Abstract
During the investigation of the development of insulin-mimetic zinc(II) complexes with a blood glucose-lowering effect in experimental diabetic animals, we found a potent bis(maltolato)zinc(II) complex, Zn(ma)2, exhibiting significant insulin-mimetic effects in a type 2 diabetic animal model. By using this Zn(ma)2 as the leading compound, we examined the in vitro and in vivo structure–activity relationships of Zn(ma)2 and its related complexes. The in vitro insulin-mimetic activity of these complexes was determined by the inhibition of free fatty acid release and the enhancement of glucose uptake in isolated rat adipocytes treated with epinephrine. A new Zn(II) complex with allixin isolated from garlic, Zn(alx)2, exhibited the highest insulin-mimetic activity among the complexes analyzed. The insulin-mimetic activity of the Zn(II) complexes examined strongly correlated (correlation coefficient=0.96) with the partition coefficient (logP) of the ligand, indicating that the activity of Zn(ma)2-related complexes depends on the lipophilicity of the ligand. The blood glucose-lowering effects of Zn(alx)2 and Zn(ma)2 were then compared, and both complexes were found to normalize hyperglycemia in KK-Ay mice after a 14-day course of daily intraperitoneal injections. However, Zn(alx)2 improved glucose tolerance in KK-Ay mice much more than did Zn(ma)2, indicating that Zn(alx)2 possesses greater in vivo anti-diabetic activity than Zn(ma)2. In addition, Zn(alx)2 improved leptin resistance and suppressed the progress of obesity in type 2 diabetic KK-Ay mice. On the basis of these observations, we conclude that the Zn(alx)2 complex is a novel potent candidate for the treatment of type 2 diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of patients suffering from diabetes mellitus (DM) was reported in the year 2002 as being approximately 173 million people worldwide [1]. DM, which is expected to be the most significant disease in the 21st century [2, 3, 4], is generally classified into two main types, namely insulin-dependent type 1 DM and non-insulin-dependent type 2 DM; the former is known to be the result of absolute insulin deficiency, and the latter is characterized by a relative insulin deficiency due to low insulin sensitivity in target cells, according to the WHO definition [5]. Although several types of insulin preparations have been developed for patients with type 1 DM, and synthetic therapeutics are available for clinical use in patients with type 2 DM, both types of treatment have been associated with problems such as physical and mental pain due to daily insulin injections and certain severe side effects, respectively [6, 7]. Therefore, the development of new approaches to the treatment of DM remain necessary in order to reduce the need for insulin injections in type 1 patients, as well as to replace the currently problematic synthetic therapeutics for type 2 DM patients.
Recently, several therapeutic agents containing gold, platinum, or zinc have been developed and used clinically for the treatment of a number of diseases, e.g. auranofin for rheumatism, cisplatin for tumors, and polaprezinc for stomach ulcers, respectively. Metal elements such as selenium, manganese, molybdenum, tungsten, vanadium, and zinc have been proposed as candidates for treating DM [8, 9, 10, 11, 12, 13, 14]. Because zinc(II) has been found to have relatively high insulin-mimetic properties and is thought to be less toxic than other metal elements [15], we chose to focus on Zn(II) in the development of a potential therapeutic agent.
In 1980, Coulston and Dandona [16] reported that zinc(II) chloride stimulated lipogenesis in rat adipocytes similar to the action of insulin. Zn(II) is known to be an essential trace element involved in the physiology of insulin [17, 18, 19, 20], and it is ubiquitously found in many proteins and metalloenzymes; to date, the insulin-mimetic effects of Zn(II) have attracted much interest among researchers in this field. Therefore, several groups have attempted to determine whether or not ZnCl2 exhibits in vivo insulin-mimetic activity. Blood glucose-lowering effects of ZnCl2 in type 1 or 2 diabetic animals have been observed; however, extremely high doses and/or the long-term administration of ZnCl2 were used. For example, Shisheva et al. [21] reported that intraperitoneal (i.p.) injection of a high dose of 100 mg (1.5 mmol) Zn/kg body weight lowered blood glucose levels in STZ-induced type 1 diabetic mice. Chen et al. [22] found that the oral administration of ZnCl2 [200 mg (3.1 mmol) Zn/kg body weight] for 8 weeks reduced fasting plasma glucose levels in type 2 diabetic ob/ob mice. Accordingly, we developed Zn(II) complexes with coordination modes such as Zn(O4), Zn(N2O2), and Zn(S2O2), which have been shown to be more effective than ZnCl2, in terms of both the absorption from the gastrointestinal system and the toxicity of Zn(II); moreover, the advantages of these complexes have been observed at lower doses as well as with short-term administration [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34].
