Abstract
Osteoporosis remains undertreated in Japan, and bone fractures are the most frequent complications imposing heavy burden on individuals and the community. This paper investigates the clinical and economic burden of fractures among osteoporosis patients in Japan. The Japan National Health and Wellness Survey 2012–2014 database was used for analysis. Respondents aged ≥ 50 years and indicated a physician diagnosis of osteoporosis (N = 1107) were categorized into three subgroups: no prior fracture (N = 693), single fracture (N = 242), and multiple (≥ 2) fractures (N = 172). Health-related quality of life (HRQoL), work productivity and activity impairment, healthcare resource utilization and associated direct and indirect costs were compared across three fracture subgroups adjusting for respondents’ sociodemographic and clinical characteristics using generalized linear regression models. The estimated fracture prevalence among respondents with osteoporosis who were ≥ 50 years was 37.4%, of whom 41.5% had multiple fractures. Relative to osteoporosis respondents with no fracture and with single fracture, those with multiple fractures reported significant higher disability in HRQoL, more healthcare resource utilization, and were associated with higher direct costs. Improved treatment of fractures among osteoporosis patients is necessary and may help reduce the clinical and economic burden in this osteoporosis population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic metabolic disease that results in low bone mass and deterioration in skeletal strength, causing an increased susceptibility and risk of subsequent bone fractures [1]. Research has found that women are at a greater risk of osteoporosis incidence than men, because women will experience more bone loss particularly when they reach menopause [2]. Other factors that were identified to contribute to the development of osteoporosis include aging, physical inactivity, reduced levels of estrogen, excessive cortisone or thyroid hormone, smoking, and excessive alcohol intake [3]. As we age, osteoporosis develops gradually over years. Studies have demonstrated that the incidence of hip fractures increased exponentially with age in many countries [2]. In Japan, it was estimated that more than 37% of its population will be over 60 years by 2050 [4]. As the population ages, osteoporosis will emerge as a significant public health problem, although it is not currently recognized as such [5]. The aging population and increased number of osteoporosis patients will pose significant societal burden including both monetary (e.g., direct medical costs and caregiver costs) and nonmonetary costs (e.g., work absenteeism) due to patients’ poor health conditions [6].
Osteoporosis has been linked with a reduced quality of life [7,8,9] and greater fears of physical harm along with a significant economic burden [6, 10, 11]. However, as noted in the call to action of Mithal et al. [12], these associations have been less studied in Asia. A previous study by Yamamoto et al. [13] suggested a significant health-related quality of life (HRQoL) and economic burden among female patients with osteoporosis in Japan. In addition, the study also showed that the presence of osteoporosis had a significant effect on HRQoL, activity impairment, and healthcare resource utilization among those with specific comorbidities/conditions (e.g., hypertension, high cholesterol, high fracture risk). However, this research was only limited to female patients, and it did not account for the number of fractures that would have a more negative effect on health outcomes.
Research on the effect of fractures caused by osteoporosis is limited in Japan. Understanding and identifying factors associated with fractures may allow for better targeting of efforts to strengthen prevention, as well as early detection and better treatment of osteoporosis. It has been shown that osteoporosis patients with prior fractures will have increased risk of future fractures, i.e., “fracture begets fracture” [14]. A study has found that 10–14% of patients will suffer another fracture each year after a hip fracture [5]. Thus, the study recommended clinicians who were caring for patients with prior fractures to consider treatment options for subsequent fracture prevention. Many health economic studies also suggested that osteoporosis therapies were more cost effective in patients with fractures [15, 16]. Therefore, assessing patient characteristics as well as clinical and economic outcomes of fractures may assist prevention and early detection of osteoporosis fractures, as well as help elucidate the clinical and economic burden associated with fractures among osteoporosis patients in Japan.
In this study, we aim to evaluate the burden of fractures in respondents aged ≥ 50 years including both men and women with osteoporosis in Japan and to compare the clinical and economic outcomes of osteoporosis by the number of fractures experienced. Results of this study will contribute to a better understanding of the knowledge gap in the clinical and economic burdens associated with fractures and unmet needs in osteoporosis management in Japan.
