Abstract
Pharmacological treatment of hypercalcemia is essential for patients with parathyroid carcinoma and intractable primary hyperparathyroidism (PHPT). Use of the calcimimetic cinacalcet hydrochloride (cinacalcet) is an option to treat such patients. We investigated the efficacy and safety of cinacalcet in Japanese patients with parathyroid carcinoma and intractable PHPT. Five Japanese patients with parathyroid carcinoma and two with intractable PHPT were enrolled in an open-label, single-arm study consisting of titration and maintenance phases. Cinacalcet doses were titrated until the albumin-corrected serum calcium concentration decreased to 10.0 mg/dL or less or until dose escalation was considered not necessary or feasible. Serum calcium concentration at the baseline was 12.1 ± 1.3 mg/dL (mean ± standard deviation; range 10.4–14.6 mg/dL) and decreased to 10.1 ± 1.6 mg/dL (range 8.6–13.3 mg/dL) at the end of the titration phase with cinacalcet at a dosage of up to 75 mg three times a day. At the end of the titration phase, at least a 1 mg/dL reduction in serum calcium concentration from the baseline was observed in five patients (three with carcinoma and two with PHPT), and it decreased to the normocalcemic range in five patients (three with carcinoma and two with PHPT). Common adverse events were nausea and vomiting. One patient discontinued participation in the study because of an adverse event, liver disorder. Cinacalcet effectively relieved hypercalcemia in 60% of the Japanese patients with parathyroid carcinoma and might be effective in those with intractable PHPT. The drug might be tolerable and safe at a dosage of at most 75 mg three times a day.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Parathyroid carcinoma, a rare cause of primary hyperparathyroidism (PHPT) [1, 2], is often intractable, with multiple recurrences and distant metastases. The 5-year survival rate after recurrence of parathyroid carcinoma is approximately 50% [3]. Uncontrolled hypercalcemia is the most critical issue among the clinical features in patients with parathyroid carcinoma, and their survival time is usually limited because of severe hypercalcemia and its related events. There are few medical management options for patients with recurrent and/or metastatic parathyroid carcinoma [4], since it is essentially unresponsive to chemotherapy and/or radiotherapy. Because the primary cause of death is parathyroid hormone (PTH)-dependent hypercalcemia but not direct tumor invasion or metastases, it is necessary to focus on direct control of PTH secretion in patients with parathyroid carcinoma.
PHPT due to benign parathyroid adenoma is a common endocrine disease and requires surgical removal of the tumor for its cure. It is, however, sometimes difficult to localize the parathyroid tumor precisely. Even though the tumor localization is confirmed with several imaging studies, it is sometimes difficult for patients with PHPT to be surgically treated because of their severe comorbidities, such as cardiovascular diseases. Uncontrolled severe hypercalcemia is the most critical issue in those patients.
Calcimimetics have been developed as allosteric modulators of the calcium-sensing receptor [5]. They directly reduce PTH secretion by binding to the calcium-sensing receptor on chief cells in parathyroid glands [5]. Cinacalcet hydrochloride (cinacalcet) is a commercially available calcimimetic and is widely prescribed to patients who are undergoing hemodialysis to relieve their secondary hyperparathyroidism. This compound has been approved for treatment of parathyroid carcinoma in many countries, because cinacalcet effectively relieves hypercalcemia in approximately two thirds of patients with parathyroid carcinoma for up to 128 weeks [6]. In addition, cinacalcet normalized serum calcium and decreased circulating PTH levels for up to 3 years in patients with PHPT [7]. In Japan, cinacalcet had not been approved for treatment of hypercalcemia with parathyroid carcinoma and intractable PHPT before this study was completed. Therefore, we investigated the efficacy and safety of cinacalcet for treatment of hypercalcemia in Japanese patients with parathyroid carcinoma and intractable PHPT.
