Abstract
Overt or subclinical thyroid dysfunction may affect the risk of fragility fractures. The aim of the present study was to assess the association of thyroid function and autoimmunity with vertebral fractures (VF) in a large sample of Greek postmenopausal women. This cross-sectional study recruited 335 euthyroid postmenopausal women, aged 35–79 years. Euthyroidism was verified by serum thyroid-stimulating hormone (TSH) within the laboratory reference range (0.4–4.5 μIU/mL). VFs were diagnosed by lumbar spine lateral radiographs, according to quantitative procedures. Serum free triiodothyronine (FT3), free thyroxine (FT4), TSH, as well as levels of anti-thyroglobulin (anti-TG) and thyroid peroxidase antibodies (anti-TPO) were compared according to the presence of VFs. Multivariate logistic regression showed that the presence of VFs was predicted independently by ln-TSH levels (OR = 0.290, p = 0.037) and positive anti-TG antibodies (OR = 3.308, p = 0.026) in models adjusted for age, menopausal age, and ln-HOMA-IR. Stepwise logistic regression analysis showed that the presence of VFs was predicted by menopausal age (OR = 1.120, p = 0.001), ln-TSH (OR = 0.312, p = 0.052), and thyroid autoimmunity (anti-TG and anti-TPO positive: OR = 6.637, p = 0.007) in a model that also included age and ln-HOMA-IR. Women with lower circulating TSH had higher risk of having a VF, independently of age, menopausal age, and insulin resistance. The presence of positive anti-TG/anti-TPO antibodies also indicated an elevated risk of fracture. Levels of thyroid hormones had no apparent effect on the risk of fracture. Further studies are necessary to establish the significance of our findings.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Osteoporotic vertebral fractures (VFs) are associated with significant disability and mortality, as well as with health and social service expenditure [1]. The spine presents a common site for osteoporotic fractures, as the lifetime probability of a major fracture at the age of menopause has been estimated to be as high as 15.1 % [2]. Moreover, VFs have been related to a marked increase of further fragility fractures [3, 4]; up to 20 % of individuals sustaining an initial VF will experience a second fracture within the following year [5]. Fracture risk is attributed to low bone density, as well as to several non-skeletal factors, which should be thoroughly evaluated in order to establish effective prevention strategies [6, 7].
Thyroid function is closely related to skeletal homeostasis. Overt and subclinical hyperthyroidism have been associated with increased risk of fracture in the general population [8–10]. Existing data, however, cannot support a clear impact of subclinical hypothyroidism on fracture risk in the general population [9, 10]. However, both subclinical hypo- and hyperthyroidism are related to higher FRAX scores of hip fracture in middle-aged women [11, 12]. Finally, both excess and deficiency of free thyroid hormones have been related to adverse effects on bone metabolism [13].
Euthyroid status is necessary for the regulation of adult bone structure [8, 14]. Fluctuation of TSH levels within the normal range has an impact on bone fractures in postmenopausal women: low normal TSH levels may increase the risk of VFs [15], hip fractures [16], or forearm and humerous fractures [17]. On the other hand, high normal TSH levels have been related with non-VFs [18]. However, a limited number of studies investigated the impact of thyroid hormones on the development of fractures, especially considering the underlying status of thyroid antibodies [15, 18, 19].
We have recently described the prevalence of vertebral fractures among Greek postmenopausal women [20]. The aim of the present study was to identify the potential association between the presence of VFs and thyroid function, as well as autoimmunity, in a sample of Greek postmenopausal euthyroid women.
Materials and methods
Subjects
This cross-sectional study included a total of 335 postmenopausal women, recruited from the Menopause Clinic of the 2nd Department of Obstetrics and Gynecology, University of Athens, Aretaieion Hospital. This clinic, active since 1998, serves both symptomatic and asymptomatic middle-aged women, providing information about menopause and offering screening and risk assessment for major morbidities of mid-life and beyond, such as osteoporosis, cardiovascular disease, cervical and breast cancer. A detailed electronic file is built for each informed, consenting woman containing demographic, life-style, and anthropometric parameters, as well as biochemical and hormonal assessments according to individual needs. All women who presented for their first evaluation between August 2007 and July 2013 and had a complete electronic file were screened for inclusion in this study. The menopausal status was defined as follicle stimulating hormone >25 mIU/mL and estradiol <50 pg/mL, after 12 consecutive months without menses.