Materials and methods
Data source
This research study used the Japan National Health and Wellness Survey (NHWS; Kantar Health, New York, NY) 2012–2014 database (N = 30,000 in each year). The NHWS is an internet-based, self-reported survey from a nationwide sample of adults (aged ≥ 18 years) using a stratified random sample framework with quotas approximating the gender and age distribution of the Japan general population. Response rates for the Japan NHWS database were 16.6% in 2012, 4.9% in 2013, and 15.4% in 2014. More details on subject recruitment and the representativeness of the NHWS sample can be found in other publications [17,18,19]. Data from 3 years of NHWS from 2012–2014 were pooled to increase sample size and statistical power. Only the most recent data were kept for respondents who had data collected in multiple years. The NHWS received Essex Institutional Review Board (Lebanon, NJ, USA) approval, and informed consents were obtained from all NHWS participants prior to joining the study [17].
Study population
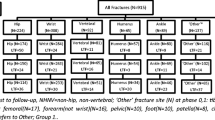
The study population for this research is all unique respondents who completed the 2012–2014 Japan NHWS aged ≥ 50 years, and self-reported a diagnosis of osteoporosis by a physician (N = 1107). The selection of study subjects for analysis is illustrated in Fig. 1.
Measures
Demographics and health characteristics
Age, sex, education, household income level, health insurance status, employment status, smoking status, exercise behaviour, alcohol use, body mass index (BMI; converted to underweight: BMI < 18.5 kg/m2, normal weight: 18.5 kg/m2 ≤ BMI < 25 kg/m2, obese: BMI ≥ 25 kg/m2, or decline to answer), and the Charlson Comorbidity Index (CCI) were analysed. The CCI is a weighted summary score that measures one’s comorbid burden with greater scores indicating greater comorbid burden [20]. The self-reported diagnosis of the following conditions was collected, and summed and weighted to calculate the CCI: chronic pulmonary disease, rheumatologic disease, diabetes without chronic complications, congestive heart failure, dementia, mild liver disease, myocardial infarction, peripheral vascular disease, cerebrovascular disease, peptic ulcer disease, diabetes with chronic complications, renal disease, hemiplegia or paraplegia, any tumours, leukaemia, lymphoma, moderate or severe liver disease, AIDS/HIV, and metastatic solid tumour. More details on each of demographics and health characteristics can be found in Table 1.
Osteoporosis diagnosis and number of prior fractures
Respondents who self-reported a diagnosis of osteoporosis by a healthcare provider were considered to have osteoporosis (henceforth ‘osteoporosis respondents’). Respondents who reported a diagnosis of osteopenia, but did not report a diagnosis of osteoporosis were excluded. All osteoporosis respondents were asked to report the number of bone fractures experienced since the age of 50. Respondents who had osteoporosis and fractures were defined as those who reported a diagnosis of osteoporosis and experienced one or more bone fractures. Furthermore, they were categorized into three groups based on the number of bone fractures experienced: (1) no prior fracture group (N = 693); (2) single fracture group (N = 242); and (3) multiple fractures group (≥ 2 bone fractures; N = 172).
Osteoporosis treatment
Respondents were also asked several questions in relation with the treatment of their osteoporosis, including diagnosing and treating physician specialty, location of receipt of medicating treatment, ever prescribed treatment, ever recommended treatment, use of calcium and/or vitamin D supplements, use of over-the-counter products, and use of herbal products. Osteoporosis respondents who reported of taking at least one type of prescription medication listed in Supplementary Table S1 were considered as currently taking prescription medication. In addition, medication adherence (low, moderate, and high adherences) was also assessed using the validated Morisky Medication Adherence Scale (MMAS-8©) [21].
Health-related quality of life (HRQoL)
HRQoL was measured using the Short-Form-36 version 2 (SF-36v2) including three metrics: (1) physical component summary (PCS); (2) mental component summary (MCS) scores (range 0–100); and (3) health state utilities derived via the SF-6D algorithm (range 0–1) [22, 23]. Higher scores indicate better quality of life.
Work productivity and activity impairment (WPAI)
The health burden on work-related activities was measured using the validated WPAI questionnaire that consists of four metrics: (1) absenteeism (percentage of work time missed in the past 7 days); (2) presenteeism (percentage of impairment experienced at work in the past 7 days); (3) overall work productivity loss (a combination of absenteeism and presenteeism); and (4) activity impairment (percentage of daily activity impairment in the past 7 days) [24]. Absenteeism, presenteeism, and overall work productivity loss were collected from currently employed NHWS respondents only, while activity impairment data were collected from all NHWS respondents. All WPAI measures range from 0 to 100%, and higher scores indicate more impairment.
