra, cross-peaks due to the geminal Hβ2–Hβ3 correlation for the putative bridging Cys-3 and Cys-21 are significantly broadened, and the corresponding correlation for Cys-9 is broadened beyond detection, indicating the presence of exchange processes. Secondly, in contrast to spectra recorded for the full-length protein [19], no mutual coupling was observed in the [113Cd,113Cd]-COSY spectrum of 113Cd2γ-Ec-1, which might again be explained by intermediate exchange processes occurring in the isolated domain.
NMR solution structure of Zn2γ-Ec-1 as part of the full-length Zn6Ec-1 protein and comparison with the separate Zn2γ-Ec-1 peptide
Spectroscopic and spectrometric studies [3, 10, 19, 45] have revealed that wheat Zn6Ec-1 is a two-domain protein. The larger C-terminal domain, termed extended β or βE, consists of 51 amino acids and embeds a mononuclear ZnCys2His2 site as well as a trinuclear Zn3Cys9 metal–thiolate cluster with similarity to the β domain of vertebrate MTs. As described already, the smaller 24 amino acids long N-terminal domain, γ-Ec-1, folds around a Zn2Cys6 cluster. Chemical shift mapping accompanied by 15N relaxation experiments was used to confirm identical folds of the βE domain in the form of the separately expressed peptide as well as being part of the full-length Zn6Ec-1 protein. To confirm that this is also true for the γ domain, chemical shift assignment and identification of close contacts from NOESY spectra were performed for the isolated peptides (CdII and ZnII isoforms) of the γ domain as well as for the corresponding part in the full-length protein. Similar chemical shifts and NOESY cross-peaks (Fig. 8) indicate analogous peptide folding. A comparison of the solution structure bundle calculated for the embedded and for the independent γ domain (ZnII isoform, Cys-9/Cys-21 bridging) is given in the electronic supplementary material. Indeed, only minor differences between the two conformations are observed.
Comparison of Zn2γ-Ec-1 with the Zn2Cys6 cluster in GAL4
As mentioned already, Zn2Cys6 clusters have so far only been structurally described in yeast transcription factors [44, 46, 47]. Amino acid sequence alignments of the latter reveal a completely conserved Cys distribution pattern and a high conservation of Lys residues, which play a major role in the interaction of these proteins with DNA (Fig. 10). In contrast, the Cys distribution pattern of the γ-Ec-1 domain differs significantly from that of the transcription factors, and only three positively charged residues, one Lys and two Arg, are present. In addition, whereas the fold of the protein backbone in the yeast transcription factors can be described as a loop, the backbone of γ-Ec-1 is S-shaped or resembles a hook (Figs. 9, 10). Taking these findings together, despite the similarity of the metal–thiolate clusters, recognition of DNA by γ-Ec-1 in the same fashion as observed for the yeast transcription factors seems unlikely.
