Abstract
Fibroblast growth factor 23 (FGF23) has been shown to work as a phosphotropic hormone. Although FGF23 reduces the serum phosphate level, it has not been established that phosphate directly regulates FGF23 production. In this study, we investigated whether phosphate can enhance Fgf23 expression using the rat osteoblastic cell line UMR-106, which has been shown to express Fgf23 in response to 1,25-dihydroxyvitamin D [1,25(OH)2D]. Phosphate increased Fgf23 expression in a dose- and time-dependent manner in the presence of 1,25(OH)2D. Phosphate also increased Fgf23 promoter activity, but showed no effect on the half-life of Fgf23 messenger RNA. Phosphonoformic acid and PD98059, an inhibitor of MEK, inhibited the effects of phosphate on Fgf23 expression and promoter activity. In addition, phosphate enhanced production of reactive oxygen species (ROS) in UMR-106 cells, and hydrogen peroxide enhanced FGF23 production in a dose- and time-dependent manner. Hydrogen peroxide also enhanced Elk1 reporter activity, a target of the MEK–extracellular-signal-regulated kinase (ERK) pathway. Furthermore, the effect of phosphate on ROS production and Fgf23 expression was inhibited by apocynin, an inhibitor of NADPH oxidase. These results indicate that phosphate directly enhances Fgf23 transcription without affecting the stability of Fgf23 messenger RNA by stimulating NADPH-induced ROS production and the MEK–ERK pathway in UMR-106 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblast growth factor 23 (FGF23) decreases proximal phosphate reabsorption by suppressing the expression of type 2a and type 2c sodium–phosphate cotransporters [1]. FGF23 also inhibits intestinal phosphate absorption by lowering the level of 1,25-dihydroxyvitamin D [1,25(OH)2D] [1]. By these actions on the kidney and the intestine, FGF23 decreases the serum phosphate level. It has been shown that excess actions of FGF23 underlie several kinds of hypophosphatemic diseases, such as X-linked hypophosphatemic rickets and tumor-induced osteomalacia [2, 3]. On the other hand, hyperphosphatemic familial tumoral calcinosis is caused by impaired actions of FGF23 [2]. These results indicate that FGF23 is a physiological humoral factor and works as a phosphotropic hormone. Therefore, the production and circulatory level of FGF23 need to be tightly regulated. Previous studies indicated that a high-phosphate diet increases FGF23 levels in both humans and animals, suggesting that phosphate increases FGF23 production [4, 5]. On the other hand, it has also been shown that acute changes in the serum phosphate level do not affect circulatory FGF23 levels in human [6]. Therefore, it is unknown whether phosphate directly regulates FGF23 production.
High FGF23 levels have been shown to be associated with various adverse events, such as cardiovascular events, left ventricular hypertrophy, progression of chronic kidney disease (CKD), and fractures [7]. However, the mechanism of these associations remains to be clarified. This problem derives at least in part from uncertainness regarding the detailed regulatory mechanisms of FGF23 production. 1,25(OH)2D was shown to enhance FGF23 production and FGF23 levels [5, 8, 9]. However, 1,25(OH)2D is clearly not the sole stimulant of FGF23 production because the FGF23 level is high in patients with CKD whose 1,25(OH)2D levels are rather low [10]. Therefore, we investigated the effects of phosphate on FGF23 production using rat osteoblast-like cells (UMR-106) which have been shown to express FGF23 [9, 11]. We also used 1,25(OH)2D3, which enhances FGF23 production, as a positive control and compared the effects of 1,25(OH)2D3 with those of phosphate.