Since the insulin-mimetic effects of Zn(II) were discovered, the mechanisms of action of Zn(II) have been examined by numerous groups. In 1982, James and Charles [35] reported that the effects of Zn(II) on both glucose oxidation and lipolysis stimulation were inhibited by extracellular catalase, largely resulting from the generation of H2O2. In 1989, Ezaki [36] demonstrated that Zn(II) stimulated both lipogenesis and glucose transport in adipocytes. In 2002, Roin et al. observed [37] that the in vivo insulin-mimetic activity of Zn(II) was mediated through the direct inhibition of endogenous glycogen synthase kinase-3β. On the other hand, we examined the mechanism of action of insulin-mimetic Zn(II) complexes in terms of free fatty acid (FFA) release in isolated rat adipocytes treated with epinephrine (adrenalin) [38]. By using several inhibitors in the insulin-signaling pathway, Zn(II) complexes have been shown to exert an effect on multiple intracellular sites; PI3-kinase (phosphatidyl inositol-3-kinase), GLUT-4 (glucose transporter-4), and PDE (phosphodiesterase) are known to be functionally related to each other [38], and this resemblance has resulted in references to this system as an “ensemble mechanism”. Based on previous observations, it is likely that the incorporation of the Zn(II) compound into cells through the cell membrane is essential for development of the insulin-mimetic activity of these complexes.
Previously, a mixture of maltol and Zn(II) was reported to enhance absorption in erythrocytes more than did free Zn(II) [39]. We found that bis(maltolato)Zn(II), Zn(ma)2, with a Zn(O4) coordination mode not only exhibited greater insulin-mimetic activity than did free Zn(II), as estimated by in vitro experiments using isolated rat adipocytes treated with epinephrine [27], but we also observed that it lowered the hypoglycemia in KK-Ay mice and GK rats with type 2 DM [26, 29].
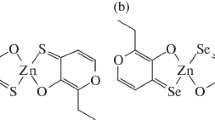
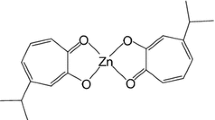
On the basis of these results, we investigated the structure–activity relationships of five Zn(ma)2-related complexes (Fig. 1) in order to develop better insulin-mimetic and anti-diabetic Zn(II) complexes than Zn(ma)2. In this paper, we propose a new potent Zn(II) complex with an allixin ligand, isolated from garlic as phytoalexin, which is a bioactive product induced in garlic by continuous environmental stress [40, 41, 42].
Materials and methods
Materials
Zinc sulfate (ZnSO4.7.0H2O, ZS), maltol (3-hydroxy-2-methyl-4-pyrone, Hma), and kojic acid (5-hydroxy-2-hydroxymethyl-4-pyrone, Hka) were purchased from Wako (Osaka, Japan). Ethyl maltol (2-ethyl-3-hydroxy-4-pyrone, Hema) was obtained from Tokyo Kasei (Tokyo, Japan). Allixin (3-hydroxy-5-methoxy-6-methyl-2-pentyl-4-pyrone, Halx) was a product of Wakunaga (Hiroshima, Japan). The bis(3-hydroxy-4-pyronato)Zn(II) complex [Zn(3hp)2] was prepared in our group according to the reported method [27]. Collagenase (type II), bovine serum albumin (BSA; fraction V), and (±)-epinephrine hydrochloride (adrenalin) were obtained from Sigma (St. Louis, Mo., USA). D-(+)-Glucose was purchased from Nacalai Tesque (Kyoto, Japan). Other reagents were of the highest purity commercially available.
Animals
Male Wistar rats (7–8 weeks old) used for biological tests of Zn(II) complexes were obtained from Shimizu (Kyoto, Japan). Male KK-Ay mice (4 weeks old) with type 2 DM weighing 20–25 g were purchased from CLEA (Tokyo, Japan). KK-Ay mice used for in vivo study were 12 weeks old. All animals were maintained on a 12-h light/dark cycle in our temperature-controlled central animal facility, and KK-Ay mice were individually housed in a cage. All animals were allowed free access to solid food (MF, Oriental Yeast, Tokyo, Japan) and tap water. All of the animal experiments were approved by the Experimental Animal Research Committee of Kyoto Pharmaceutical University (KPU) and were performed according to the Guidelines for Animal Experimentation at KPU.
Preparation and characterization of Zn(II) complexes
The Zn(II) complexes were prepared according to an analogous method for the preparation of Zn(ma)2 [27]. A new bis(allixinato)Zn(II) complex, Zn(alx)2, was prepared by the addition of an aqueous solution of LiOH·H2O (3.0 mmol) to the aqueous mixture of allixin (3.0 mmol) and ZnSO4·7.0H2O (1.5 mmol), followed by stirring for 10 h at pH 7–8 and room temperature. The resultant white precipitate was washed with the proper amount of water and was dried overnight in vacuo. The structures of the Zn(II) complexes were estimated by elemental analyses, IR, UV, and mass spectra. Elemental analyses for carbon and hydrogen were performed by the Analytical Center at KPU. The IR spectra were measured as KBr disks at 400–4600 cm−1 on a Shimadzu FT-IR 8100A spectrometer (Shimadzu, Kyoto, Japan). The UV spectra were measured with an Agilent-8453 spectrometer (Yokogawa, Tokyo, Japan). Low-resolution mass spectra were obtained at the Analytical Center of KPU with a JEOL JMS-SX 102AQQ spectrometer (JEOL, Tokyo, Japan) in FAB(+) mode using thioglycerol or 3-nitrobenzyl alcohol as the matrix material.