Before recruitment, patients underwent the routine first-visit evaluation of our clinic, which includes PAP smear and transvaginal sonography, breast examination and mammography, fasting blood glucose, thyroid–liver–renal function tests, lipid profile, and bone densitometry. Clinical neurological examination was also performed to exclude the presence of neurologic disorders, which may have affected fall risk. Exclusion criteria were the presence of gynecological malignancy, neurological disorders, clinically overt CVD, thromboembolism, and a previous diagnosis of DM or thyroid dysfunction. Since hyperthyroidism is an established risk factor for osteoporotic fractures, we decided to exclude women with thyroid hormones beyond the laboratory reference range, in order to evaluate only euthyroid women. Furthermore, we excluded women with secondary causes of osteoporosis (e.g. Paget’s bone disease, renal osteodystrophy, hyperparathyroidism), as well as pre- and perimenopausal women. Women with adherence and retention concerns (e.g., alcoholism) were not included in the study. Renal function was assessed using both serum levels of creatinine and the Cockcroft-Gault equation, GFR = [(140 − age) × (actual weight in kg)] × 0.85 (if female)/(72 × serum creatinine) [21]. Institutional review board approval was obtained by the ethics committee of Aretaieion Hospital.
Anthropometric measurements
Weight and height were measured in the morning and in light clothing in order to estimate the body mass index (BMI). Weight was measured on an electronic scale, and height was measured in a stadiometer in the upright position. BMI was calculated using the equation BMI = body weight (kg)/height2 (m2).
Biochemical and hormone assays
Serum glucose was measured using glucose assay (Abbott) with coefficient of variation (CV) ≤5 % and sensitivity 2.5 mg/dL. Serum levels of total calcium, phosphorus, magnesium, and hemoglobin A1c (HbA1c) were assessed ezymatically by an autoanalyzer (ARCHITECT ci4100 Integrated System, Abbott Diagnostics Laboratories, Abbott Park, IL, USA, Abbott 65205, Wiesbaden, Germany). Serum levels of insulin were measured on an Architect i1000 analyzer (Abbott Ireland, Diagnostics Division, Lisnamuck, Longford, Ireland). The total CV% ranged from 1.9 to 5.2, and the analytical sensitivity was 1 μU/mL. Creatinine levels were determined by an enzymatic method. The total CV% ranged from 1.9 to 5.2, and the analytical sensitivity was 1 μIU/mL. TSH 3rd gen levels, free triiodothyronine (FT3), and free thyroxin (FT4) were measured with the Abbott Architect i1000 analyzer. The total coefficient of variation (CV%) and analytical sensitivity (AS) were as follows: TSH, CV% 1.7–5.3 and AS 0.0025 μIU/mL; FT3, CV% 2.3–5.0 and AS 1.0 pg/mL; FT4, CV% 3.6–7.8 and AS 0.4 ng/dL. Anti-myeloperoxidase antibodies (anti-TPO) and antithyroglobulin antibodies (anti-TG) were evaluated using the microparticle enzyme immunoassay kits “anti-TPO Abbott Axsym” and “anti-TG Abbott Axsym”, respectively, on an Axsym analyzer (Abbott Laboratories). Reference ranges were: TSH 0.40–4.5 μIU/ml; insulin <25 mIU/L; FT4 0.71–1.85 ng/dL; FT3 1.7–3.7 pg/mL; anti-TPO, below 5 IU/mL, antithyroglobulin below 5 IU/mL. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: fasting insulin (microunits per milliliter) × fasting glucose (millimoles per liter)/22.5.