Materials and methods
Materials
Dulbecco’s modified Eagle’s high-glucose medium (DMEM), fetal bovine serum (FBS), and 0.25 % trypsin were from GIBCO, Life Technologies (Carlsbad, CA, USA). 1,25(OH)2D3 was purchased from Calbiochem (Tokyo, Japan). PD98059, apocynin, and phosphonoformic acid (PFA) were purchased from Sigma-Aldrich (Tokyo, Japan), and H2O2 was from Wako (Tokyo, Japan). Indicated concentrations of sodium phosphate buffer (0.1 M Na2HPO4/NaH2PO4, pH 7.4) were added to the culture to examine the effects of phosphate.
Cell cultures
Rat osteogenic sarcoma cells (UMR-106) were obtained from ATCC. UMR-106 cells were cultured in DMEM containing 10 % FBS. Cell cultures were maintained at 37 °C with 5 % CO2 and were passaged with 0.25 % trypsin every 3 days.
Real-time PCR
UMR-106 cells were seeded on 48-well plates at 3 × 104 cells per well. After 6 h, the cells were treated with the indicated concentrations of 1,25(OH)2D3, phosphate, or H2O2 for various times. If the incubation time was longer than 48 h, the medium was changed at 48 h. PD98059, apocynin, or PFA was added to the medium 30 min before the addition of sodium phosphate buffer. Total RNA was isolated utilizing an RNAqueous-4PCR kit (Ambion, Life Technologies, Carlsbad, CA, USA), treated with DNase (Ambion), and reverse-transcribed using PrimeScript RT master mix (perfect real time) (Takara, Tokyo, Japan). The complementary DNA from 100 ng of total RNA was subjected to real-time PCR using TaqMan fast universal PCR master mix (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). Reactions were run on and analyzed with an ABI StepOnePlus instrument (Applied Biosystems, Tokyo, Japan). The messenger RNA (mRNA) levels were normalized to the Gadph mRNA level. The primer sets were obtained from Applied Biosystems (Fgf23, no. Rn00590709_m1; Gadph, no. Rn99999916_s1).
Fgf23 promoter/reporter assays
We amplified 5′-flanking regions of the rat Fgf23 gene with 2,800, 1,200 and 560 bp by PCR and subcloned them into a pGL3-basic vector (Promega, Madison, WI) to create Fgf23 promoter/firefly luciferase reporter constructs (−2800bp_luc, −1200bp_luc, and −560bp_luc, respectively). UMR-106 cells on 48-well plates were transfected with these reporter vectors (0.3 µg) and pRL-TK (0.01 µg) (Promega) by using Attractene transfection reagent (QIAGEN, Tokyo, Japan). After 24 h, the cells were treated with 1,25(OH)2D3 and/or sodium phosphate buffer. After a further 48 h, luciferase activities were measured with a dual-luciferase reporter assay system (Promega).
Analysis of mRNA stability
Fgf23 mRNA levels were analyzed by real-time PCR at 0, 1, 2, and 4 h after treatment with actinomycin D, and the half-life of Fgf23 mRNA was calculated.
Elk1 luciferase reporter assays
Elk1 is a nuclear target for the Raf–MEK–extracellular-signal-regulated kinase (ERK) signaling cascade [12]. To confirm whether extracellular phosphate activates the Raf–MEK–ERK pathway in UMR-106 cells, we constructed an Elk1 luciferase reporter vector (Elk1_luc). Two DNA oligonucleotides containing an Elk1 binding site (5′-CTTTGCAAAATGCAGGAATTGTTTTCACAGTTTTGCAAAATGCAGGAATTGTTTTCACAGTC-3′ and 5′-TCGAGACTGTGAAAACAATTCCTGCATTTTGCAAAACTGTGAAAACAATTCCTGCATTTTGCAAAGGTAC-3′) were annealed and subcloned into a pGL3-basic vector (Promega, Madison, WI) [13]. Luciferase activities were analyzed as previously described.
Analysis of reactive oxygen species production
Reactive oxygen species (ROS) production was analyzed using a pGL4.37 vector that contains antioxidant response element (Promega, Madison, WI, USA) [14]. Cells were incubated in DMEM containing 2 % FBS, and luciferase activities were analyzed as previously described.