Measurement of the partition coefficients (logP) of maltol and its related compounds
The partition coefficients (logP) of maltol and its related compounds were determined by the “handshake” method in a chloroform/saline system [39]. After shaking for 1 min at room temperature, the mixture was centrifuged at 3000 rpm for 30 s. The two resulting phases were separated. The concentrations of maltol and its related compounds in each phase were monitored at the characteristic wavelength of approximately 265–280 nm due to the pyrone ring. The partition coefficients were calculated by the equilibrium concentrations of the maltol and its related compounds in chloroform and saline.
Evaluation of the in vitro insulin-mimetic activity of Zn(II) complexes in isolated rat adipocytes treated with epinephrine
The in vitro insulin-mimetic activity of Zn(II) complex was determined by both FFA-release inhibitory and glucose-uptake enhancing abilities in isolated rat adipocytes treated with epinephrine, according to previously reported methods [43, 44]. Briefly, epididymal fat pads, excised from male Wistar rats (7 weeks) anesthetized with ether, were cut into appropriately sized pieces and were incubated with collagenase in Krebs Ringer Bicarbonate (KRB) buffer (120 mM NaCl, 1.27 mM CaCl2, 1.2 mM MgSO4, 4.75 mM KCl, 1.2 mM KH2PO4, and 24 mM NaHCO3; pH 7.4) containing 2% BSA at 37 °C with gentle shaking at 100 cycle/min for 1 h. At the end of the incubation period, the prepared cells were filtered through sterilized cotton gauze and washed three times with the KRB buffer. The cells (1.5–2.0×106 cells/mL) were incubated at 37 °C for 30 min with Zn(II) complexes at various concentrations (0.1–1.0 mM) in KRB buffer containing 2% BSA and 2% DMSO. A 10 μM dose of epinephrine was then added to the reaction mixtures and the resulting solutions were incubated at 37 °C for 3 h. The mixtures were centrifuged at 3000 rpm for 10 min at 4 °C. As regards the outer solution of the cells, FFA and glucose levels were determined using a FFA kit (NEFA C-test; Wako, Osaka, Japan) and an automatic glucose analyzer (Fuji Dry Chem; Fuji, Tokyo, Japan), respectively [45]. The glucose uptake levels were evaluated according to the decrease in glucose concentration in the medium. The inhibition of FFA release was evaluated with respect to the apparent IC50 value, i.e. the 50% inhibitory concentration of the Zn(II) complex upon the release of FFA from isolated rat adipocytes treated with epinephrine during a 3-h incubation period. The enhancement of glucose uptake associated with the Zn(II) complex was evaluated using the EC50 value, i.e. the 50% enhancing concentration of the Zn(II) complex upon the maximal glucose uptake level in glucose uptake during a 3-h incubation. The number of cells was counted in a hemacytometer after trypan blue staining.
In vivo evaluation of Zn(II) complexes on blood glucose-lowering and anti-diabetic effects in KK-Ay mice
KK-Ay mice with type 2 DM received daily i.p. injections of Zn(II) complexes for 14 days, and their blood glucose levels, body weight, food intake, and water consumption were monitored daily at 2.00 p.m. Blood samples used for the analysis of blood glucose levels were obtained from the tail vein of KK-Ay mice, and blood glucose levels were measured using the glucose oxidase method (Glucocard; Arkray, Kyoto, Japan). As regards i.p. injection of each Zn(II) complex suspended in 5% acacia, the doses were 4.5 mg (68.8 μmol) of the Zn/kg body weight for the first two days, and then the dose was adjusted to approximately 2–4.5 mg (30.6–68.8 μmol) of Zn/kg body weight according to the blood glucose level observed for the next 12 consecutive days. After daily i.p. injections of the Zn(II) complexes for 14 days, blood samples collected from orbital exsanguinations of the mice under anesthesia with ether were centrifuged at 5000 rpm for 10 min at 4 °C, and serum samples for the analyses of urea nitrogen (UN), glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), triglycerides (TG), total cholesterol (TCHO), FFA, insulin, and leptin levels were separated. The serum UN, GPT, GOT, TG, and TCHO levels were determined by a Fuji Dry Chem analyzer. The serum FFA and insulin levels were measured by a NEFA C-test and Glazyme insulin-EIA test, respectively. Serum leptin levels were determined by AN’ALYZA (TECHNE, Minneapolis, Minn., USA). In addition, HbA1c levels in the blood obtained from the tail vein of the mice after the administration of Zn(II) complexes were determined by using a DCA 2000 system (Bayer, Tokyo, Japan).