Materials and methods
Patients
Patients with parathyroid carcinoma whose serum calcium concentrations were higher than 11.3 mg/dL at screening within 28 days before the first administration of cinacalcet were eligible for the study, because such hypercalcemic patients are usually advised to have parathyroid surgery if possible and feasible and because hypercalcemia in patients with parathyroid carcinoma is prone to serious exacerbation. Those with intractable PHPT whose serum calcium concentrations were higher than 12.5 mg/dL at screening were enrolled into the study because such hypercalcemic patients are sometimes treated with antihypercalcemic agents if they cannot have parathyroid surgery. Patients whose parathyroid tumors could not be localized before surgery or those who were in relapse after surgery or in whom parathyroidectomy was not allowed to be performed because of their complications were assigned to have intractable PHPT.
The major exclusion criteria were (1) diagnosed malignancy except for parathyroid carcinoma, nonmelanomatous skin cancers, or in situ cervical cancer within 5 years before enrollment, (2) being treated with chemotherapy for cancers other than parathyroid carcinoma, and (3) having hypercalcemia caused by malignancy other than parathyroid carcinoma.
The study was approved by the institutional or independent ethics committee and was conducted in accordance with the Declaration of Helsinki. Each patient gave written informed consent.
This study was registered with ClinicalTrials.gov (NCT01460030).
Study design
This was an open-label, single-arm, intra-individual dose-titration study. The study consisted of a dose-titration phase and a maintenance phase.
The treatment with cinacalcet was initiated at 25 mg twice daily. Cinacalcet doses were titrated (sequential doses of 25 mg twice a day, 50 mg twice a day, 75 mg twice a day, and 75 mg three or four times a day) until the serum calcium concentration decreased to 10.0 mg/dL or less or until dose escalation was considered not feasible because of adverse events [6]. Cinacalcet was administered at the same dose for at least 2 weeks before dose escalation. When dose escalation was considered not necessary or feasible, the patients underwent a transition from the dose-titration phase to the maintenance phase. Dose adjustments were permitted during the maintenance phase in the same manner as in the titration phase if needed. Clinic visits were scheduled weekly during the titration phase and every 8 weeks during the maintenance phase. The study was completed when the last patient was in the maintenance phase for 4 months.
Use of intravenously administered bisphosphonate was prohibited from 1 week before the initiation of the study treatment to the end of the study. Orally administered bisphosphonates that had been given since before initiation of the treatment with cinacalcet continued to be administered at the same dose. Use of calcitonin for treatment of hypercalcemia and other drugs that could affect calcium and bone metabolism was prohibited once the treatment with cinacalcet was initiated.
The primary end points of the study were the proportion of patients experiencing a reduction in serum calcium concentration of 1 mg/dL or greater from the baseline at the end of the titration phase, and the proportion of patients experiencing a reduction in serum calcium concentration into the reference range (10.3 mg/dL or less). Baseline values were measured on the first day of cinacalcet treatment before its administration. The secondary end points included serum intact PTH (iPTH) concentrations, adverse events, laboratory tests, and vital signs.
Assessments
Serum calcium concentrations were measured on the day of initiation of the treatment with cinacalcet (day 1) and once weekly thereafter during the titration phase. Measurement of the serum iPTH concentrations and biochemical, hematological, and electrocardiographic parameters was performed on day 1 and every 4 weeks thereafter, and at the end of the titration phase.
During the maintenance phase, measurement of the serum calcium concentrations, serum iPTH concentrations, and biochemical, hematological, and electrocardiographic parameters was performed every 8 weeks.
Blood samples were collected in the morning after patients had fasted overnight and before they took cinacalcet.
Serum calcium concentrations were corrected for albumin with use of the following equation if the serum albumin concentration was less than 4.0 g/dL: corrected calcium concentration (mg/dL) = measured calcium concentration (mg/dL) + 4 − serum albumin concentration (g/dL).
Statistical analysis
Categorical data were summarized as the number and percentage of participants, and continuous data were summarized with use of descriptive statistics; that is., mean, standard deviation (SD), and median.
Adverse events were classified by preferred term according to the Medical Dictionary for Regulatory Activities, Japanese version (MedDRA/J; version 15.1) and were graded by severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4, Japan Clinical Oncology Group edition (CTCAE v4-JCOG).
All analyses were performed by the company Kyowa Hakko Kirin using SAS version 9.2.