VF assessment
Lumbar spine lateral radiographs were obtained according to quantitative procedures [22]. A single operator, blinded to the identity of the patient, performed the morphometric analysis. The heights of each vertebra, namely the anterior (Ha), posterior (Hp), and middle (Hm), were determined by placement of six points using a cursor and backlit digitizing board. The presence of morphometric VFs was defined using ratios of vertebral height for each vertebral body from T4 to L4: the Ha/Hp (wedge) ratio, the Hm/Hp ratio, and the ratio of the above vertebrae Hp/Hp, as well as the ratio of the below vertebrae Hp/Hp. A height ratio decrease of ≥20 % was the threshold for diagnosing a VF, according to the visual criteria defined by Genant [22]. A vertebral body is considered fractured when at least one of its ratios falls below 3 SDs from normative mean values.
Statistical analysis
Statistical analysis of the data was performed using Statistical Package for the Social Sciences version 15.0 (SPSS Inc, Chicago IL, USA) and Stata statistical software package version 9.2 (StataCorp LP, College Station, TX, USA). Ln-transformed values of original parameters were used whenever necessary to improve normality in distribution for skewed parameters. Student’s t test for independent samples was used for comparisons between mean (or mean log-transformed) values of quantitative parameters between patients with and without prevalent fractures; if normality in distribution was still considered problematic after logarithmic transformation, the non-parametric Wilcoxon–Mann–Whitney test for independent samples was used instead. Fisher’s exact test and χ 2-analysis were used for comparisons of categorical parameters between women with and without prevalent fractures. Thyroid hormones were described as “low-normal” or “high-normal”, if the estimated levels were below or higher than the respective median value for each hormone; levels of thyroid antibodies were dichotomized as “positive” and “negative” if the value of the antibody was higher or lower than fivefold the upper normal limit, respectively [23]. On a secondary level, the impact of thyroid hormones and TSH on the risk of VFs has been also evaluated in the subgroup of patients with negative anti-thyroid antibodies. Multiple logistic regression was applied to further investigate possibly significant associations of parameters with presence/absence of prevalent fractures, while each hormonal parameter was then added, separately. In addition, stepwise logistic regression was used to assess the combined effect of significant indices of thyroid function on the risk of VFs. All regression models were a priori adjusted for risk factors that met the threshold of confounding (i.e. p < 0.1). A cut-off point of p < 0.05 was marked as “statistically significant”.
Results
Study sample demographics
The main demographic and anthropometric characteristics of the 335 euthyroid peri- and postmenopausal patients are presented in Table 1. At least one prevalent VF was identified in 24 (7.2 %) women in our study. No significant differences were observed regarding smoking frequency, alcohol consumption, frequency of physical exercise, level of dietary calcium intake, parity, and number of abortions or mean BMI values, comparing women with prevalent VFs to women without fracture (data not shown).
Comparison between groups
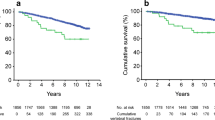
Age and biochemical measurements in the whole study sample and according to the prevalence of lumbar fractures are summed up in Table 2. Women with prevalent VFs were older, with a higher menopausal age and lower levels of ln-HOMA-IR compared to women without fractures (age: 61.2 ± 8.0 vs 56.4 ± 6.9 years, p = 0.001; menopausal age: 13.8 ± 9.1 vs 8.7 ± 6.5 years, p < 0.001; ln-HOMA-IR: 1.16 ± 1.35 vs 1.49 ± 1.71, p = 0.049). Women with prevalent VFs exhibited lower levels of FT3 compared to women without VFs (2.27 ± 0.48 vs 2.60 ± 0.60 pg/mL, p = 0.016, t test). Moreover, women with at least one VF had almost significantly lower levels of ln-insulin (5.20 ± 1.34 vs 6.52 ± 1.64 μIU/mL, p = 0.063, t test) and ln-TSH (1.08 ± 1.52 vs 1.27 ± 1.72 μIU/mL, p = 0.085, t test). In addition, the development of VFs associated with positive anti-thyroid antibodies (anti-TG: 46 vs 27.9 %, χ 2 = 0.104). Women with TSH levels < median value of 1.30 μIU/mL had significantly higher prevalence of VFs compared to women with TSH levels >1.30 μIU/mL (10.7 vs 3.6 %, p = 0.018, Fisher’s exact test; Fig. 1). In addition, women with FT3 levels < median value of 2.51 pg/mL had significantly higher prevalence of VFs compared to women without VFs (11.4 vs 2.9 %, p = 0.009, Fisher’s exact test). Women with positive anti-TPO and anti-TG antibodies presented the highest risk of VF compared to women with only one positive category of antibodies or women without antibodies (Fig. 2). No major differences in any other biochemical measurement were noted between patients having and those not having prevalent lumbar fractures (p > 0.05).