Statistical analyses
Student’s t test was used to evaluate the statistical significance between two groups. One-way ANOVA and Williams’s test for multiple sample comparisons were used for more than three groups using Dr. SPSS II for Windows (Tokyo, Japan). All values are expressed as the mean ± the standard error of the mean. P values less than 0.05 were considered to be statistically significant.
Results
Regulation of Fgf23 expression by 1,25(OH)2D3
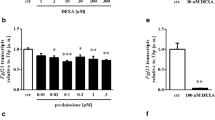
1,25(OH)2D3 was shown to enhance Fgf23 expression in UMR-106 and ROS 17/2.8 cells [8, 9]. However, the mechanism of this stimulatory effect of 1,25(OH)2D3 on FGF23 production has not been fully addressed. Therefore, we first analyzed the effects of 1,25(OH)2D3 on Fgf23 expression in UMR-106 cells. As previously reported, 1,25(OH)2D3 increased Fgf23 mRNA levels in a dose-dependent manner (Fig. 1a). Fgf23 expression was not detectable in cells with vehicle or treated with 0.1 nM 1,25(OH)2D3, but Fgf23 mRNA expression from cells treated with 100 nM 1,25(OH)2D3 was about 25-fold higher than that from cells treated with 1 nM 1,25(OH)2D3.
Effects of 1,25-dihydroxyvitamin D3 [1,25(OH) 2 D 3 ] on Fgf23 messenger RNA (mRNA) expression and Fgf23 promoter activity. a After UMR-106 cells had been treated with the indicated concentrations of 1,25(OH)2D3 for 48 h, Fgf23 mRNA was evaluated by real-time PCR analysis. Two asterisks P < 0.01 versus vehicle. b UMR-106 cells were treated with the indicated concentrations of 1,25(OH)2D3 for 48 h, and the relative luciferase activity of −560bp_luc was evaluated. One asterisk P < 0.05 versus vehicle, two asterisks P < 0.01 versus vehicle
To clarify the mechanism of this stimulatory effect of 1,25(OH)2D3 on Fgf23 expression, we created several Fgf23 promoter/firefly luciferase reporter constructs (−2800bp_luc, −1200bp_luc, and −560bp_luc). All of these Fgf23 promoters exhibited functional activities in transiently transfected UMR-106 cells (data not shown). However, −560bp_luc showed the highest luciferase activity. Therefore, we next evaluated the effects of 1,25(OH)2D3 on Fgf23 promoter activity using −560bp_luc. 1,25(OH)2D3 resulted in a dose-dependent stimulation of luciferase activity in UMR-106 cells transfected with −560bp_luc (Fig. 1b). The maximal increase was approximately twofold with 100 nM 1,25(OH)2D3. The increase of promoter activity was strongest 48 h after treatment with 100 nM 1,25(OH)2D3 (data not shown). These results suggested that the 5′ flanking region from -560 had an important promoter activity, reacting with 1,25(OH)2D3.
Messenger RNA (mRNA) levels are determined by both transcriptional activity of the gene and the stability of the mRNA produced. Therefore, we next analyzed whether 1,25(OH)2D3 also influenced mRNA stability. RNA synthesis was inhibited using actinomycin D, and Fgf23 mRNA levels were analyzed by real-time PCR at 0, 1, 2 and 4 h after the inhibition of transcription. In cells treated with 100 nM 1,25(OH)2D3, the half-life of Fgf23 mRNA (2.35 ± 0.14 h) was significantly longer than that of Fgf23 mRNA in cells treated with 1 nM 1,25(OH)2D3 (1.37 ± 0.07 h), indicating that posttranscriptional regulation of Fgf23 expression by 1,25(OH)2D3. Therefore, these results indicate that 1,25(OH)2D3 enhances Fgf23 expression by both transcriptional and posttranscriptional mechanisms.