Healthcare resource utilization
Healthcare resource utilization-related outcomes were considered in terms of the self-reported number of healthcare providers (practitioner/family practitioners, internists, dentists, and more specialized physicians) seen, the number of emergency room (ER) visits, and the number of hospitalizations due to respondents’ own medical conditions in the past 6 months.
Direct and indirect costs
Indirect costs were calculated using the human capital method by integrating WPAI measures (absenteeism and presenteeism) and hourly wage rates from the Japan Basic Survey on Wage Structure, 2011 [25, 26]. Direct costs were estimated by multiplying unit costs for physician visit, emergency room visit, and hospitalization obtained from Ministry of Health, Labour and Welfare, Japan [27,28,29] by the number of visits in the past 6 months, where the unit costs were the average costs per person per healthcare facility visit for any conditions and treatments. Both indirect and direct costs were annualized.
Statistical analysis
The underlying distributions of respondent characteristics and health outcomes for osteoporosis respondents were summarized using means and standard deviations for interval/ratio variables and using counts and percentages for nominal variables. Pearson’s Chi-square test was used to compare nominal respondent characteristic variables and one-way ANOVA test for interval/ratio respondent characteristic variables among osteoporosis respondents with no, single, and multiple fractures. The association between the increased number of fractures experienced and health outcomes was examined through generalized linear models (GLMs). In GLMs, fracture severity (none, single and multiple) was used as the primary predictor of health outcomes, and the “none” category was treated as the reference category. Other covariates included in the model were age, gender, body mass index (BMI), marital status, education, employment, household income, health insurance status, smoking status, alcohol consumption, exercise behaviours, and existing comorbidity burdens measured by CCI to adjust for potential confounding. Normal distributions were specified in the GLMs for normally distributed outcomes, i.e., MCS, PCS and health utilities (SF-6D), while negative binomial distributions were specified for positive outcomes with highly skewed distributions, i.e., WPAI-related, healthcare resource utilization-related, and cost-related health outcomes. Adjusted relative difference and relative risks (RRs) as were appropriate for GLM function specified and adjusted means for all health outcomes by the number of fractures with their corresponding 95% confidence intervals (CIs) and p values were reported. Pairwise comparisons of adjusted means among those with varying number of fractures were adjusted using Bonferroni correction. Statistical significance was assessed at a significance level of 0.05. All data analysis was performed using IBM SPSS Statistics 23 [30].
Results
A total of 1107 respondents aged 50 years and older reporting a diagnosis of osteoporosis were identified (1.3%; 95% CI 1.2–1.4%). Among all osteoporosis respondents, 414 respondents reported an experience of prior fractures (37.4%; 95% CI 34.5–40.2%). Of those who had prior fractures, 41.5% (95% CI 36.8–46.3%) were found to have multiple fractures. Most of the osteoporosis respondents were female (91.6%; 95% CI 90.0–93.2%), and on average aged 66.6 years (SD = 6.3).
Both the single fracture and multiple fractures groups were more likely to be older, less likely to be married/living with partners, and more likely to have lower household income compared with the no fracture group (Table 1). In addition, the multiple fracture group was found to have more comorbidity burdens (CCI 0.64 ± 2.67) compared with both no fracture (CCI 0.29 ± 0.69) and single fracture groups (CCI 0.30 ± 0.72; Table 1), p = 0.003.
The diagnosis and treatment characteristics of all osteoporosis respondents are summarized in Table 2. Majority of osteoporosis respondents were diagnosed by orthopaedists (63.5%). Prescription medication and calcium/vitamin D supplements use were common. Of all osteoporosis respondents, 66.9 and 46.3% used prescription medication and calcium/vitamin D supplements to treat their osteoporosis, respectively. The use of over-the counter products and herbal products to treat osteoporosis was, however, extremely uncommon among osteoporosis respondents (2.5 and 0.9%, respectively). Of those who were currently taking a prescription medication, more than half of them received the prescription medication from clinics (55.2%), and only 22.3% of them showed high adherence to osteoporosis medications, as measured by the MMAS-8©. A total of 366 osteoporosis respondents reported that they were not currently taking any prescription medications. However, 53.5% of them reported that they had ever used a prescription medication to treat their osteoporosis. Of those who have never used a prescription medication (N = 171), a large number of them reported that their doctors have never recommended any prescription medications (84.2%).