Oral glucose tolerance test
After administration of the Zn(II) complexes, an oral glucose tolerance test (OGTT) was performed. The KK-Ay mice were fasted for 12 h and glucose at a dose of 1 g/kg body weight was given orally. Blood samples were obtained from the tail vein at 0, 30, 45, 60, 90, and 120 min after glucose administration. Blood glucose levels were measured by using a Glucocard.
Statistical analysis
All experimental results are expressed as the mean values±standard deviations (SD). Statistical analysis was performed by analysis of variance (ANOVA) at a 1% or 5% significance level of difference.
Results
Preparation and characterization of Zn(II) complexes with maltol and its related ligands
All Zn(II) complexes with maltol and its related compounds were prepared according to the methods used for the preparation for Zn(ma)2 [27]. The physicochemical parameters of prepared Zn(II) complexes are summarized in Table 1. In the IR spectra of all maltol-related ligands, bands due to O–H and C=O stretching frequencies were found at approximately 3000–3250 and 1650 cm−1, respectively. The O–H stretching frequency bands disappeared and the C=O stretching frequency bands shifted in the Zn(II) complexes, and therefore both coordination of the deprotonated hydroxyl as well as the carbonyl groups of maltol-related ligands to Zn(II) were indicated. The MS spectra of Zn(II) complexes showed a molecular weight corresponding to a structure consisting of a Zn:ligand ratio of 1:2, as supported by elemental analyses. However, no peaks due to the molecular weight of the Zn(ka)2 and Zn(3hp)2 complexes were obtained because of the low ionization properties created in FAB mode. We previously reported that Zn(ma)2 was in octahedral and square-pyramidal geometries in a unit cell, and also that two ligands coordinated to Zn(II) in the trans form, as revealed by X-ray crystal structure analysis. These results suggest that the structures of the Zn(II) complexes were estimated to have a common Zn(O4) coordination mode at the binding ratio of Zn(II):ligand=1:2 when Zn(ma)2 was in the trans forms, as shown in Fig. 1.
The partition coefficients (logP) of the ligands were determined in a chloroform/saline system using a conventional method, and the resulting logP were in the following order: Halx (1.65±0.03)>Hema (1.24±0.03)>Hma (0.60±0.01)>H3hp (−0.19±0.01)>Hka (−1.32±0.10).
In vitro insulin-mimetic activity of Zn(ma)2 and its related complexes
Inhibitory activity of FFA release from isolated rat adipocytes treated with epinephrine
The inhibitory activities of the Zn(II) complexes were evaluated by using isolated rat adipocytes treated with epinephrine according to a previously reported method [43]. The concentration-dependent inhibitory effects of Zn(II) complexes on FFA release from isolated rat adipocytes treated with epinephrine are shown in Fig. S1 (Supplementary material), from which the IC50 values of the Zn(II) complexes were calculated (Table 2). The inhibitory activities of Zn(alx)2, Zn(ma)2, Zn(ema)2, and Zn(3hp)2 were higher than that of ionic ZnSO4, and no inhibitory activity was exhibited in Zn(ka)2. In particular, Zn(alx)2 exhibited the highest inhibitory activity among all of the complexes studied, whereby the order of inhibitory activity of the complexes was as follows: Zn(alx)2 (IC50=0.37±0.03 mM)>Zn(ema)2 (0.44±0.05 mM)>Zn(ma)2 (0.54±0.07 mM)>Zn(3hp)2 (0.57±0.02 mM)>>Zn(ka)2 (no activity). In order to elucidate the relationship between the obtained in vitro insulin-mimetic activity and lipophilicity of the ligands in Zn(II) complexes, their IC50 values on FFA release were plotted against the partition coefficients (logP) of the ligands. A good linear correlation was observed, as shown in Fig. 2 (r=0.96).
Relationship between the IC50 values (mM) of Zn(II) complexes and the partition coefficients of their ligands (logP). The correlation coefficient of the linear repression was greater than 0.96 for a total of five points in triplicate measurements. Data are expressed as the mean value±SD for three experiments
Glucose-uptake activity in isolated rat adipocytes treated with epinephrine
Because Zn(alx)2 exhibited the highest FFA-release inhibitory activity among the complexes studied, the glucose-uptake enhancing activity of Zn(alx)2 was compared with that of the leading complex, Zn(ma)2. As shown in Fig. 3, both Zn(II) complexes induced concentration-dependent increases in glucose uptake. The glucose-uptake enhancement of Zn(alx)2 (EC50=0.60±0.02 mM) was found to be higher than that of Zn(ma)2 (EC50=1.00±0.05 mM). These results suggest that Zn(alx)2 had the highest in vitro insulin-mimetic activity among all of the complexes studied, depending on the lipophilicity of the ligand.