Regulation of Fgf23 expression by phosphate
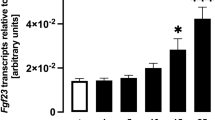
We next analyzed the effects of phosphate on Fgf23 expression. When UMR-106 cells were treated with up to 5 mM added phosphate, Fgf23 mRNA was barely detectable by real-time PCR. Then, we analyzed Fgf23 mRNA levels in the presence of 1 nM 1,25(OH)2D3. As shown in Fig. 2a, phosphate clearly increased Fgf23 mRNA levels after 72 h treatment. This increase of Fgf23 mRNA levels caused by phosphate was dose dependent and specific for phosphate, because 5 mM sodium sulfate did not increase Fgf23 mRNA levels (Fig. 2b). When PFA was added to the culture, the Fgf23 mRNA level was less than that of cells treated with 1 nM 1,25(OH)2D3 alone. PFA completely abolished the stimulatory effect of phosphate on the Fgf23 mRNA level, indicating that phosphate stimulated Fgf23 expression by entering cells (Fig. 2b).
Effects of phosphate on Fgf23 messenger RNA (mRNA) in UMR-106 cells. a The indicated amount of phosphate was added to the culture of UMR-106 cells in the presence of 1 nM 1,25-dihydroxyvitamin D3 [1,25(OH) 2 D 3 ] and Fgf23 mRNA was evaluated by real-time PCR. One asterisk P < 0.05 versus without phosphate addition, two asterisks P < 0.01 versus without phosphate addition. b UMR-106 cells were treated for 96 h, and Fgf23 mRNA was evaluated by real-time PCR. One asterisk P < 0.05 versus cells treated with 1 nM 1,25(OH)2D3 alone, two asterisks P < 0.01 versus cells treated with 1 nM 1,25(OH)2D3 alone. c Cells were treated for 48 h, and the relative luciferase activity of −560bp_luc was evaluated. In this luciferase assay, 1,25(OH)2D3 was not added. Asterisk P < 0.05 versus vehicle alone. PFA phosphonoformic acid, Pi inorganic phosphate
To investigate whether phosphate stimulated Fgf23 transcription, Fgf23 promoter activities were analyzed as described before. Phosphate enhanced luciferase activity of −560bp_luc in a dose-dependent manner (Fig. 2c). Sodium sulfate did not enhance the Fgf23 promoter activity, indicating that the effect of phosphate on the Fgf23 promoter was specific (Fig. 2c). Furthermore, PFA abolished the effect of phosphate on Fgf23 promoter activity, again indicating that phosphate enhanced Fgf23 transcription by entering cells (Fig. 2c). We also analyzed the effects of phosphate on Fgf23 mRNA stability. However, phosphate did not affect the half-life of Fgf23 mRNA (9.16 ± 2.34 h without phosphate, 6.68 ± 1.56 h with 5 mM phosphate). Therefore, phosphate seemed to enhance Fgf23 expression by stimulating transcription of Fgf23.
Mechanism of the effect of phosphate on Fgf23 expression
We further analyzed how phosphate enhanced Fgf23 transcription. Phosphate was shown to activate the Raf–MEK–ERK pathway in chondrocytes [15]. Therefore, we first analyzed the effects of phosphate on this pathway. Phosphate significantly enhanced Elk1_luc activity, indicating that phosphate actually activated the Raf–MEK–ERK pathway in UMR-106 cells (Fig. 3a). In addition, PD98059, a MEK inhibitor, inhibited the stimulatory effect of phosphate on the Fgf23 mRNA level (Fig. 3b), and also inhibited −560bp_luc luciferase activity stimulated by phosphate in a dose-dependent manner (Fig. 3c). These results indicate that phosphate enhanced FGF23 production through the ERK pathway.