Results
Patient demographics and cinacalcet doses
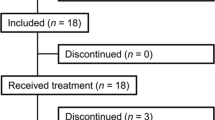
Demographic and baseline characteristics of the patients are shown in Table 1. Seven patients (five with parathyroid carcinoma and two with intractable PHPT) were enrolled in the study and received cinacalcet. There were four men and three women aged 65.9 ± 7.6 years (mean ± SD; range 51–74 years). All five patients with parathyroid carcinoma had undergone parathyroidectomy and had gross local and/or distant metastases. Four of the seven patients had been previously treated with orally administered bisphosphonates.
After initiation of the treatment with cinacalcet, one patient with parathyroid carcinoma (14.3%) discontinued participation in the study during the titration phase because of an adverse event (liver disorder) and six patients (85.7%) completed the titration phase but only four, among them two with parathyroid carcinoma, could undergo transition to the maintenance phase.
At the end of the titration phase, three patients received cinacalcet at 25 mg twice daily, and each of remaining three patients received cinacalcet at 50 mg twice daily, 75 mg twice daily, or 75 mg three times daily. The duration of the titration phase was 54.2 ± 22.3 days (mean ± SD; range 29–92 days). The maintenance phase lasted 113–501 days.
Changes in serum calcium concentration
Individual changes in serum calcium concentrations during the study are shown in Fig. 1. Five of the seven patients (71.4%), three with parathyroid carcinoma and two with intractable PHPT, experienced a reduction of serum calcium concentration of 1 mg/dL or greater from the baseline value at the end of the titration phase (Fig. 1a). Those five patients also experienced a reduction in serum calcium concentration to 10.3 mg/dL or less at the end of the titration phase (Fig. 1a). Among the remaining two patients, one failed to complete the titration phase because of to liver dysfunction, and the other could not tolerate further dose escalation because of gastritis even though the serum calcium levels of the patient did not reach the target.
The serum calcium levels during the maintenance phase were not apparently exacerbated in four patients who received cinacalcet.
The serum calcium concentration was 12.1 ± 1.3 mg/dL (mean ± SD; range 10.4–14.6 mg/dL) at the baseline and decreased to 10.1 ± 1.6 mg/dL (range 8.6–13.3 mg/dL) at the end of the titration phase. The decrease in the mean serum calcium concentrations in all seven patients was 16.5%. In the five patients with parathyroid carcinoma, the decrease in serum calcium concentration was 12.4%.
Changes in serum intact PTH
Individual changes in serum iPTH concentrations during the study are shown in Fig. 2. Serum iPTH concentrations at the end of the titration phase or at the time when participation in the study was discontinued were lower than the baseline values in four of the seven patients, three with parathyroid carcinoma and one with intractable PHPT (Fig. 2a). The serum iPTH concentrations at the baseline and at the end of the titration phase were 433.6 ± 379.8 pg/mL (n = 7) and 357.8 ± 234.6 pg/mL (n = 6) (mean ± SD) respectively.
Although at the end of the titration phase the mean serum iPTH level was less than that at the initiation of treatment, the serum iPTH levels fluctuated in patients with parathyroid carcinoma (Fig. 2b). In particular, they were consistently higher in one patient with parathyroid carcinoma than at the baseline (Fig. 2b), even though the serum calcium levels were less than those at the baseline throughout the maintenance phase (Fig. 1b).
Changes in serum phosphorus and creatinine concentrations
The serum phosphorus concentrations at the baseline and at the end of the titration phase or at the time when participation in the study was discontinued were 2.2 ± 0.6 mg/dL and 2.5 ± 0.7 mg/dL (mean ± SD) respectively (Table 2). The serum phosphorus levels somewhat increased during the treatment with cinacalcet, although the difference from the baseline value was not statistically significant. The serum creatinine concentrations at the baseline and at the end of the titration phase or at the time when participation in the study was discontinued were 0.86 ± 0.22 mg/dL and 0.93 ± 0.25 mg/dL (mean ± SD) respectively, and the difference was not statistically significant (Table 2). Those data were available in only two patients during the maintenance phase (Table 2).