Concentration-dependent glucose-uptake enhancement by Zn(ma)2 (circles) and Zn(alx)2 (triangles) in isolated rat adipocytes treated with epinephrine in the presence of 5 mM glucose and 2% DMSO for 3 h. The estimated EC50 values of Zn(ma)2 and Zn(alx)2 were 1.00±0.03 and 0.60±0.02 mM, respectively. **Significance at P<0.01 versus Zn(ma)2
Effects of Zn(II) complexes on blood glucose levels and glucose tolerance in type 2 DM KK-Ay mice
The blood glucose-lowering effect of Zn(alx)2, which showed the greatest amount of in vitro insulin-mimetic activity, was compared with that of Zn(ma)2. As shown in Fig. 4A, the blood glucose levels of KK-Ay mice treated with Zn(alx)2 dropped from hyperglycemic levels (approximately 25 mM) to an almost normal range (below 8 mM) after 2–3 days of treatment, and these levels remained within the normal glucose levels during an administration period of 14 days. After the cessation of administration of the complex, the blood glucose levels were maintained at almost normal levels (approximately 10 mM) for at least 10 days, but returned to a hyperglycemic state at 1 month after the end of the administration period (Table S1; Supplementary material). The blood glucose-lowering effects of Zn(alx)2 were almost equal to those of Zn(ma)2; however, the HbA1c levels of KK-Ay mice treated with Zn(alx)2 were significantly lower than those of the mice treated with Zn(ma)2 (Fig. 4B).
Changes in blood glucose (A) and HbA1c (B) levels in control KK-Ay mice and KK-Ay mice treated with Zn(ma)2 or Zn(alx)2 by daily i.p. injections for 14 days. Doses were 4.5 mg (68.8 μmol) Zn/kg of the body weight for the first two days, and the doses were adjusted to 2.0–4.5 mg (30.6–68.8 μmol) Zn/kg according to daily changes in blood glucose levels. Data are expressed as the mean value±SD for 4–5 mice. *Significance at P<0.05 versus the control. **Significance at P<0.01 versus the control. ● Significance at P<0.01 versus Zn(ma)2
In order to examine whether or not Zn(alx)2 improved glucose tolerance in type 2 DM KK-Ay mice, an OGTT was performed after treatment with the Zn(II) complexes. As shown in Fig. 5, the blood glucose levels of the control KK-Ay mice were elevated to a maximal concentration of 300 mg/dL (16.7 mM) at 30 min after the administration of glucose, after which the levels gradually decreased. In contrast, the elevation in the blood glucose levels of KK-Ay mice treated with Zn(alx)2 for 14 days were significantly lower than those of the control KK-Ay mice, demonstrating that the effect of Zn(alx)2 was much greater than that of Zn(ma)2. These results indicated that Zn(alx)2 exhibited greater anti-diabetic activity than Zn(ma)2.
Oral glucose tolerance tests (OGTT) for the control KK-Ay mice and KK-Ay mice after daily i.p. administration of Zn(ma)2 and Zn(alx)2. OGTT tests were performed on mice that had fasted for 12 h, and then they were given an oral glucose solution at a dose of 1 g/kg body weight. Each symbol is expressed as the mean value±SD (n=4–6). *Significance at P<0.05 versus the control. **Significance at P<0.01 versus the control. # Significance at P<0.01 versus Zn(ma)2
Effects of Zn(alx)2 on body weight and serum parameters of KK-Ay mice
The increased rate of body weight gain (%) of KK-Ay mice treated with Zn(alx)2 (−5.0±0.8%) was significantly lower than that of the control KK-Ay mice (9.1±1.8%) (Fig. 6A), demonstrating that Zn(alx)2 suppressed the progression of obesity in type 2 diabetic KK-Ay mice. Serum UN, GOT, GPT, TG, and TCHO levels in KK-Ay mice treated with Zn(alx)2 were measured after a 14-day course of treatment, and were compared with the corresponding levels in control animals (Table S2). Serum UN (23±3 mg/dL), which indicates the degree of renal disturbance, decreased significantly more than did serum UN in control KK-Ay mice (37±4 mg/dL). GOT and GPT levels, which both indicate the degree of liver disturbance, did not differ from those of the control KK-Ay mice. Serum TG (102±32 mg/dL), which indicates the level of lipid metabolism, decreased more significantly than the TG levels in control KK-Ay mice (149±17 mM); however, serum TCHO levels did not differ significantly between groups. Other parameters, i.e. serum leptin (2.0±1.1 ng/mL) and insulin (35±7 pM) levels, were significantly reduced compared with those of the control KK-Ay mice (leptin=42±4 ng/mL and insulin=168±53 pM); these results were indicative of improvement in both insulin and leptin resistance (Fig. 6B and C). These findings also demonstrated that the Zn(alx)2 complex had anti-diabetic potency due to its blood glucose-lowering effect, its ability to improve lipid metabolism and insulin and leptin resistance, and its suppression of the progress of obesity in type 2 DM KK-Ay mice.