Effects of phosphate on the extracellular-signal-regulated kinase pathway. a After UMR-106 cells had been were treated with or had not been treated with phosphate, the relative luciferase activity of Elk1_luc was evaluated. Two asterisks P < 0.01 versus cells without phosphate. b UMR-106 cells were treated with phosphate and/or PD98059 (a MEK inhibitor) for 96 h and Fgf23 messenger RNA (mRNA) was evaluated by real-time PCR. Two asterisks P < 0.01 versus cells without phosphate, cross P < 0.05 versus cells treated without PD98059. c Cells were treated with phosphate and/or PD98059 for 48 h, and the relative luciferase activity of −560bp_luc was evaluated. Asterisk P < 0.05 versus vehicle alone. In the luciferase assays (a, c), 1,25-dihydroxyvitamin D3 [1,25(OH) 2 D 3 ] was not added. Pi inorganic phosphate
Phosphate was also shown to increase the levels of ROS, and ROS are well known to have diverse effects on cellular functions [16]. Therefore, we analyzed whether ROS has a role in the regulation of Fgf23 expression caused by phosphate. H2O2 is frequently used as a positive control for ROS. Therefore, we treated UMR-106 cells with H2O2. H2O2 increased Fgf23 mRNA expression in a time- and dose-dependent manner and also enhanced Fgf23 promoter activity (Fig. 4). H2O2 also increased Elk1_luc activity, suggesting that ROS enhanced Fgf23 expression through the ERK pathway (Fig. 5a). The stimulatory effect of H2O2 on Fgf23 mRNA expression was abrogated by the addition of PD98059 (Fig. 5b). Finally, we examined whether phosphate actually enhanced ROS production in UMR-106 cells and how phosphate affected ROS production. It was shown that phosphate enhanced ROS production through NADPH oxidase in osteoblasts and the inhibition of NADPH oxidase stimulated osteoblastic differentiation [17, 18]. Therefore, we analyzed the effect of apocynin, an inhibitor of NADPH oxidase, on ROS and FGF23 production. As shown in Fig. 6a, phosphate enhanced ROS production as evaluated by antioxidant reporter activity, and apocynin almost completely inhibited the ROS production by phosphate in UMR-106 cells. Apocynin also inhibited the stimulatory effect of phosphate on Fgf23 expression (Fig. 6b). There results indicate that phosphate enhanced Fgf23 mRNA expression by the ROS–ERK pathway in UMR-106 cells.
Effects of H2O2 on fibroblast growth factor 23 production. a After UMR-106 cells had been treated with 100 µM H2O2 and 1 nM 1,25-dihydroxyvitamin D3 [1,25(OH) 2 D 3], Fgf23 messenger RNA (mRNA) was evaluated by real-time PCR. Asterisk P < 0.05 versus untreated cells. b Cells were treated with H2O2 for 48 h, and Fgf23 mRNA was evaluated by real-time PCR. Asterisk P < 0.05 versus untreated cells. c Cells were treated for 36 h, and the relative luciferase activity of −560bp_luc was evaluated. A significant increase of the promoter activity was also observed in cells treated for 48 h. In this luciferase assay, 1,25(OH)2D3 was not added. Asterisk P < 0.05 versus vehicle alone
Relationship between reactive oxygen species and the extracellular-signal-regulated kinase pathway. a After UMR-106 cells had been treated with or not treated with H2O2, the relative luciferase activity of Elk1_luc was evaluated. In this luciferase assay, 1,25-dihydroxyvitamin D3 [1,25(OH) 2 D 3 ] was not added. Asterisk P < 0.05 versus vehicle alone. b UMR-106 cells were treated with H2O2 and/or PD98059 for 48 h, and Fgf23 messenger RNA (mRNA) was evaluated by real-time PCR. Asterisk P < 0.01 versus cells without H2O2, cross P < 0.05 versus cells treated without PD98059