Vital signs and electrocardiographic parameters
No remarkable changes were observed in the blood pressure or pulse rate throughout the study period. The QT and corrected QT intervals tended to be prolonged after initiation of the treatment with cinacalcet compared with the baseline data, all of which were, however, within the reference range in all patients.
Adverse events
All patients (n = 7) experienced at least one adverse event (Table 3). Adverse events that occurred in at least two patients included nausea (n = 4, 57.1%), vomiting (n = 3, 42.9%), and gastroesophageal reflux disease (n = 2, 28.6%), and they were considered to be related to cinacalcet by the investigators. As serious adverse events, pneumonia and vomiting were observed, and both were resolved by appropriate treatments. The pneumonia was diagnosed as obstructive pneumonia caused by metastasis of the parathyroid carcinoma and was considered not to be related to cinacalcet. Vomiting at grade 3 (MedDRA/J and CTCAE v4-JCOG) as a serious adverse event in one patient was considered to be related to cinacalcet. One patient discontinued participation in the study because of an adverse event, liver disorder at grade 2 (MedDRA/J and CTCAE v4-JCOG), which was considered to be related to cinaclacet, during the titration phase. There were no deaths (Table 3).
Discussion
The symptoms associated with intractable PHPT are usually due to hypercalcemia in addition to episodes of urolithiasis and/or bone fragility fractures. The same is true for parathyroid carcinoma, because tumor invasion and/or distant metastases are typically asymptomatic. Since approximately 50% of patients with parathyroid carcinoma survive more than 10 years after diagnosis even in the absence of effective anticancer therapies [8], the patients need a feasible therapy that provides continuous and appropriate control of serum calcium levels to alleviate symptoms related to hypercalcemia. Until recently, we had no adequate options to control hypercalcemia constantly, because chemotherapy and/or radiotherapy is essentially ineffective and because treatment with calcitonin in combination with glucocorticoids [9] or intravenously administered bisphosphonates [10–12] provides only temporally relief of hypercalcemia. Calcimimetics are theoretically attractive options for treatment of hypercalcemic patients with parathyroid carcinoma because they decrease PTH secretion and serum calcium level [13, 14]. Cinacalcet, a calcimimetic, has been approved for treatment of hypercalcemic patients with parathyroid carcinoma in many countries, including the USA and EU countries, on the basis of its efficacy and safety data obtained from some clinical trials [6, 8]. Although cinacalcet had not been approved for treatment of hypercalcemia with parathyroid carcinoma and intractable PHPT in Japan before this study, it was approved in February 2014 primarily on the basis of the results presented here and in previous reports [6, 8, 15].
The present study demonstrated the clinical efficacy and safety profile of cinacalcet when it was administered to Japanese patients with parathyroid carcinoma and intractable PHPT. At the end of the titration phase, serum calcium concentration fell by at least 1 mg/dL in 60% of the patients with parathyroid carcinoma in the present study, a value similar to the 62% of 29 patients with the disease examined in the USA [6]. The mean decrease in serum calcium concentrations in patients with parathyroid carcinoma was 12.3% in the present study, a value was similar to the 12.1% reduction in a previous report [6]. Therefore, the efficacy of cinacalcet in decreasing serum calcium levels in Japanese patients with parathyroid carcinoma was similar to that in patients with parathyroid carcinoma in the USA. This might also be valid in patients with intractable PHPT. Both patients with intractable PHPT in the present study fulfilled the primary end point of the reduction in serum calcium concentration, as was reported for 15 of 17 patients with the disease in Europe, the USA, and Canada, who showed a decrease in serum calcium level by 1.0 mg/dL or more [15].
In the previous study in the USA, among 29 patients with parathyroid carcinoma enrolled, only 19 patients completed the titration phase and entered the maintenance phase of the study [6]. In the present study, only one patient was lost during the titration phase and the remaining four patients with parathyroid carcinoma completed the study, although several adverse events occurred. Thus, the safety profile of cinacalcet in Japanese patients with parathyroid carcinoma was tolerable but not worse than that in patients in the USA, although the number of patients in the present study was smaller than that in the US study [6].