Discussion
When epinephrine binds to the β-receptor of adipocytes, adenylate cyclase is activated to transform ATP into cyclic adenosine 3′,5′-monophosphate (cAMP), which in turn activates certain protein kinases and lipase. The activated lipase then hydrolyzes triglycerides to free fatty acids (FFA), which are then released from the cell [46]. When insulin is added to this system, this hormone binds to the α-subunit of the insulin receptor and catalyzes autophosphorylation, which in turn stimulates tyrosine kinase in the β-subunit of insulin receptor. The insulin receptor tyrosine kinase then phosphorylates the insulin receptor substrate (IRS). Following these reactions, signal information is conveyed to downstream locations such as PI3-K and PDE, and GLUT-4 is then translocated to the surface of the cell membrane. Consequently, glucose uptake is enhanced and FFA release is subsequently inhibited in isolated rat adipocytes treated with epinephrine [15, 47, 48].
Zn(II) is known to have effects similar to those of insulin [8, 16, 35]. Recently, we found that Zn(II) compounds had multiple intracellular active sites (i.e., the ensemble mechanism) such as PI3-kinase, GLUT-4, and PDE [38]. In addition, Zn(II) has been reported to activate PDE, as determined by X-ray structure analyses and other in vitro experiments [49, 50, 51]. In the present study, we examined the insulin-mimetic activity of five Zn(ma)2-related complexes in terms of both the inhibition of FFA release and the enhancement of glucose uptake in isolated rat adipocytes treated with epinephrine [43, 44], and found that the insulin-mimetic activity of Zn(II) complexes was strongly correlated with the partition coefficient (logP) of the ligand, indicating that the insulin-mimetic activity of the Zn(II) complexes depended on the lipophilicity of the ligand (Fig. 2). These results suggest the possibility that the insulin-mimetic active sites of Zn(II) are primarily in the cells, thus supporting the hypothesis that the lipophilicity of the ligand in Zn(II) complexes is an important factor for developing the insulin-mimetic activity of these complexes. On the basis of these results, it was revealed that Zn(alx)2 exhibited the highest insulin-mimetic activity among all of the complexes considered here; this relatively high level of activity was due to the high partition coefficient of the ligand (logP=1.65±0.03). Previously, we suggested that the stability constant (logβ) of Zn(II) complexes was an important factor for the development of their insulin-mimetic activity [30]. However, no difference between the stability constants of Zn(alx)2 and Zn(ma)2 have thus far been observed (data to be reported), indicating that the insulin-mimetic activity of Zn(ma)2-related complexes predominantly depends on the permeability of the complex through the cell membrane.
After conducting the present in vitro assay, we evaluated the blood glucose-lowering effects of Zn(alx)2 in type 2 diabetic KK-Ay mice, a model that closely resembles DM in humans, by comparing the effects of Zn(alx)2 with those of Zn(ma)2. Yellow KK-Ay mice were transferred the Ay allele into the inbred KK strain by repetitive back-crossing. The introduction of the Ay allele causes hereditary hyperglycemia, hyperleptinemia, and massive obesity [52]. Both Zn(alx)2 and Zn(ma)2 complexes dramatically lowered hyperglycemia in KK-Ay mice by daily i.p. injections at a dose of 4.5 mg (68.8 μmol) Zn/kg body weight for the first two days, and the levels were maintained by adjusting the daily doses to 2–4.5 mg (30.6–68.8 μmol) Zn/kg after the third day for 12 days (Fig. 4A). Zn(alx)2 had a more significant effect than Zn(ma)2 in terms of both lowering the high levels of HbA1c and improving glucose tolerance in KK-Ay mice (Fig. 4B). In addition, both Zn(II) complexes reduced hyperinsulinemia in KK-Ay mice. These results also indicated that the insulin-mimetic and anti-diabetic effects of Zn(alx)2 were greater than those of Zn(ma)2, and were thus in good agreement with the results of the in vitro evaluations. Moreover, the blood glucose-lowering effect was maintained for at least 10 days after cessation of treatment of Zn(alx)2. We previously examined the metallokinetics in the blood of normal rats given