Logistic regression
The odds ratios of having a VF according to biochemical and hormonal parameters are presented in Table 3. After adjusting for age, years since menopause, and ln-HOMA-IR, as possible confounders, ln-transformed serum levels of TSH associated positively with the odds of having a fracture (p = 0.037). In addition, levels of high-normal levels of TSH are associated with lower risk of VFs compared to low-normal levels of TSH (OR = 0.188, p = 0.015). The impact of TSH on the development of VFs remains the same, even in the subgroup of patients with negative anti-thyroid antibodies (ln-TSH: OR = 0.201, p = 0.029), adjusting for age, menopausal age, and insulin resistance. Moreover, the presence of anti-TG antibodies exhibited a positive association with the development of VFs, implying lack of protection. In addition, the simultaneous presence of both positive anti-TPO and anti-TG antibodies associated with the risk of VFs (OR = 6.328, p = 0.009). Ln-transformed levels of insulin and HOMA-IR tended to affect the occurrence of VFs, after adjusting for the same confounders (p = 0.083 and 0.062, respectively). Levels of FT3 and FT4 presented no significant association with the risk of VFs in the total sample or in the subgroup of patients with negative anti-thyroid antibodies, in models of multivariate analysis adjusted for age, menopausal age, and insulin resistance (data not shown).
Stepwise logistic regression analysis showed that the risk of VFs was predicted by menopausal age and both levels of ln-TSH and positive anti-TG antibodies, in a stepwise multivariate logistic regression model that included also age and ln-HOMA-IR (model χ 2 = 20.070; ln-TSH: OR = 0.277, p = 0.026; positive anti-TG: OR = 3.273, p = 0.030). Moreover, the risk of VFs was predicted by the combined presence of anti-TG and anti-TPO (OR = 6.637, p = 0.007) as well as menopausal age (OR = 1.12, p = 0.001) and ln-TSH (OR = 0.312, p = 0.052), in a stepwise multiple regression analysis model that included also age and ln-HOMA-IR as independent variables.
Discussion
The present study shows that higher TSH levels within the normal range are associated with lower risk of vertebral fracture in this sample of postmenopausal Greek women. Moreover, positive anti-thyroid antibodies further increased the risk of fracture. These associations occurred independently of confounders such as age, menopausal age, and BMI.
It is important to stress that lower TSH levels were associated with higher frequency of VFs within the euthyroid range, as we included women with normal thyroid function in the analyses. Elevated risk of hip fracture was observed in women with TSH levels within the low normal range (0.35–1.6 μIU/mL), according to a recent study of both genders that included 9421 women, aged ≥65 years [16]. In addition, the results of the OPENTHYRO cohort described an association between the risk of fractures and decreasing levels of TSH within the euthyroid range [17]. Concerning women, the same study showed that low-normal TSH was a significant predictor of hip, spine, forearm, and humerous fractures [17]. Similarly, the presence of VFs has been associated with lower levels of TSH within the normal range in a smaller cohort of 130 euthyroid postmenopausal women. In that study, women with low-normal TSH were almost three times more probable to have a vertebral fracture compared to the rest of the population [15].
On the contrary, a smaller number of studies found no association between thyroid function within the normal range and vertebral fractures. In the Osteoporosis and Ultrasound Study (OPUS), which included a large sample of 1278 euthyroid, elderly postmenopausal women, although they identified a 35 % reduction in incident non-vertebral fractures in women with TSH levels within the high-normal range, they observed no association between TSH levels and incident or prevalent VFs; however, they used a narrower reference range to define euthyroidism (0.15–3.64 μΙU/mL) compared to our study [24]. Furthermore, no association between TSH levels within the normal range and bone fractures has been identified in a large mixed-gender sample of patients with chronic disabling diseases [25].