The amount of cinacalcet in a tablet available in Japan is less than that in a tablet available in Europe and the USA. Tablets containing 25 mg or 75 mg cinacalcet are prescribed in Japan, whereas tablets containing 30 mg, 60 mg or 90 mg are prescribed in other countries. In the present study, at most 75 mg cinacalcet three times a day was needed to control hypercalcemia. The dosage of the drug given to Japanese patients was not more than that given to patients in the USA [6]. The data from the present study indicate that patients may be safely treated with cinacalcet a dosage of 75 mg three times a day, although it is uncertain if the maximal dosage in the USA of 90 mg four times a day is tolerable and safe for Japanese patients.
Cinacalcet could not control hypercalcemia in two patients; one patient who discontinued the study treatment because of an adverse event (liver disorder) and another patient for whom dose escalation was not allowed because of an adverse event (gastritis). In addition, it has been reported that cinacalcet does not increase bone mineral density [7]. Thus, treatment with intravenously administered bisphosphonates along with cinacalcet may be an option expected to have an additive effect of relieving hypercalcemia as well as increasing bone mineral density by the action of bisphosphonates.
In conclusion, cinacalcet effectively and safely relieved hypercalcemia in a sustained manner in 60% of the Japanese patients with parathyroid carcinoma and might be more consistently effective in those with intractable PHPT. The drug might be tolerable and safe at a dosage of at most 75 mg three times a day.
References
Cordeiro AC, Montenegro FL, Kulcsar MA, Dellanegra LA, Tavares MR, Michaluart P Jr, Ferraz AR (1998) Parathyroid carcinoma. Am J Surg 175:52–55
Fraker DL (2000) Update on the management of parathyroid tumors. Curr Opin Oncol 12:41–48
Shane E (2001) Parathyroid carcinoma. J Clin Endocrinol Metab 86:485–493
Stock JL, Marcus R (2001) Medical management of primary hyperparathyroidism in the United States. In: Bilezikian JP (ed) The parathyroids. Academic, San Diego, pp 459–474
Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, Van Wagenen BC, Delmar EG, Balandrin MF (1998) Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 95:4040–4045
Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM et al (2007) Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 92:3803–3808
Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA et al (2005) Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 90:135–141
Collins MT, Skarulis MC, Bilezikian JP, Silverberg SJ, Spiegel AM, Marx SJ (1998) Treatment of hypercalcemia secondary to parathyroid carcinoma with a novel calcimimetic agent. J Clin Endocrinol Metab 83:1083–1088
Au WY (1975) Calcitonin treatment of hypercalcemia due to parathyroid carcinoma: synergistic effect of prednisone on long-term treatment of hypercalcemia. Arch Intern Med 135:1594–1597
Newrick PG, Braatvedt GD, Webb AJ, Sheffield E, Corrall RJM (1994) Prolonged remission of hypercalcaemia due to parathyroid carcinoma with pamidronate. Postgrad Med J 70:231–232
Mann K (1985) Oral biphosphonate therapy in metastatic parathyroid carcinoma. Lancet 1:101–102
Jungst D (1984) Disodium clodronate effective in management of severe hypercalcaemia caused by parathyroid carcinoma. Lancet 2:1043
Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, Silver J (2006) Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the trans acting factor AUF1. J Am Soc Nephrol 7:107–112
Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, Rishton G, Scully S, Qi M, Harris R, Lacey D, Martin D (2004) Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 308:627–635
Marcocci C, Chanson P, Shoback D, Bilezikian J, Fernandez-Cruz L, Orgiazzi J, Henzen C, Cheng S, Sterling LR, Lu J, Peacock M (2009) Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J Clin Endocrinol Metab 94:2766–2772
Acknowledgements
The authors thank all the investigators, staff, and patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was sponsored by Kyowa Hakko Kirin Co., Ltd.
About this article
Cite this article
Takeuchi, Y., Takahashi, S., Miura, D. et al. Cinacalcet hydrochloride relieves hypercalcemia in Japanese patients with parathyroid cancer and intractable primary hyperparathyroidism. J Bone Miner Metab 35, 616–622 (2017). https://doi.org/10.1007/s00774-016-0797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-016-0797-0