Zn(ma)2 [29], and found that the bioavailability (bioabsorption) of Zn(ma)2 (37.3%) was higher than that of ZnCl2 (23.6%), and the distribution volume (Vd) of Zn(ma)2 (112±47 mL/kg) exhibited a higher tendency than that of ZnCl2 (72±8 mL/kg). These results indicated that complexation of ZnCl2 in terms of the increase in lipophilicity related to the increased incorporation of Zn(II) into the cells in whole tissues. Thus, it is assumed that the zinc levels accumulated in the tissues of KK-Ay mice treated with Zn(alx)2, which is an analogue of Zn(ma)2 and has a higher lipophilicity than Zn(ma)2 (Fig. 2), are higher than that of KK-Ay mice treated with ionic Zn(II) and Zn(ma)2. Therefore, the prolonged blood glucose-lowering effect of Zn(alx)2 might be due to the enhancement of the lipophilicity of Zn(alx)2. On the other hand, Zn(alx)2 suppressed the progress of obesity in KK-Ay mice (Fig. 6A), where the serum leptin levels of KK-Ay mice treated with Zn(alx)2 complex were found to be dramatically reduced from hyperleptinemic levels to the normal range for KK-Ay mice (Fig. 6B and C). Leptin, which is primarily synthesized and secreted from white adipose tissue, is a hormone protein that regulates body weight, glucose metabolism, and insulin sensitivity via the leptin receptor in the brain and peripheral tissues [53, 54, 55, 56, 57, 58, 59, 60]. It is clear that the discovery of leptin has fuelled considerable research on obesity, because this hormone is strongly related to the development of type 2 DM [61]. In the present study, a reduction in leptin levels in KK-Ay mice treated with Zn(alx)2 was observed, indicating the regulation of leptin sensitivity by this complex, which was present in target organs. It was therefore revealed that Zn(alx)2 regulated excessive food and water consumptions (Table S3) through the improvement of leptin resistance in KK-Ay mice, which in turn suppressed the progress of obesity. Moreover, the leptin levels of KK-Ay mice treated with Zn(alx)2 paralleled and was correlated with the insulin levels (Fig. 6B and C). It has recently been suggested that both leptin and insulin may mutually modulate secretion and production [62]. In keeping with that line of reasoning, we suggest here that Zn(alx)2 may regulate leptin levels by exerting an influence on insulin levels. However, there is still a great need for detailed investigations of the mechanism of action responsible for the insulin-mimetic activity of Zn(alx)2 in type 2 DM mice.
In conclusion, we have described here a new Zn(alx)2 complex, which was found to exhibit more potent insulin-mimetic activity than a Zn(ma)2 complex according to both in vitro and in vivo examinations. In addition, no elevations in serum UN, GOT, and GPT levels were observed, suggesting the low toxicity of the complex in these mice. The observed features of Zn(alx)2 administration depended on the relatively high lipophilicity of the ligand, which is related to the incorporation of the complex into cells throughout the entire body. The Zn(alx)2 complex thus shows great potential as a possible agent to treat type 2 DM.
References
Wild S, Roglic G, Sicree R, Green A, King H (2003) Global burden of disease. WHO, Geneva
DeFronzo RA, Bonadonna RC, Ferrannini E (1992) Diabetes Care 15:318–368
Yki-Jarvinen H (1992) Endocrine Rev 13:415–431
Vinik A, Pittenger G, Rafaeloff R, Rosenberg L, Duguid W (1996) Diabetes Rev 4:235–263
WHO Study Group (1985) Diabetes mellitus. (Technical report series 727) WHO, Geneva
Wagenaar LJ, Kuck EM, Hoekstra JB (1999) Neth J Med 55:4–12
Luft D, Schmulling RM, Eggstein M (1978) Diabetologia 30:75–87
Ezaki O (1990) J Biol Chem 265:1124–1128
Schwarz K, Mertz W (1959) Arch Biochem Biophys 85:292–295
Shechter Y, Karlish SJD (1980) Nature 284:556–558
Rubenstein AH, Levin NW (1962) Nature 194:188–189
Elberg G, Libman J, Shanzer A, Gefel D, Shechter Y (1995) Endocrine 3:631–637
Sakurai H (2002) Chem Rec 2:237–248
Thompson KH, McNeill JH, Orvig K (1999) Chem Rev 99:2561–2572
Sakurai H, Kojima Y, Yoshikawa Y, Kawabe K, Yasui H (2002) Coord Chem Rev 226:187–198
Coulston L, Dandona P (1980) Diabetes 29:665–667
Faure P, Roussel A, Coudray C, Richard MJ, Halimi S, Favier A (1992) Biol Trace Elem Res 15:1377–1410