Hyperthyroidism is an established risk factor for osteoporotic fractures [26]. Controversy, however, exists whether milder subclinical conditions associated with low or low-normal TSH have an impact on bone health of older adults. Our results, along with other studies [11, 19, 20] indicate that these conditions might indeed pose a risk factor for osteoporotic fractures. Recent evidence indicates that TSH may have a direct protective role against osteoporosis. Results from histomorphometric analyses showed that TSH signaling slows bone resorption, by inhibiting osteoclasts differentiation in vitro [27, 28]. Similarly, Baliram et al. [29] indicated that TSH increases osteoblast differentiation and regulates osteoprotegerin production in vitro, with the latter possibly inhibiting osteoclastic resorption locally. The same group, trying to answer the question whether the increased thyroid hormones are the only pathogenetic factor leading to osteoporosis, compared two groups of hyperthyroid mice: (1) animals lacking the TSH receptor and (2) wild-type animals [30]. The researchers observed that net bone loss was more pronounced in mice lacking TSH-receptor versus wild type, indicating the protective role of TSH signaling in the regulation of bone turnover [30].
Positive anti-thyroid antibodies presented as an additional predictor of high fracture risk, especially in association with low-normal levels of TSH in this sample of postmenopausal women. According to our knowledge, the impact of anti-thyroid antibodies on the development of VFs in the euthyroid population has not been investigated yet. On the other hand, positive anti-TPO antibodies have been associated with FRAX score in postmenopausal women with subclinical hypothyroidism [12], but not in perimenopausal women with subclinical hyperthyroidism [11]. In addition, the presence of anti-TPO antibodies has been associated with worse quantitative calcaneous ultrasound parameters, in a large sample of Chinese men [31]. The combined assessment of antithyroid antibodies seems more rational, taking into account that anti-TPO and anti-TG antibodies fluctuate in parallel [32]. It has to be noted that positive anti-TG per se was associated with the presence of vertebral fractures, independently of age, menopausal age, and insulin resistance. However, the clinical implications of this finding cannot be elucidated, since anti-TPO represents a superior clinical marker of disease compared to anti-TG [32].
Levels of free thyroid hormones were not associated with the risk of VF, adjusting for age, menopausal age, and insulin resistance, according to our findings. Evidence on the impact of circulating free thyroid hormones on the risk of VFs is limited. No significant effect of serum FT4 levels on the risk of VFs has been identified in a cohort of 130 euthyroid postmenopausal women, in agreement with our findings [15]. A direct association between levels of free thyroid hormones and the risk of non-vertebral fractures has been observed in a population based sample of 2374 euthyroid postmenopausal women [18]. In addition, high levels of FT4 have been associated with lower quantitative calcaneous parameters in a large sample of Chinese men [31], as well as with lower lumbar spine BMD in perimenopausal women [33]. Finally, according to the results of a population-based cohort study, elevated levels of TSH (>4.0 μIU/mL) have been associated with higher risk of hip fracture in women (HR = 1.75, 95 % 1.24–2.46) [19].
The present study has some limitations. First, the cross-sectional design does not permit the detection of causality. Women of our sample were recruited from an outpatient clinic and were thus more health concerned than the general population. Moreover, we did not evaluate bone turnover markers so that no definite conclusions can be drawn regarding the effect of thyroid function on bone metabolism. Finally, women with positive antithyroid antibodies cannot be defined as cases with euthyroid Hashimoto’s, since the analysis has been performed retrospectively and data regarding sonographic assessment of the thyroid was sparse.
In conclusion, this cross-sectional study of Greek postmenopausal women, serum TSH within the euthyroid range had a significant inverse association with prevalent VFs. Positive antithyroid antibodies emerged as another significant predictor of fracture risk. The risk of VF was even higher for women with positive antithyroid antibodies and low-normal TSH levels. These associations remained significant even after adjusting for traditional risk factors, such as age, menopausal age, and insulin resistance. Our findings, although indicative, may have implications on the evaluation of thyroid profile with respect to osteoporotic fracture risk.