After adjusting for relevant demographic and health characteristic variables, significant decrements in health utilities (SF-6D) were observed with the increased number of fractures (Table 3, Fig. 2 and Table S2). Respondents with single fracture did not differ on MCS, but they had 1.81 (p = 0.002) lower PCS compared with those without fracture. In addition, respondents with multiple fractures had 4.16 lower MCS and 3.18 lower PCS than those without any fractures, all p < 0.001. Respondents with multiple fractures did not differ significantly on PCS than those with single fracture.
Adjusted mean scores for a) physical component scores, b) mental component scores of the SF36v2, and c) health utility scores (SF-6D) by fracture subgroup (N = 1107). Points are adjusted mean scores with bars representing the 95% confidence intervals for adjusted means. Points within each panel not sharing the same shape (circle, square and triangle) are significantly different at adjusted p value < 0.05 using Bonferroni correction method. Please refer to the y-axis on the left for panel a) and b), and y-axis on the right for panel c)
Regarding WPAI-related outcomes, no significant difference was observed between respondents with single fracture and those without fracture controlling for confounding covariates (Table 3, Fig. 3 and Table S2). Moreover, osteoporosis respondents with multiple fractures were found to have significantly higher absenteeism (RR 4.0; p < 0.001) and activity impairment (RR 1.3; p = 0.001) than those without fracture, but significantly higher absenteeism only compared with those having single fracture (adjusted mean difference: 5.73; p = 0.01). Finally, indirect costs, which were calculated from observed overall work productivity impairment and national average wage rate, were not significantly different among various fracture subgroups (Table 3, Fig. 4, and Table S2), as indicated by the non-significant difference observed in overall work productivity impairment among different fracture subgroups.
Adjusted mean scores for a) absenteeism (N = 236), b) presenteeism (N = 237), c) overall work impairment (N = 236), and d) activity impairment by fracture subgroup (N = 1107). Points are adjusted mean scores with bars representing the 95% confidence intervals for adjusted means. Points within each panel not sharing the same shape (circle, square, and triangle) are significantly different at adjusted p value < 0.05 using Bonferroni correction method. Please refer to the y-axis on the left for panel a) and y-axis on the right for panel b). On panel d), the single fracture group is not statistically different from the no fracture group (0) as well as the multiple fractures group (2+) representing by a point with a round circle overlapping on a square
Adjusted mean scores for a) indirect costs (N = 236) and b) direct costs (N = 1107). Points are adjusted mean scores with bars representing the 95% confidence intervals for adjusted means. Points within each panel not sharing the same shape (circle, square and triangle) are significantly different at adjusted p value < 0.05 using Bonferroni correction method. Please refer to the y-axis on the left for panel a) and y-axis on the right for panel b)
After adjustments, the number of hospitalizations increased concomitantly with the increased number of fractures (adjusted means: 0.6 (no fracture); 1.2 (single fracture); 2.5 (multiple fracture); all groups p < 0.05; Table 3, Fig. 5, and Table S2). In addition, respondents with multiple fractures were found to have significantly higher number of healthcare provider visits (RR 1.4; p < 0.001) and emergency room visits (RR 2.2; p < 0.001) than those without fracture, but no significant difference was observed between respondents with multiple fractures and single fracture. Last but not least, directs costs were significantly different among respondents with no, single and multiple fractures (Table 3, Fig. 4 and Table S2).
Adjusted mean scores for a) healthcare provider visits, b) emergency room visits, and c) number of hospitalizations (N = 1107). Points are adjusted mean scores with bars representing the 95% confidence intervals for adjusted means. Points within each panel not sharing the same shape (circle, square and triangle) are significantly different at adjusted p value < 0.05 using Bonferroni correction method. On panel a) and b), the single fracture group is not statistically different from the no fracture group (0) as well as the multiple fractures group (2+) representing by a point with a round circle overlapping on a square
Discussion
Previous studies in Japan used the NHWS data set to estimate the burden of osteoporosis in women [13, 31], but osteoporosis and fractures are not restricted to postmenopausal women only. It has been continuously reported that men are also at risk of osteoporosis, and they have more osteoporosis-related complications compared with women [32, 33]. The present study examined osteoporosis and fractures associated with both male and female respondents, and men were found to account for approximately 9% of the total osteoporosis cases. Our study, therefore, generated a broader view of the prevalence and burden of osteoporosis as well as fractures in the Japan population aged 50 years and older.