Phosphate enhances Fgf23 expression by enhancing reactive oxygen species produced through NADPH oxidase a Reactive oxygen species production was examined using a reporter containing antioxidant response element. Asterisk P < 0.05 versus vehicle alone, cross P < 0.05 versus cells treated without apocynin. In this luciferase assay, 1,25-dihydroxyvitamin D3 [1,25(OH) 2 D 3 ] was not added. b UMR-106 cells were treated with phosphate and/or apocynin for 48 h, and Fgf23 mRNA was evaluated by real-time PCR. Two asterisks P < 0.01 versus cells without phosphate, cross P < 0.05 versus cells treated without apocynin. Pi inorganic phosphate
Discussion
Here we have shown that 1,25(OH)2D3 increased Fgf23 expression by enhancing both Fgf23 transcription and Fgf23 mRNA stability, whereas phosphate stimulated Fgf23 expression by promoting transcription of Fgf23 in UMR-106 cells. These results support the concept that FGF23 is a phosphotropic hormone and there is a negative-feedback system between serum phosphate and FGF23 production. It had already been shown that FGF23 decreases the serum 1,25(OH)2D level and that 1,25(OH)2D stimulates FGF23 production [1, 5, 8, 9]. Therefore, several mechanisms seem to be able to modulate Fgf23 expression, and there are multiple negative-feedback loops that regulate FGF23 production and circulatory levels.
1,25(OH)2D3 was shown to enhance Fgf23 transcription and increase FGF23 levels in vivo [5]. Liu et al. [8] reported that 1,25(OH)2D3 enhanced mouse Fgf23 promoter activity, and they identified vitamin D response element between −1,180 and −1,156 bp of murine Fgf23. Our results also indicated that 1,25(OH)2D3 enhanced rat Fgf23 promoter activity. However, we showed the stimulatory effect of 1,25(OH)2D3 on the Fgf23 promoter using −560bp_luc, suggesting that the vitamin D response elements of mouse and rat Fgf23 genes are different. In addition to this stimulatory effect of 1,25(OH)2D3 on Fgf23 transcription, our results indicate that 1,25(OH)2D3 enhanced Fgf23 mRNA stability. However, the observed increase of Fgf23 mRNA expression was much higher than that expected from these transcriptional and posttranscriptional effects of 1,25(OH)2D3. Therefore, it is plausible that unidentified regions in the Fgf23 gene determine the responsiveness to 1,25(OH)2D3 in addition to the promoter up to -560 in UMR-106 cells.
It has previously been shown that 1,25(OH)2D3 increases Fgf23 expression in UMR-106 cells [9]. However, the production of FGF23 was not shown in that article [9]. We tried to detect FGF23 by both Western blotting and ELISA. However, we could detect neither full-length FGF23 nor processed fragments of FGF23 in the conditioned medium or cell lysate of UMR-106 cells treated with 1,25(OH)2D and/or phosphate. It is not clear why the UMR-106 cells used in this study did not produce FGF23. In addition, we used only UMR-106 cells in this study because there are no other cell lines that can consistently produce Fgf23. It is therefore unclear whether these findings obtained in UMR-106 cells will be applicable to other cells and in vivo.
FGF23 is now considered to work as a phosphotropic hormone. Therefore, it is reasonable to think that the production and circulatory levels of FGF23 are regulated by phosphate as calcium modulates parathyroid hormone expression and secretion. However, it has not been widely recognized that phosphate directly affects Fgf23 expression. While phosphate was shown to enhance FGF23 expression in one report, the mechanism of this effect of phosphate is unknown [19]. Here we show that phosphate enhanced Fgf23 expression after 72 h. These results are consistent with those of our previous in vivo study that acute changes of serum phosphate did not modify FGF23 levels [6]. Therefore, the regulation of Fgf23 expression by phosphate seems to be a rather slow response. Although our results indicate that phosphate enhances Fgf23 expression by entering cells and stimulating the ERK pathway through ROS, further studies are necessary to clarify the precise regulatory mechanisms of FGF23 production.