a Amino acid sequence of γ-Ec-1 and alignment of the sequences from the three yeast transcription factors GAL4, LAC9, and PPR1 with the species name and Protein Data Bank accession code given. Cys residues are highlighted with a black background and Lys and Arg residues are highlighted with a gray background. NMR solution structure of b Cd2GAL4 [44] and c Zn2γ-Ec-1 with the metal ions drawn as yellow spheres or blue-grey spheres and the Cys residues presented in stick mode. The N-terminus of each structure is positioned at the (upper) right side, respectively
Conclusions
Complementing our investigation of the Zn4βE-Ec-1 domain including the determination of its solution structure by NMR [10, 19], we have now completed the second part of the puzzle by presenting a study of the properties and the solution structure of the γ domain of wheat Ec-1. ESI–MS experiments in conjunction with F-AAS measurements and metal ion titrations followed by UV spectroscopy clearly confirm the ability of the N-terminal Ec-1 fragment to coordinate two ZnII or CdII ions even in the absence of the βE domain. Tetrahedral tetrathiolate coordination of the bound ZnII ions was established by EXAFS measurements in addition to the presence of a short Zn–Zn distance of 3.16 Å. pH titrations of Zn2γ-Ec-1 and Cd2γ-Ec-1 revealed higher apparent pK a values of the Cys residues than previously determined for the βE domain and full-length Ec-1. This earlier protonation of thiolate ligands is paralleled by an increased peptide backbone flexibility compared with that of the βE domain [10]. A pH titration of an equimolar mixture of the γ domain and βE domain yielded intermediate pK a values, which, however, differ from the values obtained with the full-length protein. This might indicate a yet unidentified interaction between the two domains in the full-length protein that increases the pH stability especially of the M II2 Cys6 cluster of the γ domain. Owing to the low percentage or even lack of regular secondary structure in MTs, the metal clusters critically contribute to the overall protein fold. Hence, only when the metal-coordinating residues have been identified can the structure be determined correctly. In the case of the γ domain, [113Cd,1H]-HSQC spectra indicated three possible metal ion-to-Cys connectivities, one of which could be eliminated during the structure calculation. On the basis of the 113Cd NMR and 1H NMR studies of the separate Zn2γ-Ec-1 and Cd2γ-Ec-1 peptides as well as of the embedded domain in the full-length protein, we propose the presence of a highly dynamic metal cluster, possibly switching between two slightly different cluster arrangements recruiting either Cys-3 or Cys-21 as the second bridging thiolate ligand. This flexibility is in line with the observed decreased rigidity of the γ domain compared with the βE domain as observed in 15N relaxation experiments [10]. Overall, the structures of the separate Zn2γ-Ec-1 and Cd2γ-Ec-1 peptides show only minor differences within the error limits In addition, when the chemical shifts of the backbone amide protons and nitrogen atoms in the 24-residue N-terminal segment of Zn6Ec-1 and in the separate Zn2γ-Ec-1 peptide were monitored, only small differences were observed. Hence, the solution structure of the separately expressed γ-Ec-1 peptide can be reliably taken as a model for the γ domain in the full-length Ec-1 protein.
References
Vallee BL (1979) In: Kägi JHR, Nordberg M (eds) Metallothionein. Birkhäuser, Basel, pp 19–40
Binz P-A, Kägi JHR (1999) In: Klaassen C (ed) Metallothionein IV. Birkhäuser, Basel, pp 7–13
Peroza EA, Freisinger E (2007) J Biol Inorg Chem 12:377–391
Hanley-Bowdoin L, Lane BG (1983) Eur J Biochem 135:9–15
Lane BG, Kajioka R, Kennedy TD (1987) Biochem Cell Biol 65:1001–1005