Study findings showed risk factors of incremental fractures among respondents on a low household income with higher comorbidity burdens who were not living with a partner or not yet married. Besides these factors, the previous studies found that low bone mineral density (BMD), vitamin D insufficiency, time since menopause and family history of fractures were also associated with osteoporotic fractures, and respondents with multiple risk factors were more susceptible to osteoporotic fractures than those with single or no risk factor [31, 34, 35]. Knowing the risk factors will help detect patients with higher risk of fractures; thus, they may benefit from early evaluation and appropriate treatment to prevent fractures.
Osteoporosis is often undertreated [15, 36], although pharmacotherapy has been proven to be efficacious in reducing fracture incidence by over 50% [37]. Similarly, this study discovered that one in three respondents who reported a diagnosis of osteoporosis did not report taking any prescription medications, leaving them to the increased risk of fractures. In addition, more than half of the NHWS osteoporosis respondents who were not currently taking any prescription medications reported that they have ever used an anti-osteoporosis prescription medication. Reasons why respondents stopped their osteoporosis treatment were unclear. One of the possible reasons might be due to the poor adherence to current osteoporosis medications among all osteoporosis respondents assessed by MMAS-8. Some other possible reasons of withdrawals from osteoporosis prescription medications might include the fear of side effects, lifestyle compatibility, the concern of the effectiveness of anti-osteoporosis drugs and lack of communication between healthcare providers and patients which was reported in the previous research [38]. Moreover, respondents with osteoporosis might have limited access to treatment, because more than 80% of them who had never taken any osteoporosis drugs reported that their doctors have never recommended an anti-osteoporosis prescription medication to them before, which may indicate a poor physician–patient communication on prevention of fractures and treatment. The development of new osteoporosis treatments with higher efficacy, fewer side effects, and a more convenient dosing schedule is necessary to address the current problem of low adherence and to increase physician recommendation and patient uptake of osteoporosis medications.
Previous studies have shown that the presence of osteoporotic fracture was associated with reduced HRQoL, increased medical costs, and increased mortality and morbidity [39,40,41,42,43]. Particularly, the presence of hip and/or vertebral fractures demonstrated significantly greater clinical and economic burdens compared with other types of fractures [40, 42, 44]. However, studies that evaluate the clinical and economic burdens associated with incremental osteoporotic fractures are limited in Japan, despite the increasing importance of research in this area due to its aging population. To our knowledge, only one Sweden study by Hallberg et al. has reported the HRQoL following multiple osteoporotic fractures: osteoporosis patients who have experienced two or more fractures had significant lower MCS and PCS [44]. The results from this Sweden study, however, were at the bivariate level, which means that the results might be biased by potential confounding factors. In addition, health outcomes including work productivity and activity impairment, healthcare resource utilization, and costs associated with more than one fractures were not investigated in this Sweden study.
On the other hand, in our study, the clinical and economic burdens including HRQoL, WPAI, healthcare resource utilization and direct and indirect costs were estimated using multivariate regression models. The advantage of the use of multivariate regression models is to improve the balance of demographic and health characteristics variables among different fracture subgroups providing a more precise knowledge of the difference in clinical and economic burdens among groups. In multivariate analysis, respondents with osteoporosis and incremental fractures demonstrated a negative association with HRQoL, particularly in SF-6D scores. In this study, osteoporosis respondents with a single fracture had significantly lower PCS scores than respondents without any fractures. Respondents with multiple fractures also had 1.3 lower PCS scores than those with a single fracture, but the effect was not statistically significant. In addition, the previous NHWS study did not find any significant difference in MCS of the SF-36v2 between respondents with and without fractures [3]. Similarly, MCS did not differ between respondents with and without a single prior fracture in this study, but we observed significantly lower MCS scores among respondents with multiple fractures compared to the other two groups. This suggests that an experience of a single fracture will result in significant negative impact on the physical components of HRQoL only, and patients will start having reduced quality of life in the mental component as they get more fractures. These findings emphasize the need for early prevention of fractures as well as better management in avoiding subsequent fractures among osteoporosis patients with prior fractures to improve patients’ quality of life. Furthermore, osteoporosis respondents with incremental fractures were associated with higher direct costs, as indicated by higher healthcare resource utilization compared with respondents with no/single fracture implying that reducing the incidence of multiple fractures would result in significant cost savings. No significant difference in the overall work productivity impairment was identified among different fracture subgroups which might be due to small sample size, because majority of the osteoporosis respondents were unemployed.