Our results indicate that at least 1,25(OH)2D and phosphate can stimulate FGF23 production by different mechanisms. These results suggest that there are other regulators and signaling pathways that modulate FGF23 production. It has been shown that the FGF23 level begins to increase far before the development of hyperphosphatemia and despite the continuous decrease of 1,25(OH)2D levels during the progression of CKD [20]. Therefore, this increase of FGF23 levels in patients with early CKD can be explained by neither circulatory phosphate levels nor 1,25(OH)2D levels. It is known that ROS levels are increased in patients with renal failure [21]. It should be clarified whether ROS are involved in the increase of FGF23 levels in patients with CKD. Although high FGF23 levels have been reported to be associated with various adverse events [7], the precise reason for these correlations is unclear. If ROS can also increase FGF23 production in vivo, it is necessary to examine whether ROS are the link between high FGF23 levels and various adverse events.
In conclusion, we have shown that 1,25(OH)2D and phosphate enhance FGF23 production in UMR-106 cells by different mechanisms. These results underline the notion that FGF23 works as a phosphotropic hormone and warrant further studies to clarify the detailed mechanisms of the effects of 1,25(OH)2D and phosphate on FGF23 production.
References
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Fukumoto S, Martin TJ (2009) Bone as an endocrine organ. Trends Endocrinol Metab 20:230–236
Carpenter TO (2012) The expanding family of hypophosphatemic syndromes. J Bone Miner Metab 30:1–9
Ferrari SL, Bonjour JP, Rizzoli R (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90:1519–1524
Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N (2005) Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280:2543–2549
Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T (2007) Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab 25:419–422
Fukumoto S, Shimizu Y (2011) Fibroblast growth factor 23 as a phosphotropic hormone and beyond. J Bone Miner Metab 29:507–514
Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD (2006) Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17:1305–1315
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK (2005) 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042
Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, Fukagawa M (2004) Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 44:250–256
Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T (2010) PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 299:F882–F889
Babu GJ, Lalli MJ, Sussman MA, Sadoshima J, Periasamy M (2000) Phosphorylation of elk-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J Mol Cell Cardiol 32:1447–1457
Li QJ, Vaingankar S, Sladek FM, Martins-Green M (2000) Novel nuclear target for thrombin: activation of the Elk1 transcription factor leads to chemokine gene expression. Blood 96:3696–3706
Hirano S, Watanabe T, Kobayashi Y (2013) Effects of arsenic on modification of promyelocytic leukemia (PML): PML responds to low levels of arsenite. Toxicol Appl Pharmacol 273:590–599
Kimata M, Michigami T, Tachikawa K, Okada T, Koshimizu T, Yamazaki M, Kogo M, Ozono K (2010) Signaling of extracellular inorganic phosphate up-regulates cyclin D1 expression in proliferating chondrocytes via the Na+/Pi cotransporter Pit-1 and Raf/MEK/ERK pathway. Bone 47:938–947
Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E (2009) Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20:1504–1512
Lee YS, Choi EM (2011) Apocynin stimulates osteoblast differentiation and inhibits bone-resorbing mediators in MC3T3-E1 cells. Cell Immunol 270:224–229
Okamoto T, Taguchi M, Osaki T, Fukumoto S, Fujita T (2013) Phosphate enhances reactive oxygen species production and suppresses osteoblastic differentiation. J Bone Miner Metab 32:393–399
Ito N, Findlay DM, Anderson PH, Bonewald LF, Atkins GJ (2013) Extracellular phosphate modulates the effect of 1α,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J Steroid Biochem Mol Biol 2013:183–186
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378
Ruiz S, Pergola PE, Zager RA, Vaziri ND (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83:1029–1041
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hori, M., Kinoshita, Y., Taguchi, M. et al. Phosphate enhances Fgf23 expression through reactive oxygen species in UMR-106 cells. J Bone Miner Metab 34, 132–139 (2016). https://doi.org/10.1007/s00774-015-0651-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0651-9