Arquilla ER, Packer S, Tarmas W, Miyamoto S (1978) Endocrinology 103:1440–1449
Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ (1983) Am J Med 75:273–277
Levine AS, McClain CJ, Handwerger BS, Brown DM, Morley JE (1983) Am J Clin Nutr 37:382–386
Shisheva A, Gefel D, Shechter Y (1992) Diabetes 41:982–988
Chen M, Lios S, Lin P, Yang V, Alexander P, Lin W (1998) Biol Trace Elem Res 61:303–311
Yoshikawa Y, Ueda E, Sakurai H, Kojima Y (2003) Chem Pharm Bull 51:230–231
Yoshikawa Y, Ueda E, Kawabe K, Miyake H, Takino T, Sakurai H, Kojima Y (2002) J Biol Inorg Chem 7:68–73
Ueda E, Yoshikawa Y, Ishino Y, Sakurai H, Kojima Y (2002) Chem Pharm Bull 50:337–340
Yoshikawa Y, Ueda E, Miyake H, Sakurai H, Kojima Y (2001) Biochem Biophys Res Commun 281:1190–1193
Yoshikawa Y, Ueda E, Kawabe K, Miyake H, Sakurai H, Kojima Y (2000) Chem Lett 874–875
Yoshikawa Y, Ueda E, Suzuki Y, Yanagihara N, Sakurai H, Kojima Y (2001) Chem Pharm Bull 49:652–654
Fugono J, Fujimoto K, Yasui H, Kawabe K, Yoshikawa Y, Kojima Y, Sakurai H (2002) Drug Metab Pharmacokinet 17:340–347
Yoshikawa Y, Kawabe K, Tadokoro M, Suzuki Y, Yanagihara N, Nakayama A, Sakurai H, Kojima Y (2002) Bull Chem Soc Jpn 75:2423–2432
Katoh A, Tsukahara T, Saito R, Ghosh KK, Yoshikawa Y, Kojima Y, Tamura A, Sakurai H (2002) Chem Lett 114–115
Kojima Y, Yoshikawa Y, Ikura N, Ueda R, Ichimura A, Doe M, Sakurai H (2003) Vitamins (Japan) 77:571–576
Yoshikawa Y, Ueda E, Sakurai H, Kojima Y (2003) Chem Pharm Bull 51:230–231
Kojima Y, Yoshikawa Y, Ueda E, Ueda R, Yamamoto S, Kumekawa K, Yanagihara N, Sakurai H (2003) Chem Pharm Bull 51:1006–1008
James MM, Charles SC (1982) J Biol Chem 257:4362–4368
Ezaki O (1989) J Biol Chem 264:16118–16122
Roin I, Oksana K, David G, Hagit EF (2002) Biochem Biophys Res Commun 295:102–106
Yoshikawa Y, Ueda E, Kojima Y, Sakurai H (2004) Life Sci 75:741–751
Hider RC, Ejim R, Taylor PD, Gale R, Huehns E, Porter JB (1990) Biochem Pharmacol 39:1005–1012
Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y (1994) Planta Med 60:417–420
Kodera Y, Ichikawa M, Yoshida J, Kashimoto N, Uda N, Sumioka I, Ide N, Ono K (2002) Chem Pharm Bull 50:354–363
Kodera Y, Ayabe M, Ogasawara K, Yoshida S, Hayashi N, Ono K (2002) Chem Pharm Bull 50:405–407
Nakai M, Watanabe H, Fujiwara C, Kakegawa H, Satoh T, Takada J, Matsushita R, Sakurai H (1995) Biol Pharm Bull 18:719–725
Adachi Y, Sakurai H (2004) Chem Pharm Bull 52:428–433
Ohkubo A, Kamei S, Yamanaka M, Arai F, Kondo A (1981) Clin Chem 27:1287–1290
Burns TW, Langley PE, Robison GA (1975) Metab Clin Exp 24:265–276
Sakurai H, Yasui H, Adachi Y (2003) Expert Opin Investig Drugs 12:1189–1203
Saltiel AR, Kahn R (2001) Nature 414:799–806
Percival MD, Yeh B, Falgueyret (1997) Biohem Biophys Res Commun 241:175–180
Xu RX, Hassell AM, Vanderwall D, Lambert M, Holmes WD, Luther MA, Rocque WJ, Milburn MV, Zhao Y, Ke H, Nolte RT (2000) Science 288:1822–1824
Huai Q, Colicelli J, Ke H (2003) Biochemistry 42:13220–13225
Kimura I (1998) Diabetes Frontier 9:439–443
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L (1994) Nature 372:425–431
Spiegelman BM, Flier JS (2001) Cell 23:531–543
Tartaglia LA (1997) J Biol Chem 272:6093–6096
Halaas JL, Gajiwal KS, Maffei M, Cohen SL, Chait BT, Rabinowithz D, Lallone RL, Burley SK, Friedman JM (1995) Science 269:543–546
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Science 269:540–543
Harris RB (1998) Biochem Biophys Res Commun 245:502–509
Ceddia RB, Koistinen HA, Zierath JR, Sweeney G (2002) FASEB J 16:1163–1176
Al-Daghri N, Bartlett WA, Jones AF, Kumar S (2002) Diabetes Obes Metab 4:147–155
Kahn BB, Flier JS (2000) J Clin Invest 106:473–481
Kieffer TJ, Habener JF (2000) Am J Physiol Endocrinol Metab 278:E1–E14
Acknowledgements
This study was supported in part by grants from the Ministry of Education, Science, Sports, and Culture of the Japanese government (Grants-in-Aid for Scientific Research (B), Scientific Research on Priority Areas, and Specially Promoted Research).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Adachi, Y., Yoshida, J., Kodera, Y. et al. A new insulin-mimetic bis(allixinato)zinc(II) complex: structure–activity relationship of zinc(II) complexes. J Biol Inorg Chem 9, 885–893 (2004). https://doi.org/10.1007/s00775-004-0590-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0590-8