This study described the overall burden of fractures among both male and female respondents with osteoporosis. The results were not compared between female and male respondents because of the small sample size among male respondents (no fracture: n = 60; single fracture: n = 20; multiple fractures: n = 13). Future studies are warranted to compare the difference in the association between incremental fractures and poor health outcomes by gender.
Limitations
The NHWS is a self-reported internet-based cross-sectional survey with inherent design limitations. First, the diagnosis of osteoporosis as well as the number of fractures experienced was purely based on self-reporting, and no clinical data were available to validate its accuracy. In addition, respondents who, in fact, had an osteoporosis but did not report a diagnosis were not included in this analysis because of the lack of the clinical information (e.g., BMD results). The causal relationships between the exposure and the outcome cannot be determined in this study, because the NHWS is a cross-sectional survey based on prevalent cases at a single point in time rather than incident cases.
Different types of bone fractures, e.g., a hip fracture or a forearm fracture, may have an impact on patients’ health outcomes in varying degrees. All NHWS respondents were asked to report the number of bone fractures experienced, but the type of bone fractures was not collected. The results from this study, therefore, showed an average effect on osteoporosis respondents with any types of bone fractures. Further justification of the association between incremental fractures and poor health outcomes stratified by the type of bone fractures is warranted. Moreover, the comparison among fracture groups may be affected by recall bias, because all respondents were asked to recall the number of fractures experienced retrospectively since the age of 50 rather than prospectively.
It should also be noted that the unit costs used for calculating direct costs among osteoporosis respondents were the average costs of all costs for any conditions and treatments per person per visit, and were not specific for osteoporosis and fracture treatment (unit costs for osteoporosis diagnosis and treatment were not available).
As a final limitation, the response rate to the Japan NHWS survey 2012–2014 was low to modest [17, 31]. Selection bias may exist if the likelihood of responding to the survey is related to both the exposure (i.e., osteoporosis and fractures) and health outcomes (e.g., HRQoL). For example, osteoporosis patients with more severe conditions might be less likely to respond to the online survey. The association of incremental fractures and poor health outcomes, therefore, could potentially be underestimated. Although NHWS implemented a stratified random sampling procedure to approximate the demographic distribution of the Japan general population, the low-to-modest response rate may limit the representativeness of the study sample as well as the generalizability of the findings. It is unclear the extent to which this NHWS sample represents the various osteoporosis subpopulations in Japan, but the results from this study would provide useful information on burden of fracture among patients with osteoporosis patients in Japan.
In conclusion, approximately 40% respondents with a diagnosis of osteoporosis experienced at least one fracture and 42% of them with multiple fractures in Japan. Despite the high proportion of fractures among osteoporosis respondents, osteoporosis respondents were undertreated. Multiple fractures were associated with significantly worse HRQoL and significantly more absenteeism, hospitalizations, and direct costs compared with single/no fracture. Given the high proportion of fractures among osteoporosis respondents, low treatment rate and additional clinical and economic burdens (particularly among respondents with multiple fractures), better management, prevention and treatment of fractures among osteoporosis patients are necessary which may help reduce the clinical and economic burden of fractures due to osteoporosis.