References
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY et al (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Ensrud KE, Schousboe JT (2011) Clinical practice. Vertebral fractures. N Engl J Med 364:1634–1642
Donaldson MG, Palermo L, Schousboe JT, Ensrud KE, Hochberg MC, Cummings SR (2009) FRAX and risk of vertebral fractures: the fracture intervention trial. J Bone Miner Res 24:1793–1799
Briggs AM, Greig AM, Wark JD (2007) The vertebral fracture cascade in osteoporosis: a review of aetiopathogenesis. Osteoporos Int 18:575–584
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23:2239–2256
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Waung JA, Bassett JH, Williams GR (2012) Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends Endocrinol Metab 23:155–162
Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR et al (2015) Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 313:2055–2065
Yan Z, Huang H, Li J, Wang J (2015) Relationship between subclinical thyroid dysfunction and the risk of fracture: a meta-analysis of prospective cohort studies. Osteoporos Int 30:30
Polovina S, Micic D, Miljic D, Milic N, Popovic V (2015) The Fracture Risk Assessment Tool (FRAX score) in subclinical hyperthyroidism. Vojnosanit Pregl 72:510–516
Polovina S, Popovic V, Duntas L, Milic N, Micic D (2013) Frax score calculations in postmenopausal women with subclinical hypothyroidism. Hormones 12:439–448
Tuchendler D, Bolanowski M (2014) The influence of thyroid dysfunction on bone metabolism. Thyroid Res 7:0012–0014
Bassett JH, Williams GR (2008) Critical role of the hypothalamic–pituitary–thyroid axis in bone. Bone 43:418–426
Mazziotti G, Porcelli T, Patelli I, Vescovi PP, Giustina A (2010) Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone 46:747–751
Leader A, Ayzenfeld RH, Lishner M, Cohen E, Segev D, Hermoni D (2014) Thyrotropin levels within the lower normal range are associated with an increased risk of hip fractures in euthyroid women, but not men, over the age of 65 years. J Clin Endocrinol Metab 99:2665–2673
Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Brix TH, Hegedus L (2014) Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures—the OPENTHYRO register cohort. J Bone Miner Res 29:2040–2050
Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R et al (2010) Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab 95:3173–3181
Svare A, Nilsen TI, Asvold BO, Forsmo S, Schei B, Bjoro T et al (2013) Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 169:845–852
LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS (2007) Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause 14:191–202
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Saltiki K, Voidonikola P, Stamatelopoulos K, Mantzou E, Papamichael C, Alevizaki M (2008) Association of thyroid function with arterial pressure in normotensive and hypertensive euthyroid individuals: a cross-sectional study. Thyroid Res 1:3
Schmidt S, Schelde B, Norgaard K (2014) Effects of advanced carbohydrate counting in patients with type 1 diabetes: a systematic review. Diabetes Med 31:886–896
Gillespie SJ, Kulkarni KD, Daly AE (1998) Using carbohydrate counting in diabetes clinical practice. J Am Diet Assoc 98:897–905
Fitch C, Keim KS (2012) Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 112:739–758
Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC (2010) The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care 33:1859–1864
Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ (2012) Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care 35:446–454
Standl E, Erbach M, Schnell O (2013) Glycemic control: a combination of lifestyle management and the use of drugs. Cardiol Ther 2:1–16
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M et al (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Shi Y, Sun M, Wang Z, Fu Q, Cao M, Zhu Z et al (2014) Association between calcaneus quantitative ultrasound (QUS) parameters and thyroid status in middle-aged and elderly Chinese men with euthyroidism: a population-based cross-sectional study. Endocrine 47:227–233
McLachlan SM, Rapoport B (2004) Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid 14:510–520
van Rijn LE, Pop VJ, Williams GR (2014) Low bone mineral density is related to high physiological levels of free thyroxine in peri-menopausal women. Eur J Endocrinol 170:461–468
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
About this article
Cite this article
Lambrinoudaki, I., Armeni, E., Pliatsika, P. et al. Thyroid function and autoimmunity are associated with the risk of vertebral fractures in postmenopausal women. J Bone Miner Metab 35, 227–233 (2017). https://doi.org/10.1007/s00774-016-0752-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-016-0752-0