Blindauer CA, Harrison MD, Parkinson JA, Robinson AK, Cavet JS, Robinson NJ, Sadler PJ (2001) Proc Natl Acad Sci USA 98:9593–9598
Braun W, Vašák M, Robbins AH, Stout CD, Wagner G, Kägi JHR, Wüthrich K (1992) Proc Natl Acad Sci USA 89:10124–10128
Narula SS, Brouwer M, Hua Y, Armitage IM (1995) Biochemistry 34:620–631
Riek R, Prêcheur B, Wang Y, Mackay EA, Wider G, Güntert P, Liu A, Kägi JHR, Wüthrich K (1999) J Mol Biol 291:417–428
Peroza EA, Schmucki R, Güntert P, Freisinger E, Zerbe O (2009) J Mol Biol 387:207–218
Palmiter RD (1998) Proc Natl Acad Sci USA 95:8428–8430
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Cell Mol Life Sci 59:627–647
Freisinger E (2008) Dalton Trans 6663–6675
Kawashima I, Kennedy TD, Chino M, Lane BG (1992) Eur J Biochem 209:971–976
Finkelstein RR, Gampala SSL, Rock CD (2002) Plant Cell 14:S15–S45
Laudencia-Chingcuanco DL, Stamova BS, You FM, Lazo GR, Beckles DM, Anderson OD (2007) Plant Mol Biol 63:651–668
Pedersen AO, Jacobsen J (1980) Eur J Biochem 106:291–295
Freisinger E (2007) Inorg Chim Acta 360:369–380
Peroza EA, Al Kaabi A, Meyer-Klaucke W, Wellenreuther G, Freisinger E (2009) J Inorg Biochem 103:342–353
Korbas M, Marsa DF, Meyer-Klaucke W (2006) Rev Sci Instrum 77:1–5
Wellenreuther G, Parthasarathy V, Meyer-Klaucke W (2010) J Synchrotron Radiat 17:25–35
Binsted N, Strange RW, Hasnain SS (1992) Biochemistry 31:12117–12125
Braunschweiler L, Ernst RR (1983) J Magn Reson 53:521–528
Bax A, Davis DG (1985) J Magn Reson 65:355–360
Kumar A, Ernst RR, Wüthrich K (1980) Biochem Biophys Res Commun 95:1–6
Macura S, Ernst RR (1980) Mol Phys. 41:95–117
Otting G (1990) J Magn Reson 86:496–508
Rance M, Bodenhausen G, Wagner G, Wüthrich K, Ernst RR (1985) J Magn Reson 62:497–510
Palmer AG, Cavanagh J, Wright PE, Rance M (1991) J Magn Reson 93:151–170
Kay LE, Keifer P, Saarinen T (1992) J Am Chem Soc 114:10663–10665
Vašák M (1998) Biodegradation 9:501–512
Wüthrich K (1986) NMR of proteins and nucleic acids. Wiley, New York
Bartels C, Xia TH, Billeter M, Güntert P, Wüthrich K (1995) J Biomol NMR 6:1–10
Keller RLJ (2004) Computer aided resonance assignment tutorial. Cantina, Goldau
Güntert P, Mumenthaler C, Wüthrich K (1997) J Mol Biol 273:283–298
Herrmann T, Güntert P, Wüthrich K (2002) J Mol Biol 319:209–227
Güntert P (2003) Progr Nucl Magn Reson Spectrosc 43:105–125
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) J Am Chem Soc 117:5179–5197
Koradi R, Billeter M, Güntert P (2000) Comput Phys Commun 124:139–147
Luginbühl P, Güntert P, Billeter M, Wüthrich K (1996) J Biomol NMR 8:136–146
Koradi R, Billeter M, Wüthrich K (1996) J Mol Graphics 14:51–55
Braun W, Wagner G, Worgotter E, Vašák M, Kägi JHR, Wüthrich K (1986) J Mol Biol 187:125–129
Messerle BA, Schaffer A, Vašák M, Kägi JHR, Wüthrich K (1992) J Mol Biol 225:433–443
Baleja JD, Thanabal V, Wagner G (1997) J Biomol NMR 10:397–401
Leszczyszyn OI, Schmid R, Blindauer CA (2007) Proteins 68:922–935
Gardner KH, Anderson SF, Coleman JE (1995) Nat Struct Mol Biol 2:898–905
Marmorstein R, Harrison S (1994) Genes Dev 8:2504–2512
Acknowledgments
We thank Peter Güntert for refining the CYANA structures with a full force field. This work was supported by the Swiss National Science Foundation (SNSF Professorship PP002-119106/1 to E.F.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Loebus and E. A. Peroza contributed equally.
An interactive 3D complement page in Proteopedia is available at http://proteopedia.org/wiki/index.php/Journal:JBIC:9.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Loebus, J., Peroza, E.A., Blüthgen, N. et al. Protein and metal cluster structure of the wheat metallothionein domain γ-Ec-1: the second part of the puzzle. J Biol Inorg Chem 16, 683–694 (2011). https://doi.org/10.1007/s00775-011-0770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-011-0770-2