References
Kanis JA, McCloskey EV, Johansson H et al (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int J Establ Results Coop Eur Found Osteoporos Natl Osteoporos Found USA 24:23–57. https://doi.org/10.1007/s00198-012-2074-y
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767. https://doi.org/10.1016/S0140-6736(02)08657-9
Sato M, Vietri J, Flynn JA, Fujiwara S (2014) Bone fractures and feeling at risk for osteoporosis among women in Japan: patient characteristics and outcomes in the National Health and Wellness Survey. Arch Osteoporos. https://doi.org/10.1007/s11657-014-0199-7
The National Institute of Population and Social Security Research (2002) Population projections for Japan, 2001–2050 .http://www.ipss.go.jp/pp-newest/e/ppfj02/ppfj02.pdf. Accessed 10 Aug 2017
Colón-Emeric CS, Saag KG (2006) Osteoporotic fractures in older adults. Best Pract Res Clin Rheumatol 20:695–706. https://doi.org/10.1016/j.berh.2006.04.004
Becker DJ, Kilgore ML, Morrisey MA (2010) The societal burden of osteoporosis. Curr Rheumatol Rep 12:186–191. https://doi.org/10.1007/s11926-010-0097-y
Martin AR, Sornay-Rendu E, Chandler JM et al (2002) The impact of osteoporosis on quality-of-life: the OFELY cohort. Bone 31:32–36
Cook DJ, Guyatt GH, Adachi JD et al (1993) Quality of life issues in women with vertebral fractures due to osteoporosis. Arthritis Rheum 36:750–756
Borgström F, Lekander I, Ivergård M et al (2013) The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS)—quality of life during the first 4 months after fracture. Osteoporos Int J Establ Results Coop Eur Found Osteoporos Natl Osteoporos Found USA 24:811–823. https://doi.org/10.1007/s00198-012-2240-2
Svedbom A, Ivergård M, Hernlund E et al (2014) Epidemiology and economic burden of osteoporosis in Switzerland. Arch Osteoporos 9:187. https://doi.org/10.1007/s11657-014-0187-y
Brown P, McNeill R, Leung W et al (2011) Current and future economic burden of osteoporosis in New Zealand. Appl Health Econ Health Policy 9:111–123. https://doi.org/10.2165/1153150-000000000-00000
Mithal A, Kaur P (2012) Osteoporosis in Asia: a call to action. Curr Osteoporos Rep 10:245–247. https://doi.org/10.1007/s11914-012-0114-3
Yamamoto LA, DiBonaventura M, Kawaguchi I (2016) The association between osteoporosis and patient outcomes in Japan. J Med Econ 19:702–709. https://doi.org/10.3111/13696998.2016.1159567
International Osteoporosis Foundation (2012) Capture the fracture: a global campaign to break the fragility fracture cycle. http://share.iofbonehealth.org/WOD/2012/report/WOD12-Report.pdf. Accessed 08 Sep 2017
Hagino H (2012) Fragility fracture prevention: review from a Japanese perspective. Yonago Acta Med 55:21–28
Silverman S, Agodoa I, Kruse M et al (2015) Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J Osteoporos. https://doi.org/10.1155/2015/627631
Sadosky AB, DiBonaventura M, Cappelleri JC et al (2015) The association between lower back pain and health status, work productivity, and health care resource use in Japan. J Pain Res 8:119–130. https://doi.org/10.2147/JPR.S76649
Montgomery W, Sato M, Nagasaka Y, Vietri J (2017) The economic and humanistic costs of chronic lower back pain in Japan. Clin Outcomes Res CEOR 9:361–371. https://doi.org/10.2147/CEOR.S134130
Mishima K, daCosta DiBonaventura M, Gross H (2015) The burden of insomnia in Japan. Nat Sci Sleep 7:1–11. https://doi.org/10.2147/NSS.S73437
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Morisky DE, Ang A, Krousel-Wood M, Ward HJ (2008) Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens Greenwich Conn 10:348–354
Brazier J, Roberts J, Deverill M (2002) The estimation of a preference-based measure of health from the SF-36. J Health Econ 21:271–292
Maruish ME (2011) User’s manual for the SF-36v2 Health Survey, 3rd edn. QualityMetric Incorporated, Lincoln
Reilly MC, Zbrozek AS, Dukes EM (1993) The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 4:353–365
Lofland JH, Pizzi L, Frick KD (2004) A review of health-related workplace productivity loss instruments. PharmacoEconomics 22:165–184
Ministry of Health, Labour and Welfare (2011) Summary report of basic survey on wage structure (Nationwide) 2011. http://www.mhlw.go.jp/english/database/db-l/dl/23gaikyo_zenkoku_Eng.pdf. Accessed 10 Apr 2017
Ministry of Health, Labour and Welfare (2011) Report of ambulatory care (unofficial English translation). http://www.mhlw.go.jp/stf/shingi/2r9852000001wj9o-att/2r9852000001wje3.pdf. Accessed 10 Apr 2017
Ministry of Health, Labour and Welfare (2012) Report of trend estimation of medical costs per hospitalization (unofficial English translation). http://www.mhlw.go.jp/bunya/iryouhoken/database/zenpan/dl/h23_doukou_03.pdf. Accessed 10 Apr 2017
Health at a Glance 2011: OECD Indicators. http://www.oecd-ilibrary.org/sites/health_glance-2011-en/04/05/index.html?itemId=/content/chapter/health_glance-2011-33-en. Accessed 10 Aug 2017
Corp IBM (2015) IBM SPSS statistics for windows. IBM Corp., Armonk
Sato M, Vietri J, Flynn JA, Fujiwara S (2014) Treatment for osteoporosis among women in Japan: associations with patient characteristics and patient-reported outcomes in the 2008–2011 Japan National Health and Wellness Surveys. J Osteoporos. https://doi.org/10.1155/2014/909153
Alswat KA (2017) Gender disparities in osteoporosis. J Clin Med Res 9:382–387. https://doi.org/10.14740/jocmr2970w
Adler RA (2014) Osteoporosis in men: a review. Bone Res 2:boneres20141. https://doi.org/10.1038/boneres.2014.1
Pinheiro MM, dos Reis Neto ET, Machado FS et al (2010) Risk factors for osteoporotic fractures and low bone density in pre and postmenopausal women. Rev Saude Publica 44:479–485
Lee SH, Khang Y-H, Lim K-H et al (2010) Clinical risk factors for osteoporotic fracture: a population-based prospective cohort study in Korea. J Bone Miner Res Off J Am Soc Bone Miner Res 25:369–378. https://doi.org/10.1359/jbmr.090722
Hagino H, Sawaguchi T, Endo N et al (2012) The risk of a second hip fracture in patients after their first hip fracture. Calcif Tissue Int 90:14–21. https://doi.org/10.1007/s00223-011-9545-6
Delmas PD, Rizzoli R, Cooper C, Reginster J-Y (2005) Treatment of patients with postmenopausal osteoporosis is worthwhile. The position of the International Osteoporosis Foundation. Osteoporos Int J Establ Results Coop Eur Found Osteoporos Natl Osteoporos Found USA 16:1–5. https://doi.org/10.1007/s00198-004-1813-0
International Osteoporosis Foundation Osteoporosis: the inside story. https://www.iofbonehealth.org/sites/default/files/PDFs/adherence_doctor_questions_guide.pdf. Accessed 13 Sep 2017
Caliri A, De FL, Bagnato GL, Bagnato GF (2007) Osteoporotic fractures: mortality and quality of life. Panminerva Med 49:21–27
Cauley JA, Thompson DE, Ensrud KC et al (2000) Risk of mortality following clinical fractures. Osteoporos Int 11:556–561. https://doi.org/10.1007/s001980070075
Gabriel SE, Tosteson AN, Leibson CL et al (2002) Direct medical costs attributable to osteoporotic fractures. Osteoporos Int 13:323–330. https://doi.org/10.1007/s001980200033
Johnell O, Kanis JA, Odén A et al (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42. https://doi.org/10.1007/s00198-003-1490-4
Lips P, van Schoor NM (2005) Quality of life in patients with osteoporosis. Osteoporos Int J Establ Results Coop Eur Found Osteoporos Natl Osteoporos Found USA 16:447–455. https://doi.org/10.1007/s00198-004-1762-7
Hallberg I, Rosenqvist AM, Kartous L et al (2004) Health-related quality of life after osteoporotic fractures. Osteoporos Int 15:834–841. https://doi.org/10.1007/s00198-004-1622-5
Acknowledgements
We would like to thank Indira Umareddy who was an employee of Kantar Health LLC during the conduct of the study for her contributions in the study design development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by Amgen Astellas Biopharma K.K. and Astellas Pharma Inc. Saeko Fujiwara received a consulting fee from Amgen Astellas Biopharma K.K. during the conduct of the study and received a consulting fee from Eli Lilly K.K., and has served as a speaker bureau of Alere Medical Co., Ltd. Pfizer Inc. and Asahi Kasei Pharma Corp. Xiahong Zhao, Cheryl Teoh, and Dena Jaffe are employees of Kantar Health LLC and received a consulting fee from Amgen Astellas Biopharma K.K. during the conduct of the study. Yurie Taguchi is an employee of Amgen Astellas Biopharma K.K. and is an ISPOR Japan councillor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Fujiwara, S., Zhao, X., Teoh, C. et al. Disease burden of fractures among patients with osteoporosis in Japan: health-related quality of life, work productivity and activity impairment, healthcare resource utilization, and economic costs. J Bone Miner Metab 37, 307–318 (2019). https://doi.org/10.1007/s00774-018-0916-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0916-1